Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Rita Xu and Version 1 by Pritam Kumar Dikshit.
Biofuel, an inexhaustible fuel source, plays a pivotal role in the contemporary era by diminishing the dependence on non-renewable energy sources and facilitating the mitigation of CO
2
emissions. Due to the many constraints in existing technology and the resulting increased costs, the production of biofuels on a large scale is a laborious process. The production of biofuels can be enhanced with intervention of nanotechnology, owing to the unique characteristics of these nanoparticles. These nanoparticles interact with the microorganisms and enzymes leading to the enhancement in biofuel production.
- biofuels
- nanomaterials
- nanoparticle interaction
1. Introduction
In view of the severity of the threat posed by catastrophic climate change, typically caused by the burning of fossil fuels, it is becoming increasingly apparent that civilizations must modify the way they generate and utilize energy. Increasing the stream of safe, clean, and benign energy will be challenging and needs adequate inventiveness and capital. There are several resource and technological choices that might reduce emissions in the transportation, shipping, electricity generation, and boiler/heating industries while strengthening energy security [1]. A crucial strategy for guiding the transition from an oil-based economy to a novel bioeconomy—a bioeconomy that seeks to achieve a more productive and sustainable global development by achieving a BCG economy—is the biorefinery approach. This approach aims to produce biofuels, platform chemicals, and bioproducts from insatiable biomass sources. As a result, this a strategy emphasizes safe and green chemical processes, aims to reduce waste discharges, creates new opportunities for markets, and makes better use of available resources. The bioethanol production from waste agricultural residues aligns with SDG-7 (Affordable and Clean Energy) and SDG-12 (Responsible Consumption and Production) by utilizing agricultural residues and non-food crops. This approach reduces waste and promotes efficient resource utilization for energy production and contributes to the global goal of achieving net zero emissions by 2050 [2,3][2][3]. However, in order to compete with petroleum in the long run and maintain economic viability, it is crucial to concentrate on producing bioethanol at a low cost with the integration of advanced technologies and multidisciplinary approach.
The market for bioethanol was estimated at USD 33.61 billion in 2021 and is anticipated to grow at a CAGR of 14.1% in the coming years, with revenues expected to reach USD 101.64 billion in 2030 [4]. Molasses from sugar cane, starchy crops, agricultural remains, and other discarded crops can all be used to make bioethanol. Manufacturers are concentrating on miscanthus, switch grass, and sugarcane bagasse as energy crops for production, with Europe, North America, Asia Pacific, Middle East, Latin America, and Africa included in the regional scope. Government regulatory agencies promoting production are the main factors driving the global bioethanol industry [5]. The industry is expanding due to rising government initiatives to generate and utilize cleaner fuels such as bio-ethanol as well as the rising demand for blending in petrol. Some of the key market players in the bioethanol industry are Archer Daniels Midland, Abengoa Bioenergy, BlueFire Ethanol Fuels Inc., Bioethanol Japan Kansai Co Ltd., CropEnergies AG, Cremer Oleo GmbH & Co., Green Plains, Green Future Innovations Inc., Petrobras Biocombustíveis, Nordzucker AG, Royal Dutch Shell PLC, RaizenEnergia, Tereos, Soufflet Group, and Valero Energy Corporation. To increase their consumer base and firmly establish their position in the market, these businesses are extending their reach across a variety of geographies and are breaking into new markets in emerging nations [6].
The introduction of lignocellulosic biomass (LB) resources as a replacement source of green renewable energy has drawn significant recognition in regard to the rising demand for biofuels due to their abundance, absence of competition with food, and ability to produce sustainable value-added compounds, including biofuels [7]. LB feedstocks provide a plentiful source of organic carbon that may be extensively used for their transformation into bio-based chemicals and biofuels with added value. LB is made up of primarily both polar and non-polar polymeric materials such as cellulose, hemicellulose, and lignin [8]. Cellulose (40–60%), hemicellulose (20–40%), and lignin (10–25%) make up the majority of the components in LB products [9]. Due to its abundance and low cost as a feedstock, LB can provide around 40% of the world’s energy demands [10]. As of now, a variety of lignocellulose biomass including rice straw [11], elephant grass [12], switch grass [13], palm wood [14], agricultural waste [15], algal biomass residues [16], and textile mill waste including cotton spinning waste [17] are utilized as bioethanol feedstocks. The cell wall’s composition, level of lignification, and cellulose’s crystallinity, are the major causes of the structural recalcitrance, enabling lignocellulose to resist chemical and biological deconstruction [18]. It is challenging to convert LB into simple sugars because cellulose, a key component of LB, is bound to lignin and hemicellulose through different bonds. A known robust component of LB called lignin prevents the use of biocatalysts and enzymes to hydrolyze polymeric constituents such as cellulose and hemicellulose. Due to this, LB pre-treatment is the only method that can completely remove lignin and allow polysaccharide components to be fully utilized. After pre-treatment, the separated cellulose/hemicellulose-rich elements can be used for enzymatic hydrolysis and subsequent fermentation, while the segregated fractions can be further used to synthesize biochemicals. At the commercial level, for the pretreatment process employed by LB biorefineries, principal cost investment, energy consumption, and whole process efficiency are often taken into account. There are now a number of pretreatment techniques broadly classified as physical, chemical, and physicochemical procedures. The chemical pretreatment uses a variety of chemicals, including acid, alkaline, organosolv, and/or ionic liquids [19]. Pretreatment frequently involves the use of acids in particular sulphuric acid and alkalies such as NaOH and CaO [20]. These techniques can effectively extract lignin or digest hemicellulose to break down lignocellulose’s structural stubbornness [21]. Their use is constrained, however, mainly due to the high-energy need and production of toxic harmful chemicals (hydroxymethyl furfural and furfural) that can impede the activity of biocatalysts employed in fermentation. Nonetheless, the acid and alkali treatment are the most widely used method for the pretreatment of biomass due to its low cost and ambient operating conditions. Additionally, the used acid/alkali in the pretreatment process must be recycled from the hydrolysate after completion of the process to minimize its hazardous impact to the environment, and reusing recycled acid/alkali can improve the process economy [22]. Furthermore, scientists are looking for the best solution that can efficiently saccharize biomass by enzymatic approaches using hydrolases (cellulases and xylanases), oxidoreductases (laccases), recombinant feruloyl esterase, etc. [23[23][24],24], and in some cases genetic lignocellulose modification [21,24][21][24]. Contrarily, using biological techniques for LB pretreatment has a number of drawbacks, including the need for a catalyst that meets the stringent constraints, a lack of stability, and expensive manufacturing and purifying procedures.
