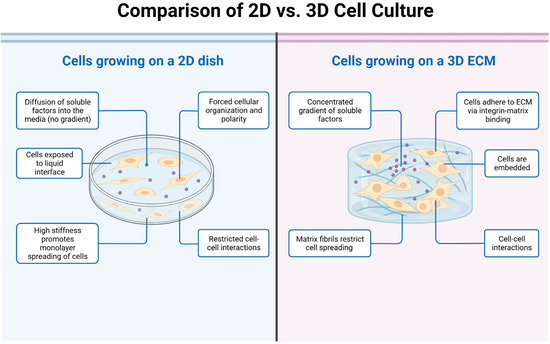
Osteosarcoma (OS) is not a uniform mass of cancer cells, but a complex, organ-like structure with diverse cell types influenced by various environmental factors. An individual with OS is subject to a multitude of complex biological, structural, mechanical, and soluble factors that may affect the effectiveness of potential therapeutics. Tumor-associated cells typically located in the vicinity of cancer cells include fibroblasts, immune cells, and endothelial cells. Structural factors include the architecture of the tumor itself (three-dimensionality), with the spherical nature of cell-to-cell interactions and the presence of extracellular matrix (ECM) key features. In addition, the mechanical forces applied by the surrounding microenvironment are important to tumor dynamics. Soluble factors may include gradients of chemicals, such as nutrients and gases, e.g., glucose and oxygen. Accordingly, the need for a more comprehensive range of OS models that precisely simulate this multifaceted tumor microenvironment is imperative for propelling advancements in drug discovery.
- osteosarcoma models
- cell lines
- 3D culture technology
- mice models
1. Two-Dimensional (2D) OS Cell Models
2. Three-Dimensional (3D) OS Cell Models
| Year | Method | Technique | Material/Technique | Cell Line | Pharmaceutical/Therapeutic | Ref. |
|---|---|---|---|---|---|---|
| 2019 | Spheroids cultures | Scaffold-free | Hanging drop technique | MG-63 | PtCl(8-O-quinoline)(dmso) (2) | [42][27] |
| 2019 | Spheroids cultures | Scaffold | High density collagen | MG-63; 148B; |
Biomimetic matrix | [43][28] |
| 2020 | Spheroids cultures | Scaffold-free | Liquid-overlay | SAOS-2 | CSCs tumoroid | [44][29] |
| 2020 | Spheroids cultures | Not Mentioned | Not Mentioned | U2OS; MG-63; |
Gamabufotalin (GBT) | [45][30] |
