Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Rita Xu and Version 1 by Charalambos Michaeloudes.
Cardiovascular disease (CVD) that includes myocardial infarction and stroke, is the leading cause of mortality worldwide. Atherosclerosis, the primary underlying cause of CVD, can be controlled by pharmacological and dietary interventions, including
n
-3 polyunsaturated fatty acid (PUFA) supplementation.
n
-3 PUFA supplementation, primarily consisting of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), has shown promise in reducing atherosclerosis by modulating risk factors, including triglyceride levels and vascular inflammation.
- n-3 polyunsaturated fatty acids
- atherosclerosis
- molecular mechanisms
1. Introduction
1.1. Cardiovascular Disease
Cardiovascular disease (CVD), including myocardial infarction and ischemic stroke, is the leading cause of death worldwide. Atherosclerosis, which entails the formation of an atheromatic plague due to build-up of lipids and fibrous tissue in the large arteries, is the primary underlying cause of CVD [1]. Stenosis caused by the atheromatic plague, as well as thrombosis due to plague rupture, can restrict blood flow in major vessels leading to myocardial infarction or ischemic stroke [2].
Myocardial infarction, caused by myocardial ischemia, involves myocardial necrosis, as well as other cell death-activating pathways due to oxygen deprivation [3]. The main pathological cause of myocardial infarctions is associated with coronary artery disease, which involves coronary artery blockage due to accumulation of atherosclerotic plagues [4]. Myocardial damage can eventually lead to heart failure, which involves anatomical and functional myocardial abnormalities that limit ventricular filling or blood ejection, and results in failure to satisfy the underlying needs of circulation [5]. Ischemic stroke involves a reduction in the blood flow to part or all of the brain, leading to tissue damage and some degree of neurological damage. The major cause of ischemic stroke is the rupture of atherosclerotic plagues and the formation of thrombosis in the carotid artery [6].
1.2. Atherosclerosis
Atherosclerosis is primarily a lipid-driven process caused by accumulation of low-density lipoprotein (LDL) and remnant lipoprotein particles in focal areas of arteries, and an associated inflammatory process particularly at regions of disturbed non-laminar flow at artery branch-points. The defined most frequent risk factors are: high LDL cholesterol (LDL-C), hypertension, diabetes mellitus, smoking, age, and family history. Sedentary lifestyle, obesity, diets high in saturated and trans fats, and specific genetic abnormalities may also increase the risk [7,8,9][7][8][9].
Atherosclerosis primarily develops due to trapping of lipids in the intima by the extracellular matrix, causing a modification that drives chronic inflammation at vulnerable sites in the arteries. This process, which is involved in all stages of atherogenic progression, starts with nascent fatty streaks in the artery intima, which develop into fibrous plaques and then into rupture-prone atherosclerotic lesions [10,11][10][11]. At the molecular level, LDL in the intima becomes oxidized and drives pro-inflammatory processes that attract monocytes to the artery wall, where they differentiate into macrophages. Macrophages take-up lipoproteins using LDL scavenging receptors, forming foam cells that create the atherosclerotic lesion. Inflammatory mediators also recruit vascular smooth muscle cells, which produce extracellular matrix proteins creating a fibrous cap that overlies the lesion, forming an atherosclerotic plague [12]. Reduced synthesis of extracellular matrix proteins by smooth muscle cells, and increased production of matrix metalloproteases by foam cells may lead to fibrous cap thinning and plague rupture leading to thrombosis [13,14,15][13][14][15]. In advanced plagues, there is increased smooth muscle cell and macrophage cell death, through apoptosis or necroptosis [16,17][16][17]. Failure of macrophages to clear apoptotic and necrotic cells by efferocytosis, leads to the formation of the necrotic core that precipitates inflammation and fibrous cap thinning [12,18][12][18].
Treatment for coronary atherosclerosis involves measures to encourage regression and stop the growth and rupture of atherosclerotic plaques. Treating risk factors such as elevated LDL-C, high blood pressure, and diabetes, through diet, exercise, smoking cessation, and pharmacological management are the key strategies for controlling atherosclerosis [19,20,21][19][20][21]. Statins are the mainstay for lowering LDL-C and preventing cardiovascular events and mortality. Angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, diuretics, beta-blockers, calcium channel blockers, and vasodilators are also used for blood pressure management [22,23,24][22][23][24].
1.3. Dietary Management of Atherosclerosis
Atherosclerosis can be controlled by a healthy diet that is rich in fibre, monounsaturated fats, oily fish, fruits, and vegetables, and low in saturated and trans fats [25]. n-3 polyunsaturated fatty acids (PUFAs) have received considerable attention for their potential in modulating key risk factors of CVD, including triglyceride levels, lipoprotein oxidation, vascular inflammation and thrombogenesis. Nonetheless, clinical studies investigating the effects of n-3-PUFAs on cardiovascular health show conflicting findings [26]. A better understanding of the factors underlying the variability in the effects of n-3-PUFAs in clinical studies will allow uresearchers to tailor supplementation regimes in order to achieve greater benefit for the patients.