These barriers restrict the use of current techniques and demand for the advancement of a quick, efficient, cost-effective, and environmentally friendly approach for LB pretreatment. As a result, it is preferable to look for better options that include sustainable and energy-efficient methods for pretreating LB materials. As a result, several techniques have recently been developed by researchers, and one of them is nanoparticles (NPs) assisted pretreatment of LB. Nanomaterials are extensively explored in a variety of applications in medicine, pollutant removal, biosensing, biomass conversion, and the production of biofuels due to their numerous advantages, including higher stability, low-cost production, recyclability, and better catalytic ability [25]. Utilizing nanobiocatalytic substances in bioprocesses is aimed at enhancing process efficiency by improving heat and mass transfer, as well as enzymatic and cellular metabolic processes. This is achieved through their vast surface areas, catalytic characteristics, and enzyme cofactor activity [26].
2. Lignocellulosic Biofuel
The initial step in the institution of sustainable biofuel production is the capability to use suitable lignocellulosic biomass as feedstock for the selected product, which is otherwise considered trash and is often simply burned, leading to environmental pollution. Lignocellulosic biomass is a plentiful, affordable, renewable, and carbon-neutral resource that can be exploited to make second-generation biofuels without affecting the food security of the world. Its production is enormous on a global scale and accounts for 181.5 billion tons per year [27,28][27][28]. Depending on the type, to varying degrees and proportions, these polymers are structured in an intricate, non-uniform, three-dimensional spatial configuration. The hydrophobic property of lignin, the crystalline structure of cellulose, and the encasement of cellulose by the lignin-hemicellulose matrix, which is firmly bound by hydrogen and covalent bonds, all have an impact on the resilience of lignocellulose [29]. Biochemical or thermochemical processes are typically used for transforming lignocellulosic biomass into bioenergy. The biochemical approach uses microorganisms and/or a variety of enzymes to reduce the feedstock into fermentable sugars, which are then fermented to make biofuels, such as bioethanol, biogas, biobutanol, biohydrogen, biodiesel, and so on. Biochemical procedures typically include very mild reaction conditions. The inclusion of a pretreatment step (chemical, physical, or biological), prior to hydrolysis improves the overall procedure and makes it commercially feasible. The thermochemical route, on the other hand, includes pyrolysis, liquefaction, and gasification to produce a variety of fuels, such as, ethanol, renewable diesel, and aviation fuel. The presence of moisture has a detrimental impact on product yields and emissions, but the thermochemical conversion can employ a wider variety of feedstock and is unaffected by lignin in the biomass.2.1. Bioethanol
Separate hydrolysis and fermentation (SHF), simultaneous saccharification and fermentation (SSF), Pre-saccharification and simultaneous saccharification and fermentation (PSSF) and consolidated bioprocessing (CBP) are the primary adopted techniques for bioethanol production [40][30]. Considering bioethanol, the saccharification-fermentation process is the main biological mechanism for converting lignocellulosic biomass into bioenergy. According to this method [41][31], biomass is hydrolyzed to create monosaccharides, which are subsequently fermented to generate ethanol. An alternative to this is gasification-fermentation, which eliminates the intricate saccharification stage, addressing a key downside of saccharification-fermentation. LB is thermally gasified to create synthetic gas (syngas), which is made of CO, H2, CO2, and N2, and is subsequently fermented to create biomaterials, such as bioethanol [42][32]. Despite decades of development and research targeted at raising the market value of biomass, commercialized bioenergy production from lignocellulose biomass still needs technological and financial advancement [9].2.2. Biobutanol
