Borrelia burgdorferi sensu lato (B. burgdorferi s.l.), which is predominantly spread by ticks, is the cause of Lyme disease (LD), also known as Lyme borreliosis, one of the zoonotic diseases affecting people. In recent years, LD has become more prevalent worldwide, even in countries with no prior records. The aptamer is an advanced technology with the potential for Borrelia antigen detection. Notably, combining the latest technology with the aptamer could enhance test sensitivity and detection limits and reduce the time required to complete the assay. Furthermore, the test can function alone or complement the conventional serological test practiced in most laboratories. In summary, a fast and convenient assay may facilitate the diagnosis of the fever-like symptom possibly caused by Lyme Borrelia infection.
- Lyme disease
- tick-borne disease
- direct detection
- aptamer
1. Introduction
2. Current Guidelines for LD Diagnostic Test
Two types of enzyme immunoassay, known as standard or modified, two-tiered serology testing (STTT/MTTT), are used to detect immunoglobulin (IgM or IgG). These methods differ in terms of the second confirmation assay; the STT uses western blotting, whereas the second enzyme immunoassay (EIA) is utilized for MTT when the sample is positive or ambiguous. Figure 1 summarises the difference between the two-tier STTT and MTTT testing [29][30][31][32][29,30,31,32].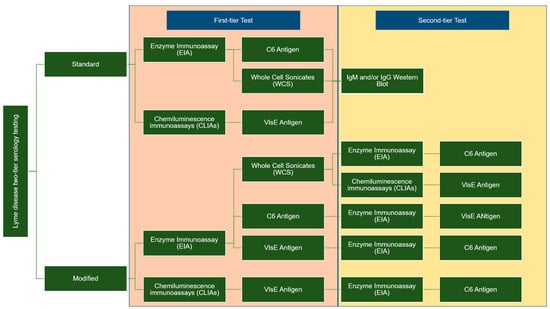
3. Direct Detection Method of Borrelia
3.1. Nucleic Acid Amplification
Most commercial kits are developed to target and conserve the genomic sequences of Borrelia species using two-step PCR (nested PCR). Borrelia species can be directly detected in patients’ samples, such as synovial fluids, cerebrospinal fluid (CSF), and blood, using polymerase chain reaction (PCR). The PCR assay targets several genes located on the genome sequence or linear plasmid of Borrelia species, such as flagellin B (flab), outer surface protein A (OspA), OspC rpoB, and 16S rDNA [38][39][40][38,39,40]. These target genes are also useful for phylogenetic analysis of Borrelia from ticks. Nevertheless, this method demonstrates various sensitivity ranges (4–100%) with 93–100% specificity [41]. Furthermore, the sensitivity ranges between 5 and 50% for EM skin biopsies compared to the CSF samples [42][43][42,43]. The low DNA recovery in patients’ samples, particularly for younger patients with limited samples, reflects the unsatisfactory sensitivity of the assay. Several PCR protocols have been established to enhance the sensitivity of the detection method in combination with the enrichment step. For instance, the loop-mediated isothermal amplification (LAMP) assay targets the 16S rRNA and has higher sensitivity (0.2 to 0.02 pg of DNA) in detecting B. burgdorferi s.l. isolated from field-collected ticks compared to conventional and nested PCR [44]. In another study, the LAMP assay targeted the flagellin (fla) gene to detect as few as 20 copies of DNA per reaction and cross-react with 11 related bacteria [45]. Thus, the success rate of the LAMP assay is equivalent to nested PCR. Further more, the high amplification efficiency of the LAMP assay is due to the continuous amplification under isothermal conditions, yielding magnesium pyrophosphate as a by-product. The white-coloured precipitation is easily observed by the naked eye or by real-time turbidity monitoring using itoring using the conventional photometer [46]. Recombinase polymerase amplification (RPA) is another example of an isothermal amplification method that utilizes the recombinase protein to unwind the double-stranded DNA molecules and the strand-displacing activity to amplify DNA targets. This process takes 20–30 min from 37 °C to 42 °C [47]. The detection limit of the RPA