2. Current Status of Knowledge
2.1. Biochemistry and Structural Morphology of n-3 PUFAs
n-3 (omega-3) PUFAs are a family of long chain cis polyunsaturated fatty acids [27]. The term n-3 reflects the fact that the first double bond is situated three carbon atoms from the methyl terminal group [27]. Alpha linolenic acid (ALA; C18:3) is precursor to the longer chain (LC) n-3 PUFAs, eicosapentaenoic acid (EPA; C20:5) and docosahexaenoic acid (DHA; C22:6) [28]. ALA is ‘essential’ and must be included in our diet since it cannot be synthesised de novo in the body. Oily fish are the main source of LC n-3 PUFAs [29]. n-6 (omega-6) FAs are the second known important family of PUFAs. Linoleic acid (LA; C18:2) is a precursor to the LC n-6 PUFA arachidonic acid (C20:4). The main sources of n-6 PUFAs include the common vegetable oils used in cooking, such as sunflower and soybean oil, as well as foods derived from livestock animals and poultry [30]. It has been widely accepted that the present Western diet is ‘deficient’ in n-3 PUFAs with a ratio of n-6 to n-3 of 15-20:1, which is far higher than the optimal 4:1 ratio and the ideal 1:1 ratio [31]. However, reducing n-6 PUFA intake is not a prerequisite to achieve the optimum ratio. According to Zhao et al., n-6 PUFA intake can be increased without inducing any adverse effect provided that adequate n-3 PUFAs are consumed. Moreover, sufficient consumption of n-6 PUFAs is important for reducing LDL-C concentrations and therefore, a sufficient intake of both n-3 and n-6 PUFAs is crucial for CVD risk reduction [32].2.2. Clinical Evidence on the Effects of n-3 PUFAs on Different CVDs
Epidemiological studies have shown a positive correlation between oily fish intake and beneficial cardiovascular effects [33]. The evidence of the benefits of oily fish is stronger in secondary than in primary prevention settings [34]. For instance, GISSI Prevenzione investigators (1999) showed that dietary supplementation with LC n-3 PUFAs (1 g/day) in patients after myocardial infarction reduced total mortality by 21% and sudden death by 45%. In addition, a recent pooled analysis of four cohort studies showed an association between oily fish intake and lower risk of mortality among patients with prior CVD [35]. These benefits have been attributed to the LC n-3 PUFAs, EPA and DHA, primarily found in oily fish [36]. ALA is generally far less effective at inducing biological effects, partly because of its inefficient conversion (<5%) to EPA and DHA in humans [28]. ALA is inefficiently converted into the LC n-3 PUFAs partly because of the large and increasing amounts of n-6 fatty acids present in our diet, which compete for the same enzymes, shown in the metabolic pathway [37,38][37][38] (Figure 1).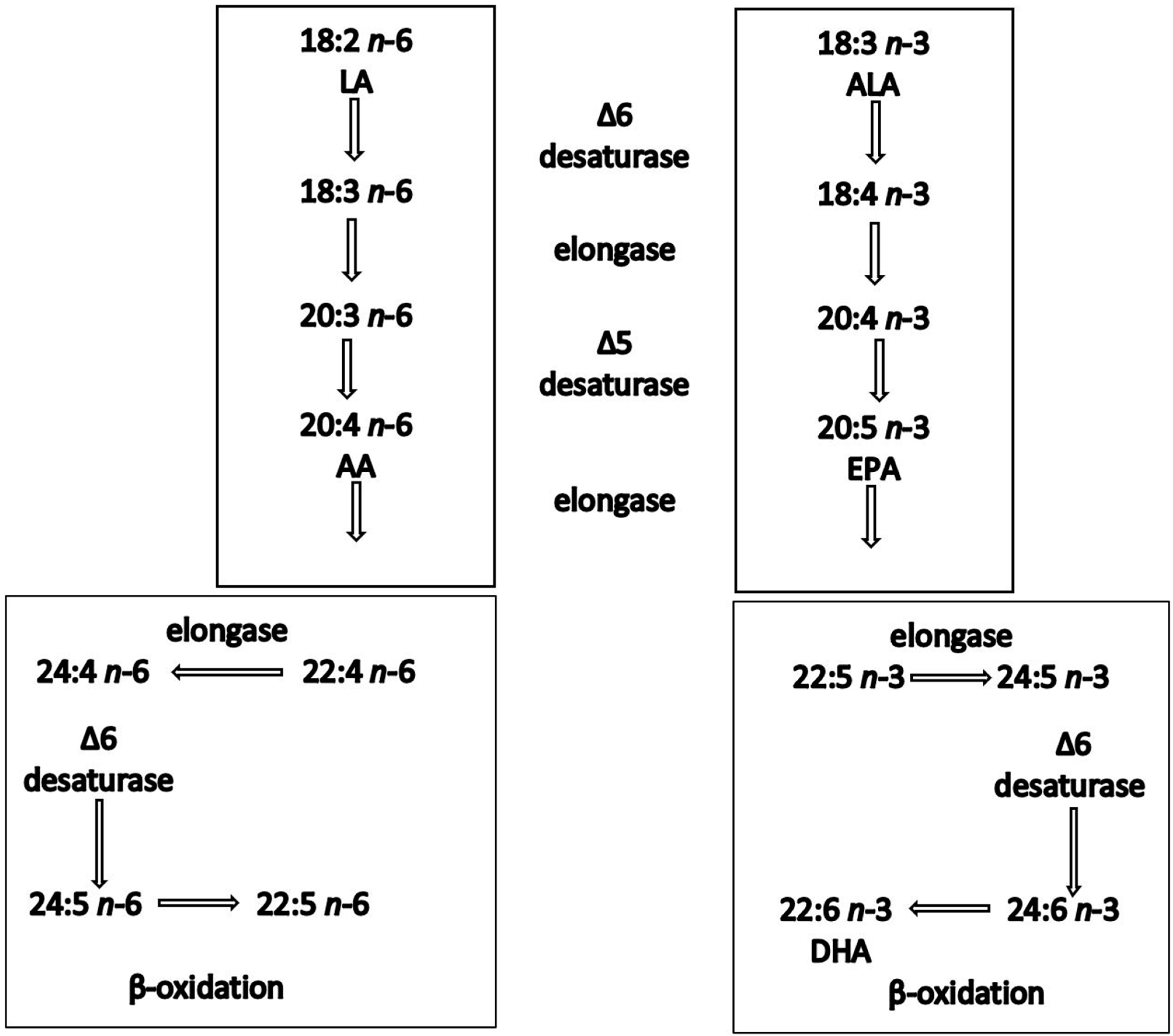
Figure 1. Synthesis of n-6 and n-3 polyunsaturated fatty acids in humans. Both linoleic acid (LA) and a-linolenic acid (ALA) are elongated, desaturated and β-oxidised using the same enzyme system.