Biobutanol (C4H10O), also known as butyl alcohol, is a renewable biofuel that has an advantage over bioethanol due to its higher energy density, immiscibility in water, lower Reid vapor pressure, low toxicity, and compatibility with existing infrastructure [43][33]. It is primarily generated by the acetone-butanol-ethanol (ABE) fermentation process, which entails the microbial fermentation of sugars from biomass feedstocks into butanol using particular bacterial strains, such as Clostridium species. Butanol’s toxicity to microorganisms, problems with butanol recovery later on, the choice of biomass, and pretreatment, which have an impact on large-scale synthesis, are the main obstacles of the ABE process [44][34]. The biobutanol industries create a variety of high-value byproducts, including plastics, fibers, solvents, and coatings. They also serve as an important precursor for many chemicals with added value including butyl acetate, acrylic acid, adhesives, and glycol ethers in addition to producing the primary transportation fuel, all of which have the potential to boost economic growth through a variety of product alternates [45][35].2.3. Biodiesel
Biodiesel is a clean energy source that reduces greenhouse gas emissions, maintains ecological balance, and is compatible with existing infrastructure. It is derived from biological sources such as edible and non-edible oils, animal fats, and waste cooking oils [46][36]. Traditional physicochemical processes include transesterification, esterification, pyrolysis, and micro-emulsion, among which transesterification, wherein triglycerides and alcohol react in the presence of a catalyst to yield fatty acids alkyl ester and glycerol at low temperature and pressure, is cost-effective and yields high-quality products. However, conventional production methods have reached their maximum efficiency, making biodiesel less competitive than petroleum-based diesel [47][37]. In recent years, there has been a growing demand for innovative, clean, and enhanced technology to accelerate the reaction times, use less energy and catalysts, and maintain excellent biodiesel quality. To that end, a variety of techniques, including microwave, ultrasonic, supercritical, hydrodynamic cavitation, reactive distillation, membrane, plasma, cosolvent, rotatory, and plug flow reactors, have been investigated for biodiesel production [48][38].2.4. Biohydrogen
Hydrogen, with its high energy density, is used in industries as a fuel and renewable energy source. However, traditional techniques such as water electrolysis and auto-thermal processes are economically unviable due to their high-power requirements. Biohydrogen, a carbon-neutral process, offers potential benefits over thermochemical and electrochemical methods [49][39]. It can be produced using pure sugars or waste substrates such as lignocellulosic biomass and microalgae. It is produced by dark fermentation, photofermentation, or a combination of these methods. However, the generation and output of biohydrogen are influenced by substrate availability, inoculum origin, and operational factors. A study by Patel et al. established a low-cost biohydrogen manufacturing technique using agricultural waste. H2 production peaked at 37 °C and pH 8.5. The highest H2 yield was measured in wheat straw pre-hydrolysate (WSPH) at 2.54 ± 0.2 mol-H2/mol-reducing sugar and in pre-treated wheat straw enzymatic-hydrolysate (WSEH) at 2.61 ± 0.1 mol-H2/mol-reducing sugar [50][40]. Advancements in technology and renewable energy sources make biohydrogen a promising option for a cleaner and more sustainable future [51][41].2.5. Biogas