| 2020 | Spheroids cultures | Not Mentioned | Not Mentioned | U2OS; | Novel imidazopyrimidine derivatives |
[46][31] |
| 2021 | Spheroids cultures | Scaffold-free | Liquid-overlay | MG-63 SW-1353 |
Ca2+-activated K+ channel KCa1.1 | [47][32] |
| 2021 | Spheroids cultures | Scaffold | PLMA-based hydrogels | hBM-MSCs; MG-63 | A co-culture model for drug screening purposes | [48][33] |
| 2021 | Spheroids cultures | Scaffold-free | Hanging drop technique | MHM; MG63; SAOS-2 |
Targeting NAMPT | [49][34] |
| 2021 | Spheroids cultures | Scaffold-free | Liquid-overlay | UMR-106 | BP-loaded MAO-coated Mg–Sr alloy pellet | [50][35] |
| 2022 | Spheroids cultures | Scaffold-free | Liquid-overlay | OHS | 224Ra/212Pb-TCMC-TP-3 | [51][36] |
| 2022 | Spheroids cultures | Scaffold-free | Liquid-overlay | SaOS2 | A novel model for early and late-stage osteosarcoma. | [52][37] |
| 2022 | Spheroids cultures | Scaffold | Polyurethane | SAOS-2 | Assess new treatments. | [53][38] |
| 2022 | Spheroids cultures | Scaffold | Gelatin and hydroxyapatite | MG-63 | The 3D GelHA models can predict the in vivo efficacy of drug targets | [54][39] |
| 2022 | Spheroids cultures | Scaffold | Collagen and chitosan | OSL08; OSL16; OSL20 | Reconstructed high-grade osteosarcoma and its immune and extracellular matrix microenvironment | [55][40] |
| 2022 | Spheroids cultures | Scaffold-free | Liquid-overlay | MG-63 | I-131 radio-nanotherapeutic | [56][41] |
| 2022 | Spheroids cultures | Scaffold | GelMA/HAMA hydrogel. | HOS; 143B; U2-OS cells |
Autophagy-targeted therapy | [57][42] |
| 2022 | Spheroids cultures | Scaffold | Sponge-like Col1/hydroxyapatite nHA | SaOS-2; G-292; U2 OS |
Cold atmospheric plasmas and PTL | [58][43] |
| 2022 | Spheroids cultures | Scaffold | Honeycomb-like GelMA hydrogel | K7M2 | Maintain tumorigenicity preferably. | [59][44] |
| 2023 | Spheroids cultures | Scaffold-free | Liquid-overlay | 143B; MG63; Saos-2 |