2.2.1. Effect on Triglycerides
According to Milte et al., circulating TG levels constitute an independent risk factor for CVD and are correlated with the severity and development of atherosclerosis [53]. However, Torrejon et al., argued that unlike the well-established role of LDL-C in the development of CVD, the role of circulating TG concentrations in CVD development remains controversial [54]. Nevertheless, according to the British Nutrition Foundation (BNF) (2005) [55], an indirect effect of TGs on CVD risk seems to be the case. Research has shown that a reduction in TG levels leads to a lower abundance of small, dense LDL-C and therefore a reduced CVD risk (BNF, 2005) [55]. The most consistent effect of n-3 PUFAs is the decrease in fasting and postprandial serum TGs [48,54][48][54]. However, the exact dose as well as exact duration of intervention, which can give the optimum TG-lowering effect is still unclear [53]. So far, the majority of the studies have demonstrated a significant reduction in TGs (25–30%) following treatment with doses ≥ 3 g/day of LC n-3 PUFAs (EPA and DHA) mainly in the form of fish oil [56]. In contrast, there is evidence showing a much lower effect following treatment with lower doses [57]. The duration of studies ranged between 6 and 104 weeks, and the optimal duration of intervention remains unclear since no difference was found in TG levels associated with longer trial duration (>16 weeks) compared to shorter trial duration (≤16 weeks) [33,58][33][58]. A double-blind randomized placebo controlled parallel study showed that DHA supplementation (3 g/day) for 45 days significantly decreased fasting TG levels of hypertriglyceridaemic men by 25–30%. Conversely, a double-blind placebo-controlled study [57] using a LC n-3 PUFA dose (1.8 g EPA and 0.3 g DHA) per day, which approximated the daily dietary intake upper limit (2 g of LC n-3 PUFAs) of the current UK guideline range (Scientific Advisory Committee on Nutrition, 2004) [59], had no effect on TG levels of normotriglyceridaemic individuals. Yusof et al. suggested that apart from the lower dose, the fact that the oil used was relatively poor in DHA might have contributed to the lack of effect. It is now believed that DHA is more potent in lowering TGs than EPA [60]. Furthermore, it has been shown that the DHA TG-lowering effect is greater in hypertriglyceridaemic individuals compared with normotriglyceridaemic individuals [61,62][61][62]. A comprehensive meta-analysis (16511 participants in 47 studies included) assessing the role of n-3 PUFAs in treating hyperlipidaemia, demonstrated an average reduction of 14% in TG levels of hypertriglyceridaemic individuals following 6-month treatment with an average daily intake of 3.25 g of EPA and/or DHA [33]. However, this dose exceeds the safety limit (3 g/day) approved by the US food and drug administration (FDA) [63].2.2.2. Effect on Low-Density and High-Density Lipoprotein Particle Size
Smaller diameter and denser LDL particles, such as LDL-3 subfraction, have been shown to be more susceptible to oxidation [50]. In addition, they have an increased ability to penetrate the intima than larger, less dense LDL particles, such as LDL-1 and LDL-2 subfractions [50] and have, therefore, been associated with an increased coronary heart disease risk [50]. A very recent systematic review concluded that small dense LDL are associated with an increased CVD risk [64]. According to Torrejon et al., particle size is significantly increased with DHA supplementation. DHA supplementation reduces plasma TGs, contributing to a reduction in the number of small, dense LDL particles and subsequently to decreased CVD risk [49]. Larger, more cholesterol-rich HDL particles (e.g., HDL-2 subfraction) are thought to facilitate a more efficient reverse cholesterol transport compared with the smaller, less buoyant subfraction HDL-3, making them more cardioprotective [50,65,66][50][65][66]. The HDL-2 subfraction has been shown to be increased in response to DHA, whilst it is not affected by EPA [54,65][54][65]. This was confirmed by a recent systematic review [67].2.2.3. Effect on ‘n-3 Index’
Harris and von Schacky (2004) were the first to propose that the content of EPA and DHA in RBC membranes, expressed as a proportion of total FAs, termed the ‘omega-3 index’, can be considered as a risk factor for coronary heart disease and sudden cardiac death mortality [51]. Specifically, an ‘omega-3 Index’ level of ≥8% is a reasonable preliminary target value for reducing coronary heart disease risk. On the other hand, an ‘omega-3 Index’ < 4% has been implicated with a 10-fold greater risk of sudden cardiac death compared with an ‘omega-3 Index’ of ≥8% (Figure 2) [68,69][68][69].
Figure 2. Proposed risk zones for the ‘omega-3 index’.
3. Factors Implicated in the Efficacy of PUFAs
3.1. Variability in the Clinical Effects of n-3 PUFAs
Following supplement uptake, n-3 PUFAs are incorporated into cell membranes and plasma lipids in a dose- and time-dependent manner, replacing other fatty acid types, including n-6 PUFAs [71]. n-3 PUFA incorporation lead to changes in cell signaling by affecting the cell membrane structure and fluidity and changing the function of cell surface receptors and ion channels. The effects of n-3 PUFAs on atherosclerosis are conferred through molecular mechanisms that involve changes in the cell membrane composition, eicosanoid synthesis, transcription factor activity and gene expression [72]. EPA and DHA are known to have differential metabolism, and divergent effects on the molecular mechanisms of atherosclerosis. Antagonism between the two types of n-3 PUFAs, may explain the conflicting findings of large clinical studies on the effects of n-3 PUFAs on CVD risk factors [73,74,75,76][73][74][75][76]. The effects of n-3 PUFAs on atherosclerosis risk factors depend on different considerations including their dose and length of supplementation, EPA/DHA composition and formulation [77]. Understanding how these factors affect their incorporation into the lipid pool, their metabolism and their downstream molecular effects will allow more effective design of n-3 PUFA supplementation strategies for CVD prevention.3.2. Incorporation of EPA and DHA into the Lipid Pool