Biogas is one of the most important renewable energy sources to solve the environmental and energy challenges and serves as a substitute to natural gas or transportation fuel. Biogas refers to a mixture of gases produced by anaerobic organisms via the fermentation of organic materials such as plant materials, agricultural waste, food waste, sewage, municipal waste, and compost without the presence of oxygen [52][42]. This process is known as bio-methanation, and it primarily produces methane and carbon dioxide with minute amounts of hydrogen sulphides and siloxanes. The main variables affecting the effectiveness of biogas production procedures include organic loading rate, pH, carbon to nitrogen ratio, temperature, retention duration, and mixing rate. According to their sensitivity to temperature, the microorganisms utilized in the bioreactor are divided into three main groups: psychrophilic (15–25 °C), mesophilic (35–40 °C), and thermophilic (55–60 °C) [53][43]. However, the actual use of lignocellulose-based material in the anaerobic digestion process is limited because of the biomass’s resistant nature, which results in poor digestion efficiency and biodegradation. Further developments, such as the use of several pretreatment techniques, microbial inoculum, and the application of chemical (NaOH and CaO) and biological (white-rot and brown-rot fungi) additives, are being prioritized in order to speed up microbial growth and the rate of biogas production [54][44]. In an investigation, researchers have studied the biogas production from pineapple waste, in which both the biogas and methane production showed significant increases (mL/day) from longer to shorter HRT. The maximal values (HRT 5 days, OLR 5 g/COD/day with recirculation) were 55,130 and 30,322 mL/day, respectively [55][45]. Its ability to harness methane from organic waste, its various applications in energy generation, cooking, and transportation, as well as its role in fertilizer production, make biogas a valuable asset in ourthe transition towards a cleaner and more sustainable energy system.3. Nanoparticle Application in Biofuel Generation
Lignocellulosic biofuels, obtained from plentiful organic sources provide a hopeful pathway for sustainable energy. Nevertheless, the conversion of these substances into biofuels encounters obstacles as a result of intricate compositions and ineffective decomposition mechanisms. Nanoparticle or nanomaterial application plays a transformative role by acting as a catalyst in the conversion of lignocellulosic materials into biofuels. NMs are defined as materials that have components or particles with at least one dimension in the nanometer scale, typically ranging from one nanometer to a few hundred nanometers. Furthermore, NMs where all dimensions are at the nanoscale are called NPs. Nanoparticles are essential for speeding up the decomposition of the resistant components in lignocellulose, which improves the overall effectiveness of biofuel manufacturing. Their catalytic characteristics greatly enhance the efficiency and standard of biofuels obtained from these renewable raw materials, representing a crucial breakthrough in sustainable energy generation. The application of nanomaterial at different process stages for improving the biofuel quality and yield is represented in Figure 1.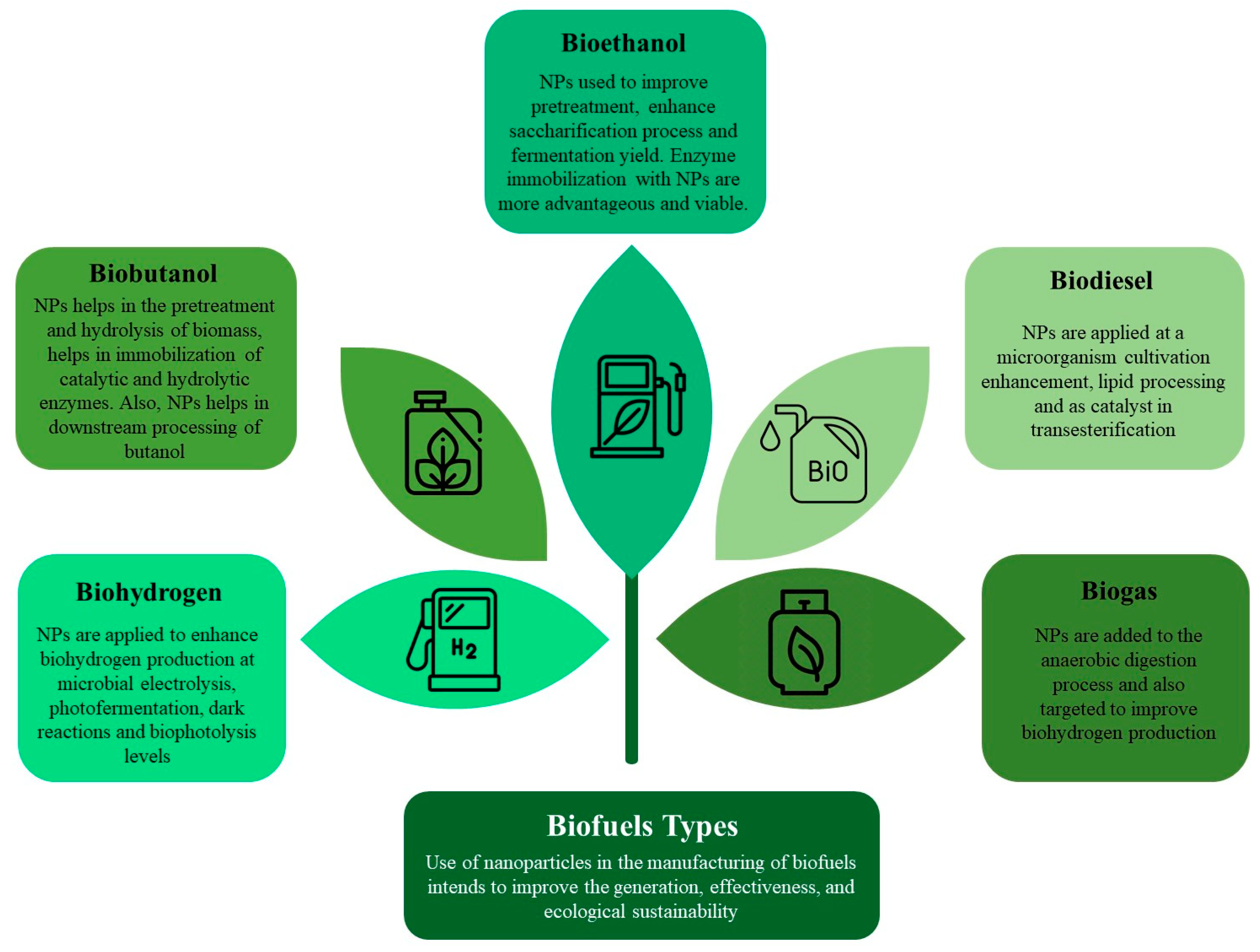
Figure 1. Possible avenues for the utilization of nanoparticles for various biofuel production.