Targeting ECM proteins. | [60][45] |
3. Murine Models
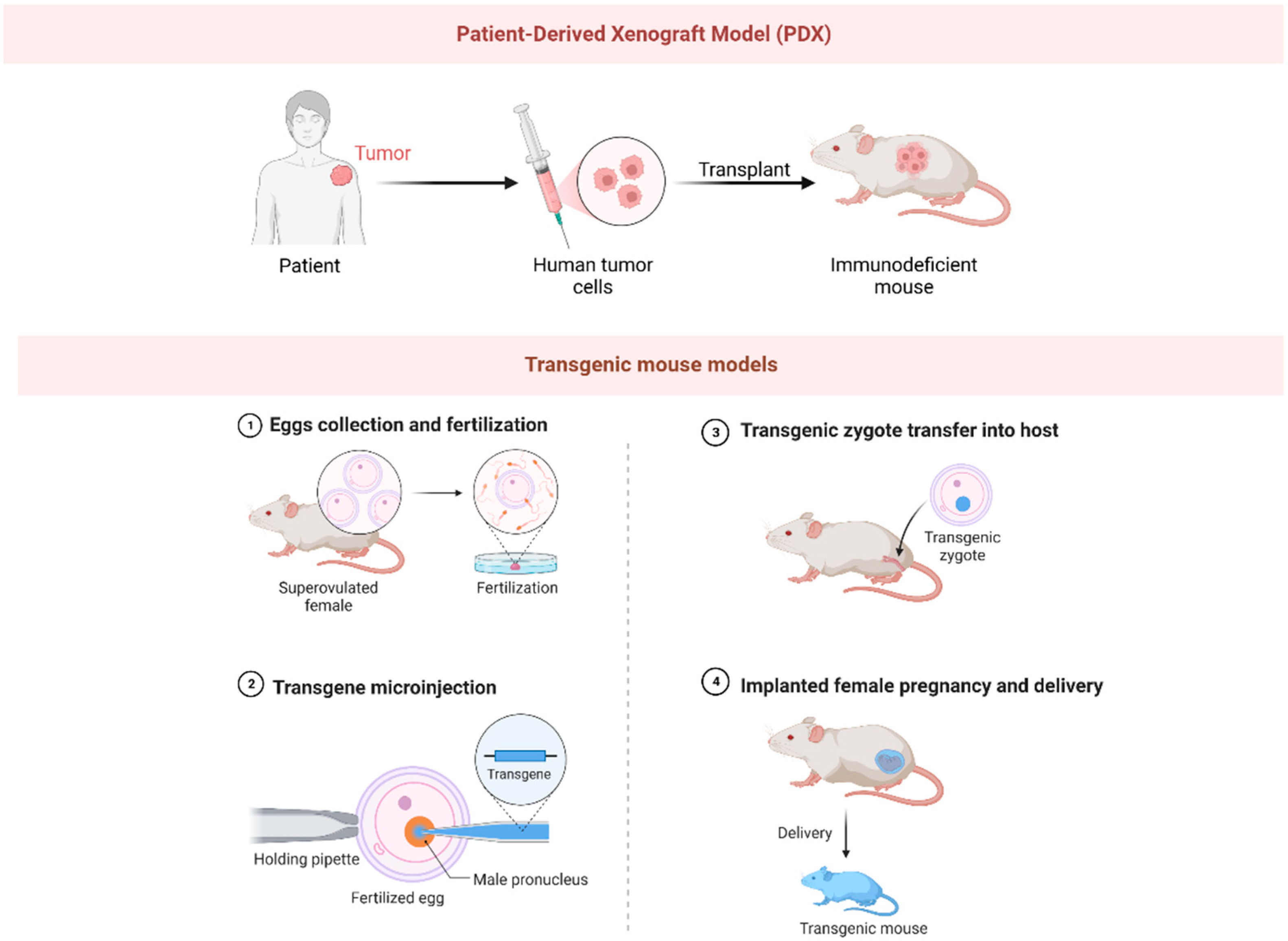
3.1. Xenograft Mouse Models
3.2. Transgenic Mouse Models
4. Canine Models
References
- Shoieb, A.M.; Hahn, K.A.; Barnhill, M.A. An in vivo/in vitro experimental model system for the study of human osteosarcoma: Canine osteosarcoma cells (COS31) which retain osteoblastic and metastatic properties in nude mice. Vivo 1998, 12, 463–472.
- Baudino, T.A. Targeted Cancer Therapy: The Next Generation of Cancer Treatment. Curr. Cancer Drug Targets 2015, 12, 3–20.
- Mohseny, A.B.; Machado, I.; Cai, Y.; Schaefer, K.-L.; Serra, M.; Hogendoorn, P.C.; Llombart-Bosch, A.; Cleton-Jansen, A.-M. Functional characterization of osteosarcoma cell lines provides representative models to study the human disease. Lab. Investig. 2011, 91, 1195–1205.
- Cruz-Ramos, M.; Zamudio-Cuevas, Y.; Medina-Luna, D.; Martínez-Flores, K.; Martínez-Nava, G.; Fernández-Torres, J.; López-Reyes, A.; Solca, F. Afatinib is active in osteosarcoma in osteosarcoma cell lines. J. Cancer Res. Clin. Oncol. 2020, 146, 1693–1700.
- Thanindratarn, P.; Li, X.; Dean, D.C.; Nelson, S.D.; Hornicek, F.J.; Duan, Z. Establishment and Characterization of a Recurrent Osteosarcoma Cell Line: OSA 1777. J. Orthop. Res. 2020, 38, 902–910.
- VanCleave, A.; Palmer, M.; Fang, F.; Torres, H.; Rodezno, T.; Li, Q.; Fuglsby, K.; Evans, C.; Afeworki, Y.; Ross, A.; et al. Development and characterization of the novel human osteosarcoma cell line COS-33 with sustained activation of the mTOR pathway. Oncotarget 2020, 11, 2597–2610.
- Ottaviano, L.; Schaefer, K.-L.; Gajewski, M.; Huckenbeck, W.; Baldus, S.; Rogel, U.; Mackintosh, C.; de Alava, E.; Myklebost, O.; Kresse, S.H.; et al. Molecular characterization of commonly used cell lines for bone tumor research: A trans-European EuroBoNet effort. Genes Chromosom. Cancer 2010, 49, 40–51.