EPA and DHA show differential metabolism-, tissue-, and compartment-specific accumulation following supplementation, which may affect their function. Supplementation with EPA and DHA leads to a dose-dependent increase in plasma, which reaches equilibrium approx. one month post-supplementation [78]. Studies suggest a very low conversion of EPA to DHA following supplementation. However, DHA supplementation increases plasma EPA concentrations, possibly due to retro-conversion or slow EPA turnover [78,79][78][79]. Following fish oil supplementation, EPA preferentially accumulates in cholesteryl esters within very low-density lipoproteins (VLDL), possibly due to increased selectivity of the enzyme lecithin-cholesterol acyltransferase to EPA [80,81,82][80][81][82]. On the other hand, DHA is a preferential substrate for diacylglycerol acyltransferase leading to increased incorporation into TG [81,82,83][81][82][83]. The source and formulation of n-3-PUFA supplements also affects their bioavailability and consequently their lipid incorporation. Studies comparing the incorporation of EPA and DHA into plasma lipids after short-term supplementation with nutritional or pharmacological doses (>3 g/day) of n-3-PUFAs, in the forms of free fatty acids, ethyl esters or re-esterified TG, have reported conflicting findings [84,85,86,87][84][85][86][87]. However, a clinical study investigating the effect of prolonged supplementation (6 months) of dyslipidemic patients on statins with moderate doses of n-3-PUFAs (1.01 g EPA and 0.67 g DHA), reported that the re-esterified triglyceride formulation showed higher incorporation into red blood cell membranes and a more effective reduction in fasting plasma TG, compared to the ethyl ester formulation [88,89][88][89].3.3. Effects of EPA and DHA on Lipoprotein Metabolism
High levels of blood TG, transported by the TG-rich VLDLs and chylomicrons, increase the risk for atherosclerosis [90]. Dietary supplementation with fish oil or with pure EPA and DHA, attenuates plasma TG by inhibiting TG synthesis and VLDL production, and inducing apolipoprotein B degradation in hepatocytes [91,92,93,94][91][92][93][94]. Furthermore, n-3-PUFAs limit the supply of plasma non-esterified fatty acids for VLDL synthesis through inhibition of intracellular lipolysis in adipocytes [95,96][95][96]. Accelerated chylomicron clearance through induction of lipolysis has also been shown to be induced by both DHA and EPA [97]. A number of studies also demonstrate that n-3-PUFAs supplementation increases HDL levels; however, there are studies showing contradicting findings [65,98,99,100][65][98][99][100]. However, a number of studies using EPA or DHA monotherapy report differential effects or efficacies of the two fatty acids on plasma lipids. The randomised cross-over study ComparED, compared the effect of DHA and EPA (2.7 g/day), formulated as re-esterified triacylglycerol, on blood lipids and inflammatory markers in subjects with abdominal obesity and subclinical inflammation. The study reported that DHA may be more effective in reducing TG, and increasing HDL- and LDL-cholesterol concentrations, compared to EPA. Apolipoprotein B levels were also reduced by DHA, but not by EPA [103][101]. A secondary analysis of the ComparED study results indicated that the increased efficacy of DHA in reducing TG does not depend on a greater effect magnitude but on the fact that a greater proportion of study subjects responded to DHA compared to EPA [104][102]. Previous studies also investigated the effect of supplementation with highly pure, free, or esterified, DHA or EPA (>3 g/day) for 4–7 weeks on the plasma lipid profile of healthy or hypertensive subjects. These studies report mixed findings, with some showing a superior effect of DHA compared to EPA supplementation and others no difference between the two n-3 PUFAs [97,105,106,107,108][97][103][104][105][106]. A meta-analysis of these studies concluded that overall DHA reduces TG and increases LDL and HDL cholesterol, to a greater extent than EPA [109][107]. High doses (3–4 g/day) of EPA or DHA were shown to be equally efficient in inducing liposome lipase activity, suggesting that the superior triglyceride-lowering effect of DHA is not due to a more efficient chylomicron/VLDL clearance [97,110][97][108].References
- Frostegard, J. Immunity, atherosclerosis and cardiovascular disease. BMC Med. 2013, 11, 117.
- Libby, P. Inflammation in atherosclerosis. Nature 2002, 420, 868–874.
- Thom, T.; Haase, N.; Rosamond, W.; Howard, V.J.; Rumsfeld, J.; Manolio, T.; Zheng, Z.J.; Flegal, K.; O’Donnell, C.; Kittner, S.; et al. Heart disease and stroke statistics—2006 update: A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2006, 113, e85–e151.
- Reimer, K.A.; Jennings, R.B.; Tatum, A.H. Pathobiology of acute myocardial ischemia: Metabolic, functional and ultrastructural studies. Am. J. Cardiol. 1983, 52, 72A–81A.
- Hajouli, S.; Ludhwani, D. Heart Failure and Ejection Fraction. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023.
- Gasbarrino, K.; Di Iorio, D.; Daskalopoulou, S.S. Importance of sex and gender in ischaemic stroke and carotid atherosclerotic disease. Eur. Heart J. 2022, 43, 460–473.
- Mohd Nor, N.S.; Al-Khateeb, A.M.; Chua, Y.A.; Mohd Kasim, N.A.; Mohd Nawawi, H. Heterozygous familial hypercholesterolaemia in a pair of identical twins: A case report and updated review. BMC Pediatr. 2019, 19, 106.
- Pahwa, R.; Jialal, I. Atherosclerosis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023.
- Dichgans, M.; Pulit, S.L.; Rosand, J. Stroke genetics: Discovery, biology, and clinical applications. Lancet Neurol. 2019, 18, 587–599.
- Sakakura, K.; Nakano, M.; Otsuka, F.; Ladich, E.; Kolodgie, F.D.; Virmani, R. Pathophysiology of atherosclerosis plaque progression. Heart Lung Circ. 2013, 22, 399–411.
- Kowara, M.; Cudnoch-Jedrzejewska, A. Pathophysiology of Atherosclerotic Plaque Development-Contemporary Experience and New Directions in Research. Int. J. Mol. Sci. 2021, 22, 3513.
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgozoglu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Primers 2019, 5, 56.
- Galis, Z.S.; Sukhova, G.K.; Lark, M.W.; Libby, P. Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J. Clin. Investig. 1994, 94, 2493–2503.
- Amento, E.P.; Ehsani, N.; Palmer, H.; Libby, P. Cytokines and growth factors positively and negatively regulate interstitial collagen gene expression in human vascular smooth muscle cells. Arterioscler. Thromb. 1991, 11, 1223–1230.
- Galis, Z.S.; Sukhova, G.K.; Kranzhofer, R.; Clark, S.; Libby, P. Macrophage foam cells from experimental atheroma constitutively produce matrix-degrading proteinases. Proc. Natl. Acad. Sci. USA 1995, 92, 402–406.
- Lin, J.; Li, H.; Yang, M.; Ren, J.; Huang, Z.; Han, F.; Huang, J.; Ma, J.; Zhang, D.; Zhang, Z.; et al. A role of RIP3-mediated macrophage necrosis in atherosclerosis development. Cell Rep. 2013, 3, 200–210.
- Geng, Y.J.; Libby, P. Evidence for apoptosis in advanced human atheroma. Colocalization with interleukin-1 beta-converting enzyme. Am. J. Pathol. 1995, 147, 251–266.
- Schrijvers, D.M.; De Meyer, G.R.; Kockx, M.M.; Herman, A.G.; Martinet, W. Phagocytosis of apoptotic cells by macrophages is impaired in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1256–1261.