3.1. Bioethanol
Application of nanomaterials in bioethanol production is numerous and encompasses all stages of the lignocellulose to bioethanol process, ranging from pretreatment to fermentation. For example, Kim et al. (2023) explored the use of cerium-doped iron oxide nanoparticles for the simultaneous pretreatment and saccharification of raw corn cob biomass [57][47]. These modified nanoparticles exhibit laccase and cellulase/hemicellulase mimicking properties, which further aids in the pretreatment. The synthesized NPs are of spherical shape with a size ranging from 40 to 70 nm. During simultaneous pretreatment and saccharification, a small amount of cellulose/hemicellulose enzymes are used. During the pretreatment process, approximately 44% of delignification was achieved due to the laccase mimicking properties of NPs. Further, the hydrolysate resulted in a maximum ethanol production of 21.7 g/L. In another study, Iron oxide NPs of size 70–100 nm synthesized with the Spinacia oleracea leaves extract for bioethanol production from Corncob yielded 53.7% ethanol [58][48].3.2. Biohydrogen
Anaerobic bacteria used in dark fermentation break down carbohydrate-rich substrate and create hydrogen. With an energy density of 140 MJ/kg, which is higher than that of coal (24 MJ/kg) and petrol (44 MJ/kg), biohydrogen (H2) has a lot of promise as a cheap, renewable, carbon-free, and ecologically friendly fuel [59][49]. The kind of raw materials used, the nutrients that are accessible (such as C, N2, PO43−, and SO42−), and other operational circumstances all have an impact on the synthesis of biohydrogen. To boost microbial development and enhance the activity of enzymes involved in producing H2, researchers are proposing novel strategies, such as the use of mixed substrates, mixed microbial culture, and usage of nanomaterials and carbon-based biomaterials [60][50]. An important way to produce bio-H2 is nanotechnology-based pretreatment on lignocellulosic biomass structures. The cost of the procedure is decreased since the chemicals are readily recyclable and usable again [61][51]. Titanium NPs produced 127% more biohydrogen when combined with sugarcane bagasse and anaerobic sludge [62][52]. In a comparable manner, using palladium NP in a mixed culture also included with glucose produced 9% H2 [63][53].3.3. Biobutanol
A sustainable, environmentally friendly and perhaps practical alternative fuel to conventional petrol is biobutanol. Zinc oxide (ZnO) NPs were utilized as a catalyst in fermentation from coffee bean husk, a byproduct of farm waste chosen as raw material, and biobutanol was effectively produced by ABE fermentation. High butanol production and 0.36 g/L alcohol, 70.5% sugars were observed with 10 g of ZnO NPs utilized as catalyst [64][54]. In another study by Gandarias et al. [65][55], employing distinct bimetallic and trimetallic catalysts made by sol-immobilization under reaction conditions of 100 °C, 6 h, at 3 bar O2, n-butanol solution yielded butyraldehyde, butyl butyrate, and butyric acid as a conversion product. In a case, Pt/C metal(s)/support NP have a 91.8% conversion rate of n-butanol with a yield of 23.2% butyraldehyde, 67.9% butyric acid, and 8.8% butyl-butyrate.3.4. Biogas
Research is ongoing to determine the effect of nanoadditives on the anaerobic digestion (AD) process and, as a result, the output of biogas. The study examined the effects of titanium dioxide, zinc oxide, and silver nanoparticles (with an average size of at least 1 dimension <100 nm) on the process of methanogenesis in mesophilic batch anaerobic digestion of primary sludge. The findings indicated that none of the NPs used had a significant impact on methane generation. The methane production rates, measured in m3 of CH4 per kilogram of volatile solids, ranged from 0.08 to 0.13. There was no statistically significant difference observed between the control groups and experimental sets for the examined NPs [66][56]. The majority of previous studies have suggested that methane production is decreased by increased NP concentrations. The results on several conductive materials, including graphene oxide (GO), carbon fibers, activated carbon, iron oxides, and biochar, support direct interspecies electron transfer, which is a state-of-the-art method to increase biomethane output. Mixed anaerobic culture with graphene oxide on the anaerobic fermentation process of assam lemon showed 219.64 mL/g VS fed improvement of biomethane yield [67][57].3.5. Biodiesel
The process of producing biodiesel can be done in a number of ways, including transesterification, pyrolysis or cracking, and micro-emulsion. The most widely used technique for producing biodiesel among them is triglyceride transesterification of methanol or ethanol in the presence of a chemical or biological catalyst. This may be done by immobilizing lipase on magnetic nanoparticles (MNPs), which will increase the triacylglycerol (TAG) conversion in the presence of a catalyst. Another way of biodiesel production is through whole cell immobilization of recombinant Aspergillus oryzae, which was engineered for the generation of biodiesel and expressed the Candida antarctica lipase B gene (r-CALB), according to a study by Adachi et al. [68][58]. In another study, solid catalyst NPs derived from oil-palm empty fruit bunches were used as a renewable catalyst for biodiesel production. The study observed the highest palm-oil to biodiesel conversion of up to 97.90% when using 1% palm bunch ash nanocatalyst, which was produced by heating empty fruit bunches at 600 °C, and a 3 h reaction time. [69][59].References
- Bauen, A. Future energy sources and systems—Acting on climate change and energy security. J. Power Sources 2006, 157, 893–901.
- Sriariyanun, M.; Gundupalli, M.P.; Phakeenuya, V.; Phusamtisampan, T.; Cheng, Y.S.; Venkatachalam, P. Biorefinery approaches for production of cellulosic ethanol fuel using recombinant engineered microorganisms. J. Appl. Sci. Eng. 2023, 27, 1985–2005.
- Jose, D.; Kitiborwornkul, N.; Sriariyanun, M.; Keerthi, K. A review on chemical pretreatment methods of lignocellulosic biomass: Recent advances and progress. Appl. Sci. Eng. Prog. 2022, 15, 6210.