assay targeting the recA gene is five copies, while the sensitivity is 50 femtograms of B. burgdorferi genomic DNA [48]. Even though the PCR sensitivity is high, this assay cannot differentiate between live and dead bacteria within the samples. The positive PCR result is unable to distinguish live bacteria in patients with persistent arthritis after antibiotic therapy [49]. In addition, PCR sensitivity is also highly variable depending on the type of starting materials, DNA extraction methods, target gene location, and PCR amplification method [50][51][52][53][54][50,51,52,53,54]. Thus, further evaluation of the PCR assay is still required to improve the assay sensitivity, and the method must be standardized to provide unambiguous diagnostic results.3.2. Direct Antigen Detection
3.2. Direct Antigen Detection
Borrelia antigen detection is a dependable, quick, accurate, and sensitive method for early LD diagnosis and improved patient care before acquiring disseminated LD. These assays are performed using Borrelia polyclonal antibodies, with the shed surface antigens detectable in the urine, blood, and various organs of infected hosts [55]. Nonetheless, the assay sensitivity, specificity, and accuracy in detecting several antigenic moieties (31 kDa, 34 kDa, 39 kDa, and 93 kDa) are reportedly low in invalidated clinical samples [56]. The assay can be enhanced by performing high-speed centrifugation to separate Borrelia membrane proteins from the rest of the serum proteins. B. burgdorferi cells that have been broken will release membrane vesicles containing membrane proteins into the blood. Precisely, high-speed centrifugation contributes to a low protein detection limit at approximately 4.0 fmol of ospA/mg of serum protein [57]. Hydrogel microparticles are widely used in biomedical research, such as being incorporated with chemical bait to mediate small target molecule sequestration and entrapment. This method helps concentrate the target molecules in the solution and eliminate other molecules through sieving. For instance, Douglas et al. reported that Acid Black 48 dye effectively acts as bait to concentrate the B. burgdorferi OspA and OspB proteins at a limit of detection (LOD) of 700 pg/mL in 10 mL of urine [58]. In another study, Remazol Brilliant Blue dye was used as bait for the Nanotrap technology. The Nanotrap particles were coupled with a Borrelia monoclonal antibody that targeted epitope OspA 236–239, with a detection rate as low as 1.7 pg/mL of Borrelia antigen. These studies supported the shedding of urinary OspA protein, which strongly correlates with the clinical diagnosis of the early and active stage of LD [59]. An earlier study found ten highly conserved amino acids in OspC that are significantly immunodominant and potential immunogens for anti-OspC antibody production [60]. In addition, this peptide contains a common sequence (PVVAESPKKP) found in most pathogenic Borrelia [60][61]. A solid-phase immobilized epitope immunoassay (SPIE-IA) of OspC protein was recently developed [62], despite the high variability of this protein within and between the Borrelia species. The developed SPIE-IA recorded a LOD as low as 17 pg/mL for OspC from infected animal blood samples. Moreover, t3.3. Biosensor
3.3. Biosensor