- Spannella, F.; Giulietti, F.; Di Pentima, C.; Sarzani, R. Prevalence and Control of Dyslipidemia in Patients Referred for High Blood Pressure: The Disregarded “Double-Trouble” Lipid Profile in Overweight/Obese. Adv. Ther. 2019, 36, 1426–1437.
- Esper, R.J.; Nordaby, R.A. Cardiovascular events, diabetes and guidelines: The virtue of simplicity. Cardiovasc. Diabetol. 2019, 18, 42.
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 140, e596–e646.
- Puttananjaiah, M.K.; Dhale, M.A.; Gaonkar, V.; Keni, S. Statins: 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) reductase inhibitors demonstrate anti-atherosclerotic character due to their antioxidant capacity. Appl. Biochem. Biotechnol. 2011, 163, 215–222.
- Li, E.C.; Heran, B.S.; Wright, J.M. Angiotensin converting enzyme (ACE) inhibitors versus angiotensin receptor blockers for primary hypertension. Cochrane Database Syst. Rev. 2014, 2014, CD009096.
- Misra, S.; Stevermer, J.J. ACE inhibitors and ARBs: One or the other--not both--for high-risk patients. J. Fam. Pract. 2009, 58, 24–27.
- Sikand, G.; Severson, T. Top 10 dietary strategies for atherosclerotic cardiovascular risk reduction. Am. J. Prev. Cardiol. 2020, 4, 100106.
- Yagi, S.; Fukuda, D.; Aihara, K.I.; Akaike, M.; Shimabukuro, M.; Sata, M. n-3 Polyunsaturated Fatty Acids: Promising Nutrients for Preventing Cardiovascular Disease. J. Atheroscler. Thromb. 2017, 24, 999–1010.
- Deckelbaum, R.J.; Worgall, T.S.; Seo, T. n-3 fatty acids and gene expression. Am. J. Clin. Nutr. 2006, 83, 1520S–1525S.
- Deckelbaum, R.J.; Akabas, S.R. n-3 Fatty acids and cardiovascular disease: Navigating toward recommendations. Am. J. Clin. Nutr. 2006, 84, 1–2.
- Sullivan, E.J. Education. Is tenure still viable today? J. Prof. Nurs. 1996, 12, 130.
- Sokola-Wysoczanska, E.; Wysoczanski, T.; Wagner, J.; Czyz, K.; Bodkowski, R.; Lochynski, S.; Patkowska-Sokola, B. Polyunsaturated Fatty Acids and Their Potential Therapeutic Role in Cardiovascular System Disorders—A Review. Nutrients 2018, 10, 1561.
- Simopoulos, A.P. The omega-6/omega-3 fatty acid ratio, genetic variation, and cardiovascular disease. Asia Pac. J. Clin. Nutr. 2008, 17 (Suppl. S1), 131–134.
- Zhao, G.; Etherton, T.D.; Martin, K.R.; Gillies, P.J.; West, S.G.; Kris-Etherton, P.M. Dietary alpha-linolenic acid inhibits proinflammatory cytokine production by peripheral blood mononuclear cells in hypercholesterolemic subjects. Am. J. Clin. Nutr. 2007, 85, 385–391.
- Eslick, G.D.; Howe, P.R.; Smith, C.; Priest, R.; Bensoussan, A. Benefits of fish oil supplementation in hyperlipidemia: A systematic review and meta-analysis. Int. J. Cardiol. 2009, 136, 4–16.
- Marik, P.E.; Varon, J. Omega-3 dietary supplements and the risk of cardiovascular events: A systematic review. Clin. Cardiol. 2009, 32, 365–372.
- Mohan, D.; Mente, A.; Dehghan, M.; Rangarajan, S.; O’Donnell, M.; Hu, W.; Dagenais, G.; Wielgosz, A.; Lear, S.; Wei, L.; et al. Associations of Fish Consumption With Risk of Cardiovascular Disease and Mortality Among Individuals With or Without Vascular Disease From 58 Countries. JAMA Intern. Med. 2021, 181, 631–649.
- Shahidi, F.; Ambigaipalan, P. Omega-3 Polyunsaturated Fatty Acids and Their Health Benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 345–381.
- Mariamenatu, A.H.; Abdu, E.M. Overconsumption of Omega-6 Polyunsaturated Fatty Acids (PUFAs) versus Deficiency of Omega-3 PUFAs in Modern-Day Diets: The Disturbing Factor for Their “Balanced Antagonistic Metabolic Functions” in the Human Body. J. Lipids 2021, 2021, 8848161.
- Barceló-Coblijn, G.; Murphy, E.J. Alpha-linolenic acid and its conversion to longer chain n-3 fatty acids: Benefits for human health and a role in maintaining tissue n-3 fatty acid levels. Prog. Lipid Res. 2009, 48, 355–374.
- Bird, J.K.; Calder, P.C.; Eggersdorfer, M. The Role of n-3 Long Chain Polyunsaturated Fatty Acids in Cardiovascular Disease Prevention, and Interactions with Statins. Nutrients 2018, 10, 775.
- Metcalf, R.G.; James, M.J.; Gibson, R.A.; Edwards, J.R.; Stubberfield, J.; Stuklis, R.; Roberts-Thomson, K.; Young, G.D.; Cleland, L.G. Effects of fish-oil supplementation on myocardial fatty acids in humans. Am. J. Clin. Nutr. 2007, 85, 1222–1228.
- Watanabe, Y.; Tatsuno, I. Prevention of Cardiovascular Events with Omega-3 Polyunsaturated Fatty Acids and the Mechanism Involved. J. Atheroscler. Thromb. 2020, 27, 183–198.
- Russo, G.L. Dietary n-6 and n-3 polyunsaturated fatty acids: From biochemistry to clinical implications in cardiovascular prevention. Biochem. Pharmacol. 2009, 77, 937–946.
- Massaro, M.; Scoditti, E.; Carluccio, M.A.; De Caterina, R. Basic mechanisms behind the effects of n-3 fatty acids on cardiovascular disease. Prostaglandins Leukot. Essent. Fat. Acids 2008, 79, 109–115.
- Lavie, C.J.; Milani, R.V.; Mehra, M.R.; Ventura, H.O. Omega-3 polyunsaturated fatty acids and cardiovascular diseases. J. Am. Coll. Cardiol. 2009, 54, 585–594.