- Bioethanol Market Share, Size, Trends, Industry Analysis Report, By Feedstock (Cereals & Starch, Wheat, Maize, Beet, Sugarcane, Others); By Industry; By Region; Segment Forecast, 2022–2030. Available online: https://www.polarismarketresearch.com/industry-analysis/bioethanol-market (accessed on 30 October 2023).
- Vaishnavi, S.; Ghosh, S.; Singh, R.; Irshath, A.; Rajan, A.P. BIOELAION-Biofuel-Based Business Model Plan. Int. J. Humanit. Soc. Sci. Manag. (IJHSSM) 2023, 3, 194–207.
- Bioethanol Market Size Global Report, 2022–2030. Available online: https://www.polarismarketresearch.com/industry-analysis/bioethanol-market (accessed on 30 October 2023).
- Toor, M.; Kumar, S.S.; Malyan, S.K.; Bishnoi, N.R.; Mathimani, T.; Rajendran, K.; Pugazhendhi, A. An overview on bioethanol production from lignocellulosic feedstocks. Chemosphere 2020, 242, 125080.
- Kommoji, S.; Gopinath, M.; Sagar, P.S.; Yuvaraj, D.; Iyyappan, J.; Varsha, A.J.; Sunil, V. Lipid bioproduction from delignified native grass (Cyperus distans) hydrolysate by Yarrowia lipolytica. Bioresour. Technol. 2021, 324, 124659.
- Singhvi, M.; Kim, B.S. Current developments in lignocellulosic biomass conversion into biofuels using nanobiotechology approach. Energies 2020, 13, 5300.
- Alio, M.A.; Tugui, O.C.; Rusu, L.; Pons, A.; Vial, C. Hydrolysis and fermentation steps of a pretreated sawmill mixed feedstock for bioethanol production in a wood biorefinery. Bioresour. Technol. 2020, 310, 123412.
- Li, J.; Tang, X.; Chen, S.; Zhao, J.; Shao, T. Ensiling pretreatment with two novel microbial consortia enhances bioethanol production in sterile rice straw. Bioresour. Technol. 2021, 339, 125507.
- Sivarathnakumar, S.; Jayamuthunagai, J.; Baskar, G.; Praveenkumar, R.; Selvakumari, I.A.E.; Bharathiraja, B. Bioethanol production from woody stem Prosopis juliflora using thermo tolerant yeast Kluyveromyces marxianus and its kinetics studies. Bioresour. Technol. 2019, 293, 122060.
- Saini, S.; Kumar, A.; Singhal, B.; Kuhad, R.C.; Sharma, K.K. Fungal oxidoreductases and CAZymes effectively degrade lignocellulosic component of switchgrass for bioethanol production. Fuel 2022, 328, 125341.
- Sathendra, E.R.; Baskar, G.; Praveenkumar, R.; Gnansounou, E. Bioethanol production from palm wood using Trichoderma reesei and Kluveromyces marxianus. Bioresour. Technol. 2019, 271, 345–352.
- Elsa, C.; Kumar, M.D.; Baskar, G. Immobilization of cellulase onto manganese dioxide nanoparticles for bioethanol production by enhanced hydrolysis of agricultural waste. Chin. J. Catal. 2015, 36, 1223–1229.
- Gengiah, K.; Moses, G.L.P.; Baskar, G. Bioethanol production from Codium tomentosum residue. Energy Sources Part A Recovery Util. Environ. Eff. 2020, 1–10.
- Ranjithkumar, M.; Rajarathinam, R.; Kumar, P.S.; Rangasamy, G.; Gurunathan, B.; Ethiraj, B.; Thanabal, V. Insight into the effective utilization of cotton spinning wastes from textile mills for the production of bioethanol. Sustain. Energy Technol. Assess. 2022, 53, 102770.
- Usmani, Z.; Sharma, M.; Gupta, P.; Karpichev, Y.; Gathergood, N.; Bhat, R.; Gupta, V.K. Ionic liquid based pretreatment of lignocellulosic biomass for enhanced bioconversion. Bioresour. Technol. 2020, 304, 123003.
- Zhao, L.; Sun, Z.F.; Zhang, C.C.; Nan, J.; Ren, N.Q.; Lee, D.J.; Chen, C. Advances in pretreatment of lignocellulosic biomass for bioenergy production: Challenges and perspectives. Bioresour. Technol. 2022, 343, 126123.
- Lv, Z.; Liu, F.; Zhang, Y.; Tu, Y.; Chen, P.; Peng, L. Ecologically adaptable Populus simonii is specific for recalcitrance-reduced lignocellulose and largely enhanced enzymatic saccharification among woody plants. GCB Bioenergy 2021, 13, 348–360.
- Kumar, A.K.; Sharma, S. Recent updates on different methods of pretreatment of lignocellulosic feedstocks: A review. Bioresour. Bioprocess. 2017, 4, 7.
- Tabka, M.G.; Herpoël-Gimbert, I.; Monod, F.; Asther, M.; Sigoillot, J.C. Enzymatic saccharification of wheat straw for bioethanol production by a combined cellulase xylanase and feruloyl esterase treatment. Enzym. Microb. Technol. 2006, 39, 897–902.