- Weinstein, J.N.; Myers, T.G.; O’Connor, P.M.; Friend, S.H., Jr.; Fornace, A.J.; Kohn, K.W.; Fojo, T.; Bates, S.E.; Rubinstein, L.V.; Anderson, N.L.; et al. An Information-Intensive Approach to the Molecular Pharmacology of Cancer. Science 1997, 275, 343–349.
- Gillet, J.-P.; Calcagno, A.M.; Varma, S.; Marino, M.; Green, L.J.; Vora, M.I.; Patel, C.; Orina, J.N.; Eliseeva, T.A.; Singal, V.; et al. Redefining the relevance of established cancer cell lines to the study of mechanisms of clinical anti-cancer drug resistance. Proc. Natl. Acad. Sci. USA 2011, 108, 18708–18713.
- Gillet, J.-P.; Varma, S.; Gottesman, M.M. The Clinical Relevance of Cancer Cell Lines. Clin. Med. 2013, 105, 452–458.
- Breslin, S.; O’driscoll, L. Three-dimensional cell culture: The missing link in drug discovery. Drug Discov. Today 2013, 18, 240–249.
- Nelson, C.M.; Bissell, M.J. Of Extracellular Matrix, Scaffolds, and Signaling: Tissue Architecture Regulates Development, Homeostasis, and Cancer. Annu. Rev. Cell Dev. Biol. 2006, 22, 287–309.
- Capes-Davis, A.; Theodosopoulos, G.; Atkin, I.; Drexler, H.G.; Kohara, A.; MacLeod, R.A.; Masters, J.R.; Nakamura, Y.; Reid, Y.A.; Reddel, R.R.; et al. Check your cultures! A list of cross-contaminated or misidentified cell lines. Int. J. Cancer 2010, 127, 1–8.
- Edmondson, R.; Broglie, J.J.; Adcock, A.F.; Yang, L. Three-Dimensional Cell Culture Systems and Their Applications in Drug Discovery and Cell-Based Biosensors. ASSAY Drug Dev. Technol. 2014, 12, 207–218.
- Duval, K.; Grover, H.; Han, L.-H.; Mou, Y.; Pegoraro, A.F.; Fredberg, J.; Chen, Z. Modeling Physiological Events in 2D vs. 3D Cell Culture. Physiology 2017, 32, 266–277.
- Guan, X.; Huang, S. Advances in the application of 3D tumor models in precision oncology and drug screening. Front. Bioeng. Biotechnol. 2022, 10, 1021966.
- Chow, T.; Wutami, I.; Lucarelli, E.; Choong, P.F.; Duchi, S.; Di Bella, C. Creating In Vitro Three-Dimensional Tumor Models: A Guide for the Biofabrication of a Primary Osteosarcoma Model. Tissue Eng. Part B Rev. 2021, 27, 514–529.
- Banerjee, D.; Singh, Y.P.; Datta, P.; Ozbolat, V.; O’Donnell, A.; Yeo, M.; Ozbolat, I.T. Strategies for 3D bioprinting of spheroids: A comprehensive review. Biomaterials 2022, 291, 121881.
- Roy, M.; Alix, C.; Bouakaz, A.; Serrière, S.; Escoffre, J.-M. Tumor Spheroids as Model to Design Acoustically Mediated Drug Therapies: A Review. Pharmaceutics 2023, 15, 806.
- Yan, H.H.; Chan, A.S.; Lai, F.P.-L.; Leung, S.Y. Organoid cultures for cancer modeling. Cell Stem Cell 2023, 30, 917–937.
- Hong, K.-J.; Seo, S.-H. Organoid as a culture system for viral vaccine strains. Clin. Exp. Vaccine Res. 2018, 7, 145–148.
- Kretzschmar, K.; Clevers, H. Organoids: Modeling Development and the Stem Cell Niche in a Dish. Dev. Cell 2016, 38, 590–600.
- Ferreira, L.; Gaspar, V.; Mano, J. Bioinstructive microparticles for self-assembly of mesenchymal stem Cell-3D tumor spheroids. Biomaterials 2018, 185, 155–173.