- Balk, E.M.; Lichtenstein, A.H.; Chung, M.; Kupelnick, B.; Chew, P.; Lau, J. Effects of omega-3 fatty acids on serum markers of cardiovascular disease risk: A systematic review. Atherosclerosis 2006, 189, 19–30.
- Liu, Y.X.; Yu, J.H.; Sun, J.H.; Ma, W.Q.; Wang, J.J.; Sun, G.J. Effects of Omega-3 Fatty Acids Supplementation on Serum Lipid Profile and Blood Pressure in Patients with Metabolic Syndrome: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Foods 2023, 12, 725.
- Kones, R.; Howell, S.; Rumana, U. n-3 Polyunsaturated Fatty Acids and Cardiovascular Disease: Principles, Practices, Pitfalls, and Promises—A Contemporary Review. Med. Princ. Pract. Int. J. Kuwait Univ. Health Sci. Cent. 2017, 26, 497–508.
- Farnier, M.; Zeller, M.; Masson, D.; Cottin, Y. Triglycerides and risk of atherosclerotic cardiovascular disease: An update. Arch. Cardiovasc. Dis. 2021, 114, 132–139.
- Kelley, D.S.; Siegel, D.; Vemuri, M.; Mackey, B.E. Docosahexaenoic acid supplementation improves fasting and postprandial lipid profiles in hypertriglyceridemic men. Am. J. Clin. Nutr. 2007, 86, 324–333.
- Harper, C.R.; Edwards, M.C.; Jacobson, T.A. Flaxseed oil supplementation does not affect plasma lipoprotein concentration or particle size in human subjects. J. Nutr. 2006, 136, 2844–2848.
- Harris, W.S.; Von Schacky, C. The Omega-3 Index: A new risk factor for death from coronary heart disease? Prev. Med. 2004, 39, 212–220.
- Harris, W.S.; Zotor, F.B. n-3 Fatty acids and risk for fatal coronary disease. Proc. Nutr. Soc. 2019, 78, 526–531.
- Milte, C.M.; Coates, A.M.; Buckley, J.D.; Hill, A.M.; Howe, P.R. Dose-dependent effects of docosahexaenoic acid-rich fish oil on erythrocyte docosahexaenoic acid and blood lipid levels. Br. J. Nutr. 2008, 99, 1083–1088.
- Torrejon, C.; Jung, U.J.; Deckelbaum, R.J. n-3 Fatty acids and cardiovascular disease: Actions and molecular mechanisms. Prostaglandins Leukot. Essent. Fat. Acids 2007, 77, 319–326.
- BNF. Cardiovascular Disease: Diet, Nutrition and Emerging Risk Factors; Blackwell Publishing: Hoboken, NJ, USA, 2005.
- Breslow, J.L. n-3 fatty acids and cardiovascular disease. Am. J. Clin. Nutr. 2006, 83, 1477S–1482S.
- Yusof, H.M.; Miles, E.A.; Calder, P. Influence of very long-chain n-3 fatty acids on plasma markers of inflammation in middle-aged men. Prostaglandins Leukot. Essent. Fat. Acids 2008, 78, 219–228.
- Sherratt, S.C.R.; Libby, P.; Budoff, M.J.; Bhatt, D.L.; Mason, R.P. Role of Omega-3 Fatty Acids in Cardiovascular Disease: The Debate Continues. Curr. Atheroscler. Rep. 2023, 25, 1–17.
- SACN. Advice on Fish Consumption: Benefits and Risks; The Stationery Office: London, UK, 2004.
- Toth, P.P.; Chapman, M.J.; Parhofer, K.G.; Nelson, J.R. Differentiating EPA from EPA/DHA in cardiovascular risk reduction. Am. Heart J. Plus Cardiol. Res. Pract. 2022, 17, 100148.
- Geppert, J.; Kraft, V.; Demmelmair, H.; Koletzko, B. Microalgal docosahexaenoic acid decreases plasma triacylglycerol in normolipidaemic vegetarians: A randomised trial. Br. J. Nutr. 2006, 95, 779–786.
- Skulas-Ray, A.C.; Wilson, P.W.F.; Harris, W.S.; Brinton, E.A.; Kris-Etherton, P.M.; Richter, C.K.; Jacobson, T.A.; Engler, M.B.; Miller, M.; Robinson, J.G.; et al. Omega-3 Fatty Acids for the Management of Hypertriglyceridemia: A Science Advisory From the American Heart Association. Circulation 2019, 140, e673–e691.
- FDA. Letter Responding to Health Claim Petition dated November 3, 2003 (MartekPetition): Omega-3 Fatty Acids and Reduced Risk of Coronary Heart Disease. 2004. Available online: http://www.cfsan.fda.gov/~dms/ds-ltr37.html (accessed on 28 September 2023).
- Chary, A.; Tohidi, M.; Hedayati, M. Association of LDL-cholesterol subfractions with cardiovascular disorders: A systematic review. BMC Cardiovasc. Disord. 2023, 23, 533.
- Buckley, R.; Shewring, B.; Turner, R.; Yaqoob, P.; Minihane, A.M. Circulating triacylglycerol and apoE levels in response to EPA and docosahexaenoic acid supplementation in adult human subjects. Br. J. Nutr. 2004, 92, 477–483.
- Wang, H.H.; Garruti, G.; Liu, M.; Portincasa, P.; Wang, D.Q. Cholesterol and Lipoprotein Metabolism and Atherosclerosis: Recent Advances In reverse Cholesterol Transport. Ann. Hepatol. 2017, 16, s27–s42.
- Innes, J.K.; Calder, P.C. The Differential Effects of Eicosapentaenoic Acid and Docosahexaenoic Acid on Cardiometabolic Risk Factors: A Systematic Review. Int. J. Mol. Sci. 2018, 19, 532.
- Kelley, D.S.; Siegel, D.; Vemuri, M.; Chung, G.H.; Mackey, B.E. Docosahexaenoic acid supplementation decreases remnant-like particle-cholesterol and increases the (n-3) index in hypertriglyceridemic men. J. Nutr. 2008, 138, 30–35.
- Harris, W.S. Omega-3 fatty acids and cardiovascular disease: A case for omega-3 index as a new risk factor. Pharmacol. Res. 2007, 55, 217–223.