- Ingle, A.P.; Chandel, A.K.; Antunes, F.A.F.; Rai, M.; da Silva, S.S. New trends in application of nanotechnology for the pretreatment of lignocellulosic biomass. Biofuels Bioproduct. Biorefining 2019, 13, 776–788.
- Wu, L.; Feng, S.; Deng, J.; Yu, B.; Wang, Y.; He, B.; Peng, H.; Li, Q.; Hu, R.; Peng, L. Altered carbon assimilation and cellulose accessibility to maximize bioethanol yield under low-cost biomass processing in corn brittle stalk. Green Chem. 2019, 21, 4388–4399.
- Zhou, Y.; Liu, B.; Yang, R.; Liu, J. Filling in the gaps between nanozymes and enzymes: Challenges and opportunities. Bioconjug. Chem. 2017, 28, 2903–2909.
- Kim, Y.K.; Lee, H. Use of magnetic nanoparticles to enhance bioethanol production in syngas fermentation. Bioresour. Technol. 2016, 204, 139–144.
- Ashokkumar, V.; Venkatkarthick, R.; Jayashree, S.; Chuetor, S.; Dharmaraj, S.; Kumar, G.; Chen, W.H.; Ngamcharussrivichai, C. Recent advances in lignocellulosic biomass for biofuels and value-added bioproducts-A critical review. Bioresour. Technol. 2022, 344, 126195.
- Peng, F.; Ren, J.L.; Xu, F.; Bian, J.; Peng, P.; Sun, R.C. Fractional study of alkali-soluble hemicelluloses obtained by graded ethanol precipitation from sugar cane bagasse. J. Agric. Food Chem. 2010, 58, 1768–1776.
- Woiciechowski, A.L.; Dalmas Neto, C.J.; Porto de Souza Vandenberghe, L.; de Carvalho Neto, D.P.; Novak Sydney, A.C.; Letti, L.A.J.; Karp, S.G.; Zevallos Torres, L.A.; Soccol, C.R. Lignocellulosic biomass: Acid and alkaline pretreatments and their effects on biomass recalcitrance—Conventional processing and recent advances. Bioresour. Technol. 2020, 304, 122848.
- López-Linares, J.C.; Romero, I.; Cara, C.; Ruiz, E.; Moya, M.; Castro, E. Bioethanol production from rapeseed straw at high solids loading with different process configurations. Fuel 2014, 122, 112–118.
- Kootstra, A.M.J.; Mosier, N.S.; Scott, E.L.; Beeftink, H.H.; Sanders, J.P. Differential effects of mineral and organic acids on the kinetics of arabinose degradation under lignocellulose pretreatment conditions. Biochem. Eng. J. 2009, 43, 92–97.
- Liu, K.; Atiyeh, H.K.; Stevenson, B.S.; Tanner, R.S.; Wilkins, M.R.; Huhnke, R.L. Continuous syngas fermentation for the production of ethanol, n-propanol and n-butanol. Bioresour. Technol. 2014, 151, 69–77.
- Algayyim, S.J.M.; Wandel, A.P.; Yusaf, T.; Hamawand, I. The impact of n-butanol and iso-butanol as components of butanol-acetone (BA) mixture-diesel blend on spray, combustion characteristics, engine performance and emission in direct injection diesel engine. Energy 2017, 140, 1074–1086.
- Huang, H.; Singh, V.; Qureshi, N. Butanol production from food waste: A novel process for producing sustainable energy and reducing environmental pollution. Biotechnol. Biofuels 2015, 8, 147.
- Liu, Y.; Yuan, Y.; Ramya, G.; Mohan Singh, S.; Thuy Lan Chi, N.; Pugazhendhi, A.; Xia, C.; Mathimani, T. A review on the promising fuel of the future—Biobutanol; the hindrances and future perspectives. Fuel 2022, 327, 125166.
- Singh, D.; Sharma, D.; Soni, S.L.; Sharma, S.; Kumar Sharma, P.; Jhalani, A. A review on feedstocks, production processes, and yield for different generations of biodiesel. Fuel 2020, 262, 116553.
- Mathew, G.M.; Raina, D.; Narisetty, V.; Kumar, V.; Saran, S.; Pugazhendi, A.; Sindhu, R.; Pandey, A.; Binod, P. Recent advances in biodiesel production: Challenges and solutions. Sci. Total Environ. 2021, 794, 148751.
- Bashir, M.A.; Wu, S.; Zhu, J.; Krosuri, A.; Khan, M.U.; Ndeddy Aka, R.J. Recent development of advanced processing technologies for biodiesel production: A critical review. Fuel Process. Technol. 2022, 227, 107120.
- Yaashikaa, P.R.; Keerthana Devi, M.; Senthil Kumar, P. Biohydrogen production: An outlook on methods, constraints, economic analysis and future prospect. Int. J. Hydrogen Energy 2022, 47, 41488–41506.
- Patel, A.K.; Debroy, A.; Sharma, S.; Saini, R.; Mathur, A.; Gupta, R.; Tuli, D.K. Biohydrogen production from a novel alkalophilic isolate Clostridium sp. IODB-O3. Bioresour. Technol. 2015, 175, 291–297.
- Ananthi, V.; Ramesh, U.; Balaji, P.; Kumar, P.; Govarthanan, M.; Arun, A. A review on the impact of various factors on biohydrogen production. Int. J. Hydrogen Energy 2022, in press.