- Nunes, A.S.; Barros, A.S.; Costa, E.C.; Moreira, A.F.; Correia, I.J. 3D tumor spheroids as in vitro models to mimic in vivo human solid tumors resistance to therapeutic drugs. Biotechnol. Bioeng. 2019, 116, 206–226.
- Neto, A.I.; Correia, C.R.; Oliveira, M.B.; Rial-Hermida, M.I.; Alvarez-Lorenzo, C.; Reis, R.L.; Mano, J.F. A novel hanging spherical drop system for the generation of cellular spheroids and high throughput combinatorial drug screening. Biomater. Sci. 2015, 3, 581–585.
- Fitzgerald, K.A.; Malhotra, M.; Curtin, C.M.; Brien, F.J.O.; Driscoll, C.M.O. Life in 3D is never flat: 3D models to optimise drug delivery. J. Control. Release 2015, 215, 39–54.
- Ruiz, M.C.; Resasco, A.; Di Virgilio, A.L.; Ayala, M.; Cavaco, I.; Cabrera, S.; Aleman, J.; León, I.E. In vitro and in vivo anticancer effects of two quinoline–platinum(II) complexes on human osteosarcoma models. Cancer Chemother. Pharmacol. 2019, 83, 681–692.
- Pavlou, M.; Shah, M.; Gikas, P.; Briggs, T.; Roberts, S.; Cheema, U. Osteomimetic matrix components alter cell migration and drug response in a 3D tumour-engineered osteosarcoma model. Acta Biomater. 2019, 96, 247–257.
- Ozturk, S.; Gorgun, C.; Gokalp, S.; Vatansever, S.; Sendemir, A. Development and characterization of cancer stem cell-based tumoroids as an osteosarcoma model. Biotechnol. Bioeng. 2020, 117, 2527–2539.
- Ma, K.; Zhang, C.; Li, W. Gamabufotalin suppressed osteosarcoma stem cells through the TGF-β/periostin/PI3K/AKT pathway. Chem. Interact. 2020, 331, 109275.
- Elie, J.; Feizbakhsh, O.; Desban, N.; Josselin, B.; Baratte, B.; Bescond, A.; Duez, J.; Fant, X.; Bach, S.; Marie, D.; et al. Design of new disubstituted imidazopyridazine derivatives as selective Haspin inhibitors. Synthesis, binding mode and anticancer biological evaluation. J. Enzym. Inhib. Med. Chem. 2020, 35, 1840–1853.
- Ohya, S.; Kajikuri, J.; Endo, K.; Kito, H.; Elboray, E.E.; Suzuki, T. Ca2+-activated K+ channel KCa1.1 as a therapeutic target to overcome chemoresistance in three-dimensional sarcoma spheroid models. Cancer Sci. 2021, 112, 3769–3783.
- Monteiro, C.F.; Custódio, C.A.; Mano, J.F. Bioengineering a humanized 3D tri-culture osteosarcoma model to assess tumor invasiveness and therapy response. Acta Biomater. 2021, 134, 204–214.
- Franceschini, N.; Oosting, J.; Tamsma, M.; Niessen, B.; Bruijn, I.B.-D.; Akker, B.v.D.; Kruisselbrink, A.B.; Palubeckaitė, I.; Bovée, J.V.M.G.; Cleton-Jansen, A.-M. Targeting the NAD Salvage Synthesis Pathway as a Novel Therapeutic Strategy for Osteosarcomas with Low NAPRT Expression. Int. J. Mol. Sci. 2021, 22, 6273.
- Li, M.; Yao, M.; Wang, W.; Wan, P.; Chu, X.; Zheng, Y.; Yang, K.; Zhang, Y. Nitrogen-containing bisphosphonate-loaded micro-arc oxidation coating for biodegradable magnesium alloy pellets inhibits osteosarcoma through targeting of the mevalonate pathway. Acta Biomater. 2021, 121, 682–694.