- Schuchardt, J.P.; Ostermann, A.I.; Stork, L.; Kutzner, L.; Kohrs, H.; Greupner, T.; Hahn, A.; Schebb, N.H. Effects of docosahexaenoic acid supplementation on PUFA levels in red blood cells and plasma. Prostaglandins Leukot. Essent. Fat. Acids 2016, 115, 12–23.
- Walker, C.G.; West, A.L.; Browning, L.M.; Madden, J.; Gambell, J.M.; Jebb, S.A.; Calder, P.C. The Pattern of Fatty Acids Displaced by EPA and DHA Following 12 Months Supplementation Varies between Blood Cell and Plasma Fractions. Nutrients 2015, 7, 6281–6293.
- Jump, D.B. The biochemistry of n-3 polyunsaturated fatty acids. J. Biol. Chem. 2002, 277, 8755–8758.
- Yokoyama, M.; Origasa, H.; Matsuzaki, M.; Matsuzawa, Y.; Saito, Y.; Ishikawa, Y.; Oikawa, S.; Sasaki, J.; Hishida, H.; Itakura, H.; et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): A randomised open-label, blinded endpoint analysis. Lancet 2007, 369, 1090–1098.
- Bhatt, D.L.; Steg, P.G.; Miller, M.; Brinton, E.A.; Jacobson, T.A.; Ketchum, S.B.; Doyle, R.T., Jr.; Juliano, R.A.; Jiao, L.; Granowitz, C.; et al. Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia. N. Engl. J. Med. 2019, 380, 11–22.
- Group, A.S.C.; Bowman, L.; Mafham, M.; Wallendszus, K.; Stevens, W.; Buck, G.; Barton, J.; Murphy, K.; Aung, T.; Haynes, R.; et al. Effects of n-3 Fatty Acid Supplements in Diabetes Mellitus. N. Engl. J. Med. 2018, 379, 1540–1550.
- Manson, J.E.; Cook, N.R.; Lee, I.M.; Christen, W.; Bassuk, S.S.; Mora, S.; Gibson, H.; Albert, C.M.; Gordon, D.; Copeland, T.; et al. Marine n-3 Fatty Acids and Prevention of Cardiovascular Disease and Cancer. N. Engl. J. Med. 2019, 380, 23–32.
- Sweeney, T.E.; Gaine, S.P.; Michos, E.D. Eicosapentaenoic acid vs. docosahexaenoic acid for the prevention of cardiovascular disease. Curr. Opin. Endocrinol. Diabetes Obes. 2023, 30, 87–93.
- Arterburn, L.M.; Hall, E.B.; Oken, H. Distribution, interconversion, and dose response of n-3 fatty acids in humans. Am. J. Clin. Nutr. 2006, 83, 1467S–1476S.
- Metherel, A.H.; Irfan, M.; Klingel, S.L.; Mutch, D.M.; Bazinet, R.P. Compound-specific isotope analysis reveals no retroconversion of DHA to EPA but substantial conversion of EPA to DHA following supplementation: A randomized control trial. Am. J. Clin. Nutr. 2019, 110, 823–831.
- Singer, P.; Berger, I.; Wirth, M.; Godicke, W.; Jaeger, W.; Voigt, S. Slow desaturation and elongation of linoleic and alpha-linolenic acids as a rationale of eicosapentaenoic acid-rich diet to lower blood pressure and serum lipids in normal, hypertensive and hyperlipemic subjects. Prostaglandins Leukot. Med. 1986, 24, 173–193.
- Subbaiah, P.V.; Kaufman, D.; Bagdade, J.D. Incorporation of dietary n-3 fatty acids into molecular species of phosphatidyl choline and cholesteryl ester in normal human plasma. Am. J. Clin. Nutr. 1993, 58, 360–368.
- Sanders, T.A.; Sullivan, D.R.; Reeve, J.; Thompson, G.R. Triglyceride-lowering effect of marine polyunsaturates in patients with hypertriglyceridemia. Arteriosclerosis 1985, 5, 459–465.
- Madsen, L.; Rustan, A.C.; Vaagenes, H.; Berge, K.; Dyroy, E.; Berge, R.K. Eicosapentaenoic and docosahexaenoic acid affect mitochondrial and peroxisomal fatty acid oxidation in relation to substrate preference. Lipids 1999, 34, 951–963.
- Nordoy, A.; Barstad, L.; Connor, W.E.; Hatcher, L. Absorption of the n-3 eicosapentaenoic and docosahexaenoic acids as ethyl esters and triglycerides by humans. Am. J. Clin. Nutr. 1991, 53, 1185–1190.
- Krokan, H.E.; Bjerve, K.S.; Mork, E. The enteral bioavailability of eicosapentaenoic acid and docosahexaenoic acid is as good from ethyl esters as from glyceryl esters in spite of lower hydrolytic rates by pancreatic lipase in vitro. Biochim. Biophys. Acta 1993, 1168, 59–67.
- Dyerberg, J.; Madsen, P.; Moller, J.M.; Aardestrup, I.; Schmidt, E.B. Bioavailability of marine n-3 fatty acid formulations. Prostaglandins Leukot. Essent. Fat. Acids 2010, 83, 137–141.
- Hedengran, A.; Szecsi, P.B.; Dyerberg, J.; Harris, W.S.; Stender, S. n-3 PUFA esterified to glycerol or as ethyl esters reduce non-fasting plasma triacylglycerol in subjects with hypertriglyceridemia: A randomized trial. Lipids 2015, 50, 165–175.
- Neubronner, J.; Schuchardt, J.P.; Kressel, G.; Merkel, M.; von Schacky, C.; Hahn, A. Enhanced increase of omega-3 index in response to long-term n-3 fatty acid supplementation from triacylglycerides versus ethyl esters. Eur. J. Clin. Nutr. 2011, 65, 247–254.
- Schuchardt, J.P.; Neubronner, J.; Kressel, G.; Merkel, M.; von Schacky, C.; Hahn, A. Moderate doses of EPA and DHA from re-esterified triacylglycerols but not from ethyl-esters lower fasting serum triacylglycerols in statin-treated dyslipidemic subjects: Results from a six month randomized controlled trial. Prostaglandins Leukot. Essent. Fat. Acids 2011, 85, 381–386.