Several single multiplexed assays have been developed to replace the second tier of serology assays. For example, the mChip-Ld assay targeted three Borrelia antigens (VlsE, PepVF, and OspC) and showed a sensitivity range between 80% and 100% in sample patients with early Lyme disease and Lyme arthritis, respectively [36]. Several biosensor applications have been developed to detect Borrelia antigen, including field-effect transistors (FETs) and microfluidics [63][64][63,64]. The attachment of B. Burgdorferi flagellar antibodies (p41) to nanotubes effectively detected antigens in buffer (1 ng/mL) using atomic force microscopy [63][64][63,64]. The assay utilized the turnoff voltage measurement following antigen exposure, which involves shifting the threshold voltage towards the more negative region. Furthermore, the device is highly selective for the target antigen on the flagellar protein. In a different study, the PPO triplex test (rP100 + PepVF + rOspC-K)-based microfluidic diagnostic device (mChip-Ld) was developed for antibody detection during early and late LD stages. The assay recorded 84% sensitivity and 92% specificity, comparable to the lab-based C6 peptide ELISA [64]. Figure 2 summarises the method used for direct detection of genetic material and soluble antigens of Borrelia.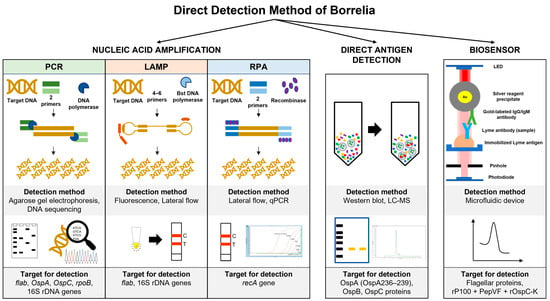
4. Aptamer for Borrelia Antigen Detection
5. Current Aptamer Development for Borrelia Antigen Detection
Only one study has used aptamer technology to directly detect Borrelia antigen using surface-enhanced Raman spectroscopy (SERS) [82]. SERS is an ultrasensitive method for single-molecule detection[64, which is a powerful tool for biosensing applications in various fields. Furthermore80, SERS peaks have narrow bandwidths and a characteristic molecular “fingerprint” compared to other techniques; thus, this method is ideal for multiplex detection81]. The unique surface plasmon resonance of metallic nanoparticles is utilized in this technology, which operates as signal-amplifying substrates and eliminates the need for pathogen culture [83]. Surface-bound, highly affine capture biomolecules provide a layer of specificity to these particles, allowing whole-cell pathogen fingerprinting. The design of highly sensitive Raman aptasensors requires rigorous control of the three-dimensional assembled configuration to maximize SERS enhancement, particularly the local amplification of the electromagnetic (EM) field. Moreover, SERS aptasensors were developed by combining SERS probes with highly sensitive and selective aptamers for recognizing target molecules [84]. In a recent study, SERS accurately identified 91% of serum samples from Lyme patients and 96% of serum samples from symptomatic controls with a LOD of 1 × 10−4 ng/mL, more than four orders of magnitude lower than serum samples from early LD patients [82]. This assay demonstrated a 50% improvement in sensitivity without significantly reducing specificity, unlike the existing Lyme diagnostic assay. In other studies, SERS identified bacteria from complex solutions with a high capture efficiency for S. aureus (88.89%) and E. coli (74.96%) within 15 min [85][86]. The latest applications of the other SERS-based aptasensor include the detection of the SARS-CoV-2 virus, Salmonella Typhimurium, H3N2 virus, Vibrio parahaemolyticus, Staphylococcus aureus, and influenza A [87][88][89][90][91][92]. Table 1 compares the available detection methods for Lyme disease and their sensitivity.| Detection Methods | Target Molecules | Sensitivity | LOD | Reference |
|---|---|---|---|---|
| Nested PCR | p66, 16S rRNA gene, fla gene, 23S rRNA gene, 5S rRNA-23S rRNA gene spacer, recA gene, OspA gene | 4–100% | ND | [93][94][95] |
| LAMP | 16S rRNA | 32.7% | 0.2 to 0.02 pg | [96] |
| fla gene | 37.5% | 20 copies | [97] | |
| RPA | recA gene | 90% | 50 femtograms | [98] |
| Hydrogel microparticles | B. burgdorferi OspA and OspB | ND | 700 pg/mL | [99] |
| Nanotrap technology | OspA 236–239 | 87.5% | 1.7 pg/mL | [100] |
| SPIE-IA | OspC | ND | 10 ng/mL–17 pg/mL | [101] |
| mChip-Ld | VlsE, PepVF, OspC | 80% to 100% | ND | [102] |
| FETs | p41 | Unavailable | 1 ng/mL | [103] |
| Microfluidics | rP100, PepVF, rOspC-K | 84% | ND | [104] |
| SERS (aptasensor) | OspA | 91–96% | 1 × 104 ng/mL | [105] |