- Tornes, A.J.K.; Stenberg, V.Y.; Larsen, R.H.; Bruland, Ø.S.; Revheim, M.-E.; Juzeniene, A. Targeted alpha therapy with the 224Ra/212Pb-TCMC-TP-3 dual alpha solution in a multicellular tumor spheroid model of osteosarcoma. Front. Med. 2022, 9, 1058863.
- Freeman, F.E.; Burdis, R.; Mahon, O.R.; Kelly, D.J.; Artzi, N. A Spheroid Model of Early and Late-Stage Osteosarcoma Mimicking the Divergent Relationship between Tumor Elimination and Bone Regeneration. Adv. Healthc. Mater. 2022, 11, 2101296.
- Negrini, N.C.; Ricci, C.; Bongiorni, F.; Trombi, L.; D’alessandro, D.; Danti, S.; Farè, S. An Osteosarcoma Model by 3D Printed Polyurethane Scaffold and In Vitro Generated Bone Extracellular Matrix. Cancers 2022, 14, 2003.
- Díaz, E.C.G.; Lee, A.G.; Sayles, L.C.; Feria, C.; Sweet-Cordero, E.A.; Yang, F. A 3D Osteosarcoma Model with Bone-Mimicking Cues Reveals a Critical Role of Bone Mineral and Informs Drug Discovery. Adv. Healthc. Mater. 2022, 11, e2200768.
- Pierrevelcin, M.; Flacher, V.; Mueller, C.G.; Vauchelles, R.; Guerin, E.; Lhermitte, B.; Pencreach, E.; Reisch, A.; Muller, Q.; Doumard, L.; et al. Engineering Novel 3D Models to Recreate High-Grade Osteosarcoma and its Immune and Extracellular Matrix Microenvironment. Adv. Healthc. Mater. 2022, 11, e2200195.
- Marshall, S.K.; Saelim, B.; Taweesap, M.; Pachana, V.; Panrak, Y.; Makchuchit, N.; Jaroenpakdee, P. Anti-EGFR Targeted Multifunctional I-131 Radio-Nanotherapeutic for Treating Osteosarcoma: In Vitro 3D Tumor Spheroid Model. Nanomaterials 2022, 12, 3517.
- Lin, Y.; Yang, Y.; Yuan, K.; Yang, S.; Zhang, S.; Li, H.; Tang, T. Multi-omics analysis based on 3D-bioprinted models innovates therapeutic target discovery of osteosarcoma. Bioact. Mater. 2022, 18, 459–470.
- Tornín, J.; Mateu-Sanz, M.; Rey, V.; Murillo, D.; Huergo, C.; Gallego, B.; Rodríguez, A.; Rodríguez, R.; Canal, C. Cold plasma and inhibition of STAT3 selectively target tumorigenicity in osteosarcoma. Redox Biol. 2023, 62, 102685.
- He, J.; Chen, C.; Chen, L.; Cheng, R.; Sun, J.; Liu, X.; Wang, L.; Zhu, C.; Hu, S.; Xue, Y.; et al. Honeycomb-Like Hydrogel Microspheres for 3D Bulk Construction of Tumor Models. Research 2022, 2022, 9809763.
- Cortini, M.; Macchi, F.; Reggiani, F.; Vitale, E.; Lipreri, M.V.; Perut, F.; Ciarrocchi, A.; Baldini, N.; Avnet, S. Endogenous Extracellular Matrix Regulates the Response of Osteosarcoma 3D Spheroids to Doxorubicin. Cancers 2023, 15, 1221.
- Beck, J.; Ren, L.; Huang, S.; Berger, E.; Bardales, K.; Mannheimer, J.; Mazcko, C.; LeBlanc, A. Canine and murine models of osteosarcoma. Vet. Pathol. 2022, 59, 399–414.