- Gabani, M.; Shapiro, M.D.; Toth, P.P. The Role of Triglyceride-rich Lipoproteins and Their Remnants in Atherosclerotic Cardiovascular Disease. Eur. Cardiol. 2023, 18, e56.
- Rustan, A.C.; Nossen, J.O.; Christiansen, E.N.; Drevon, C.A. Eicosapentaenoic acid reduces hepatic synthesis and secretion of triacylglycerol by decreasing the activity of acyl-coenzyme A:1,2-diacylglycerol acyltransferase. J. Lipid Res. 1988, 29, 1417–1426.
- Wang, H.; Chen, X.; Fisher, E.A. n-3 fatty acids stimulate intracellular degradation of apoprotein B in rat hepatocytes. J. Clin. Investig. 1993, 91, 1380–1389.
- Bordin, P.; Bodamer, O.A.; Venkatesan, S.; Gray, R.M.; Bannister, P.A.; Halliday, D. Effects of fish oil supplementation on apolipoprotein B100 production and lipoprotein metabolism in normolipidaemic males. Eur. J. Clin. Nutr. 1998, 52, 104–109.
- Yuan, M.; Zhang, Y.; Hua, T.; Liu, X.L.; Liu, T.; Yuan, R.Y.; Li, G.P.; Zhu, Y.; Zhang, X. Omega-3 polyunsaturated fatty acid supplementation improves lipid metabolism and endothelial function by providing a beneficial eicosanoid-pattern in patients with acute myocardial infarction: A randomized, controlled trial. Clin. Nutr. 2021, 40, 445–459.
- Rustan, A.C.; Hustvedt, B.E.; Drevon, C.A. Dietary supplementation of very long-chain n-3 fatty acids decreases whole body lipid utilization in the rat. J. Lipid Res. 1993, 34, 1299–1309.
- Lorente-Cebrian, S.; Bustos, M.; Marti, A.; Fernandez-Galilea, M.; Martinez, J.A.; Moreno-Aliaga, M.J. Eicosapentaenoic acid inhibits tumour necrosis factor-alpha-induced lipolysis in murine cultured adipocytes. J. Nutr. Biochem. 2012, 23, 218–227.
- Park, Y.; Harris, W.S. Omega-3 fatty acid supplementation accelerates chylomicron triglyceride clearance. J. Lipid Res. 2003, 44, 455–463.
- Chen, H.; Deng, G.; Zhou, Q.; Chu, X.; Su, M.; Wei, Y.; Li, L.; Zhang, Z. Effects of eicosapentaenoic acid and docosahexaenoic acid versus alpha-linolenic acid supplementation on cardiometabolic risk factors: A meta-analysis of randomized controlled trials. Food Funct. 2020, 11, 1919–1932.
- Borja-Magno, A.; Guevara-Cruz, M.; Flores-Lopez, A.; Carrillo-Dominguez, S.; Granados, J.; Arias, C.; Perry, M.; Sears, B.; Bourges, H.; Gomez, F.E. Differential effects of high dose omega-3 fatty acids on metabolism and inflammation in patients with obesity: Eicosapentaenoic and docosahexaenoic acid supplementation. Front. Nutr. 2023, 10, 1156995.
- Hande, L.N.; Kjellmo, C.; Pettersen, K.; Ljunggren, S.; Karlsson, H.; Cederbrant, K.; Marcusson-Stahl, M.; Hovland, A.; Lappegard, K.T. Effect of n-3 Polyunsaturated Fatty Acids on Lipid Composition in Familial Hypercholesterolemia: A Randomized Crossover Trial. Biomedicines 2022, 10, 1809.
- Allaire, J.; Couture, P.; Leclerc, M.; Charest, A.; Marin, J.; Lepine, M.C.; Talbot, D.; Tchernof, A.; Lamarche, B. A randomized, crossover, head-to-head comparison of eicosapentaenoic acid and docosahexaenoic acid supplementation to reduce inflammation markers in men and women: The Comparing EPA to DHA (ComparED) Study. Am. J. Clin. Nutr. 2016, 104, 280–287.
- Allaire, J.; Vors, C.; Harris, W.S.; Jackson, K.H.; Tchernof, A.; Couture, P.; Lamarche, B. Comparing the serum TAG response to high-dose supplementation of either DHA or EPA among individuals with increased cardiovascular risk: The ComparED study. Br. J. Nutr. 2019, 121, 1223–1234.
- Egert, S.; Kannenberg, F.; Somoza, V.; Erbersdobler, H.F.; Wahrburg, U. Dietary alpha-linolenic acid, EPA, and DHA have differential effects on LDL fatty acid composition but similar effects on serum lipid profiles in normolipidemic humans. J. Nutr. 2009, 139, 861–868.
- Grimsgaard, S.; Bonaa, K.H.; Hansen, J.B.; Nordoy, A. Highly purified eicosapentaenoic acid and docosahexaenoic acid in humans have similar triacylglycerol-lowering effects but divergent effects on serum fatty acids. Am. J. Clin. Nutr. 1997, 66, 649–659.
- Nestel, P.; Shige, H.; Pomeroy, S.; Cehun, M.; Abbey, M.; Raederstorff, D. The n-3 fatty acids eicosapentaenoic acid and docosahexaenoic acid increase systemic arterial compliance in humans. Am. J. Clin. Nutr. 2002, 76, 326–330.
- Woodman, R.J.; Mori, T.A.; Burke, V.; Puddey, I.B.; Watts, G.F.; Beilin, L.J. Effects of purified eicosapentaenoic and docosahexaenoic acids on glycemic control, blood pressure, and serum lipids in type 2 diabetic patients with treated hypertension. Am. J. Clin. Nutr. 2002, 76, 1007–1015.
- Wei, M.Y.; Jacobson, T.A. Effects of eicosapentaenoic acid versus docosahexaenoic acid on serum lipids: A systematic review and meta-analysis. Curr. Atheroscler. Rep. 2011, 13, 474–483.
- Klingel, S.L.; Metherel, A.H.; Irfan, M.; Rajna, A.; Chabowski, A.; Bazinet, R.P.; Mutch, D.M. EPA and DHA have divergent effects on serum triglycerides and lipogenesis, but similar effects on lipoprotein lipase activity: A randomized controlled trial. Am. J. Clin. Nutr. 2019, 110, 1502–1509.
More