- Olatunji, K.O.; Ahmed, N.A.; Ogunkunle, O. Optimization of biogas yield from lignocellulosic materials with different pretreatment methods: A review. Biotechnol. Biofuels 2021, 14, 159.
- Okolie, J.A.; Nanda, S.; Dalai, A.K.; Kozinski, J.A. Techno-economic evaluation and sensitivity analysis of a conceptual design for supercritical water gasification of soybean straw to produce hydrogen. Bioresour. Technol. 2021, 331, 125005.
- Mao, C.; Feng, Y.; Wang, X.; Ren, G. Review on research achievements of biogas from anaerobic digestion. Renew. Sustain. Energy Rev. 2015, 45, 540–555.
- Pattharaprachayakul, N.; Kesonlam, N.; Duangjumpa, P.; Rungsardthong, V.; Suvajittanont, W.; Lamsal, B. Optimization of hydraulic retention time and organic loading rate in anaerobic digestion of squeezed pineapple liquid wastes for biogas production. Appl. Sci. Eng. Prog. 2021, 14, 468–476.
- Dikshit, P.K.; Kumar, J.; Das, A.K.; Sadhu, S.; Sharma, S.; Singh, S.; Gupta, P.K.; Kim, B.S. Green synthesis of metallic nanoparticles: Applications and limitations. Catalysts 2021, 11, 902.
- Kim, M.; Singhvi, M.S.; Kim, B.S. Eco-friendly and rapid one-step fermentable sugar production from raw lignocellulosic biomass using enzyme mimicking nanomaterials: A novel cost-effective approach to biofuel production. Chem. Eng. J. 2023, 465, 142879.
- Rekha, B.; Saravanathamizhan, R. Catalytic conversion of corncob biomass into bioethanol. Int. J. Energy Res. 2021, 45, 4508–4518.
- Hughes, J.P.; Rowley-Neale, S.; Banks, C. Enhancing the efficiency of the hydrogen evolution reaction utilising Fe3P bulk modified screen-printed electrodes via the application of a magnetic field. RSC Adv. 2021, 11, 8073–8079.
- Srivastava, N.; Srivastava, M.; Malhotra, B.D.; Gupta, V.K.; Ramteke, P.W.; Silva, R.N.; Shukla, P.; Dubey, K.K.; Mishra, P.K. Nanoengineered cellulosic biohydrogen production via dark fermentation: A novel approach. Biotechnol. Adv. 2019, 37, 107384.
- Chandel, H.; Kumar, P.; Chandel, A.K.; Verma, M.L. Biotechnological advances in biomass pretreatment for bio-renewable production through nanotechnological intervention. Biomass Convers. Biorefinery 2022, 4, 1–23.
- Jafari, O.; Zilouei, H. Enhanced biohydrogen and subsequent biomethane production from sugarcane bagasse using nano-titanium dioxide pretreatment. Bioresour. Technol. 2016, 214, 670–678.
- Mohanraj, S.; Anbalagan, K.; Kodhaiyolii, S.; Pugalenthi, V. Comparative evaluation of fermentative hydrogen production using Enterobacter cloacae and mixed culture: Effect of Pd (II) ion and phytogenic palladium nanoparticles. J. Biotechnol. 2014, 192, 87–95.
- Saka, A.; Jule, L.T.; Gudata, L.; Shuma, S.; Nagaprasad, N.; Subramanian, K.; Afessa, G.; Ramaswamy, K. Preparation of biobutanol via coffee bean harsh extracts by zinc oxide nanoparticle as catalyst. Biomass Convers. Biorefinery 2022, 1–10.
- Gandarias, I.; Nowicka, E.; May, B.J.; Alghareed, S.; Armstrong, R.D.; Miedziak, P.J.; Taylor, S.H. The selective oxidation of n-butanol to butyraldehyde by oxygen using stable Pt-based nanoparticulate catalysts: An efficient route for upgrading aqueous biobutanol. Catal. Sci. Technol. 2016, 6, 4201–4209.
- Sakarya, K.; Akyol, Ç.; Demirel, B. The effect of short-term exposure of engineered nanoparticles on methane production during mesophilic anaerobic digestion of primary sludge. Water Air Soil Pollut. 2015, 226, 100.
- Kundu, D.; Banerjee, S.; Karmakar, S.; Banerjee, R. A new insight on improved biomethanation using graphene oxide from fermented Assam lemon waste. Fuel 2022, 309, 122195.
- Adachi, D.; Hama, S.; Nakashima, K.; Bogaki, T.; Ogino, C.; Kondo, A. Production of biodiesel from plant oil hydrolysates using an Aspergillus oryzae whole-cell biocatalyst highly expressing Candida antarctica lipase B. Bioresour. Technol. 2013, 135, 410–416.
- Husin, H.; Asnawi, T.M.; Firdaus, A.; Husaini, H.; Ibrahim, I.; Hasfita, F. Solid catalyst nanoparticles derived from oil-palm empty fruit bunches (OP-EFB) as a renewable catalyst for biodiesel production. IOP Conf. Ser. Mater. Sci. Eng. 2018, 358, 012008.
More