- Rygaard, J.; Poulsen, C.O. Heterotransplantation Of A Human Malignant Tumour To “Nude” Mice. Acta Pathol. Microbiol. Scand. 1969, 77, 758–760.
- Budach, V.; Stuschke, M.; Budach, W.; Molls, M.; Sack, H. Radioresponsiveness of a human soft tissue sarcoma xenograft to different single and fractionated regimens. Strahlenther. Onkol. 1989, 165, 513–514.
- Higuchi, T.; Igarashi, K.; Yamamoto, N.; Hayashi, K.; Kimura, H.; Miwa, S.; Bouvet, M.; Tsuchiya, H.; Hoffman, R.M. Osteosarcoma Patient-derived Orthotopic Xenograft (PDOX) Models Used to Identify Novel and Effective Therapeutics: A Review. Anticancer Res. 2021, 41, 5865–5871.
- Sampson, V.B.; Kamara, D.F.; Kolb, E.A. Xenograft and genetically engineered mouse model systems of osteosarcoma and Ewing’s sarcoma: Tumor models for cancer drug discovery. Expert Opin. Drug Discov. 2013, 8, 1181–1189.
- Gao, H.; Korn, J.M.; Ferretti, S.; Monahan, J.E.; Wang, Y.; Singh, M.; Zhang, C.; Schnell, C.; Yang, G.; Zhang, Y.; et al. High-throughput screening using patient-derived tumor xenografts to predict clinical trial drug response. Nat. Med. 2015, 21, 1318–1325.
- Higuchi, T.; Igarashi, K.; Yamamoto, N.; Hayashi, K.; Kimura, H.; Miwa, S.; Bouvet, M.; Tsuchiya, H.; Hoffman, R.M. Review: Precise sarcoma patient-derived orthotopic xenograft (PDOX) mouse models enable identification of novel effective combination therapies with the cyclin-dependent kinase inhibitor palbociclib: A strategy for clinical application. Front. Oncol. 2022, 12, 957844.
- Bruheim, S.; Bruland, O.S.; Breistol, K.; Maelandsmo, G.M.; Fodstad, Ø. Human osteosarcoma xenografts and their sensitivity to chemotherapy. Pathol. Oncol. Res. 2004, 10, 133–141.
- Gill, J.; Gorlick, R. Advancing therapy for osteosarcoma. Nat. Rev. Clin. Oncol. 2021, 18, 609–624.
- Wang, G.; Zhang, M.; Meng, P.; Long, C.; Luo, X.; Yang, X.; Wang, Y.; Zhang, Z.; Mwangi, J.; Kamau, P.M.; et al. Anticarin-β shows a promising anti-osteosarcoma effect by specifically inhibiting CCT4 to impair proteostasis. Acta Pharm. Sin. B 2022, 12, 2268–2279.
- Kopp, L.M.; Malempati, S.; Krailo, M.; Gao, Y.; Buxton, A.; Weigel, B.J.; Hawthorne, T.; Crowley, E.; Moscow, J.A.; Reid, J.M.; et al. Phase II trial of the glycoprotein non-metastatic B-targeted antibody–drug conjugate, glembatumumab vedotin (CDX-011), in recurrent osteosarcoma AOST1521: A report from the Children’s Oncology Group. Eur. J. Cancer 2019, 121, 177–183.
- Isakoff, M.S.; Goldsby, R.; Villaluna, D.; Krailo, M.D.; Hingorani, P.; Collier, A.; Morris, C.D.; Kolb, E.A.; Doski, J.J.; Womer, R.B.; et al. A phase II study of eribulin in recurrent or refractory osteosarcoma: A report from the Children’s Oncology Group. Pediatr. Blood Cancer 2019, 66, e27524.
- Gill, J.; Zhang, W.; Zhang, Z.; Roth, M.; Harrison, D.J.; Rowshan, S.; Erickson, S.; Gatto, G.; Kurmasheva, R.; Houghton, P.; et al. Dose-response effect of eribulin in preclinical models of osteosarcoma by the pediatric preclinical testing consortium. Pediatr. Blood Cancer 2020, 67, e28606.
- Liao, N.; Koehne, T.; Tuckermann, J.; Triviai, I.; Amling, M.; David, J.-P.; Schinke, T.; Luther, J. Osteoblast-specific inactivation of p53 results in locally increased bone formation. PLoS ONE 2021, 16, e0249894.
- Wang, J.; Aldahamsheh, O.; Ferrena, A.; Borjihan, H.; Singla, A.; Yaguare, S.; Singh, S.; Viscarret, V.; Tingling, J.; Zi, X.; et al. The interaction of SKP2 with p27 enhances the progression and stemness of osteosarcoma. Ann. N. Y. Acad. Sci. 2021, 1490, 90–104.
- Ferrena, A.; Wang, J.; Zhang, R.; Karadal-Ferrena, B.; Al-Hardan, W.; Singh, S.; Borjihan, H.; Schwartz, E.; Zhao, H.; Yang, R.; et al. SKP2 knockout in Rb1/p53 deficient mouse models of osteosarcoma induces immune infiltration and drives a transcriptional program with a favorable prognosis. bioRxiv 2023.
- Zheng, B.; Ren, T.; Huang, Y.; Sun, K.; Wang, S.; Bao, X.; Liu, K.; Guo, W. PD-1 axis expression in musculoskeletal tumors and antitumor effect of nivolumab in osteosarcoma model of humanized mouse. J. Hematol. Oncol. 2018, 11, 16.
- Withrow, S.J.; Wilkins, R.M. Cross talk from pets to people: Translational osteosarcoma treatments. ILAR J. 2010, 51, 208–213.
- Fan, T.M.; Roberts, R.D.; Lizardo, M.M. Understanding and Modeling Metastasis Biology to Improve Therapeutic Strategies for Combating Osteosarcoma Progression. Front. Oncol. 2020, 10, 13.
- Chirio, D.; Sapino, S.; Chindamo, G.; Peira, E.; Vercelli, C.; Riganti, C.; Manzoli, M.; Gambino, G.; Re, G.; Gallarate, M. Doxorubicin-Loaded Lipid Nanoparticles Coated with Calcium Phosphate as a Potential Tool in Human and Canine Osteosarcoma Therapy. Pharmaceutics 2022, 14, 1362.
- Yang, Y.-T.; Yuzbasiyan-Gurkan, V. Sorafenib and Doxorubicin Show Synergistic Effects in Human and Canine Osteosarcoma Cell Lines. Int. J. Mol. Sci. 2022, 23, 9345.
- Regan, D.P.; Chow, L.; Das, S.; Haines, L.; Palmer, E.; Kurihara, J.N.; Coy, J.W.; Mathias, A.; Thamm, D.H.; Gustafson, D.L.; et al. Losartan Blocks Osteosarcoma-Elicited Monocyte Recruitment, and Combined With the Kinase Inhibitor Toceranib, Exerts Significant Clinical Benefit in Canine Metastatic Osteosarcoma. Clin. Cancer Res. 2022, 28, 662–676.
- Witta, S.; Collins, K.P.; Ramirez, D.A.; Mannheimer, J.D.; Wittenburg, L.A.; Gustafson, D.L. Vinblastine pharmacokinetics in mouse, dog, and human in the context of a physiologically based model incorporating tissue-specific drug binding, transport, and metabolism. Pharmacol. Res. Perspect. 2023, 11, e01052.
- Becker, M.; Volk, H.; Kunzmann, P. Is Pet Health Insurance Able to Improve Veterinary Care? Why Pet Health Insurance for Dogs and Cats Has Limits: An Ethical Consideration on Pet Health Insurance. Animals 2022, 12, 1728.
