Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Rita Xu and Version 1 by Beom Soo Kim.
Some collaborative studies advocate the unique characteristics of unconventional materials, including carbon nanotubes, nanosheets, nanoparticles, conducting polymers, integrated nano polymers, nano enzymes, and zero-dimensional nanomaterials/carbon dots (CDs) at the atomic and molecular level to generate efficient energy from various biomass substrates. Nanotechnology-based catalysts are considered a crucial tool for revolutionizing various energy-related applications.
- carbon dots
- biomass
- precursors
- biofuel cell
1. Introduction
Researchers are concerned about how the preservation of the ecosystem and human health is threatened by the rise in atmospheric pollution and rising energy consumption. To tackle these issues, several technologies are being actively pursued, such as renewable energy sources, alternative energy storage [1,2,3][1][2][3], biofuel cells [4], sustainability [5], advanced nanocatalysts such as two dimensional (2D) materials, one dimensional (1D) materials, zero-dimensional materials, or carbon dots (CDs) [6,7,8,9,10][6][7][8][9][10].
In the field of electronics, nanomaterials such as ambipolar graphene quantum dots and other 2D materials play a significant role in excellent carrier mobility in phototransistors with excellent light-harvesting properties. The distinctive structure of 3D graphene allows it to efficiently absorb light while maintaining excellent electrical conductivity [11,12][11][12]. Additionally, there has been significant interest in the use of CDs, specifically group IV–VI quantum dots, due to their excellent light-harvesting capabilities in the infrared region. These quantum dots can be conveniently integrated with silicon substrates through a solution process. This integration method offers a practical and efficient approach to incorporating quantum dots into silicon-based devices [12]. CDs emit efficiently in the blue-green range, with the peak shifting towards longer wavelengths as excitation increases. A limited understanding of photoluminescence (PL) in CDs hinders researchers due to the complex structure and variability of the PL centers [13]. Nevertheless, theoretical calculations offer insight into the excited states and electronic structures of different CDs in the context of their optical properties [14].
According to several reports [8,15[8][15][16],16], CDs were first introduced in 2004, and Sun’s research group later gave the name ‘fluorescent CDs’ in 2006 [17]. Xu’s group found CDs during the downstream of single-wall nanotubes (SWNTs) by the gel phoresies of carbon soot [6,8,15,16][6][8][15][16]. CDs have quasi-spherical even shapes with ultra nano size (almost less than 10 nm) that are primarily made of sp2 or sp3 amorphous carbon along with nanocrystalline graphene layers and some functional groups such as O (almost 5 to 50 wt%), S, −NH2, N, −OH, and −COOH [15[15][18],18], which mostly depends on the technique involved [15,16,19][15][16][19]. Due to their ubiquitous optical, electrical, thermal, biological, and physicochemical properties [20], CDs are potential replacements for traditional bio-based nanomaterials in several domains [21,22,23,24][21][22][23][24]. Additionally, CDs possess excellent electron-transferring abilities due to the uniform dispersion quality of quantum dots [16]. The increased usage of CDs has been hindered by numerous debates due to the rapid publication of emerging nanomaterials, which creates significant obstacles to comprehending their natural properties, thus substantially impeding their widespread adoption [22].
Among the several nanomaterials like graphene, graphene oxide [10[10][25],25], and reduced graphene oxide, CDs are becoming a subject of growing interest in various fields and are being examined as a potential substitute for traditional energy storage materials, specifically semiconductor quantum dots, enzymatic biofuel cells, and electronic devices [26] due to their high tunable band gap, higher surface-to-volume ratio, and quantum confinement effect [27].
Some researchers have reported CDs as carbon quantum dots (CQDs)/multi-layered graphene quantum dots (m-GQDs) due to the uncertainty surrounding the classical quantum confinement effect. This is because there is some uncertainty regarding whether the quantum confinement effect is present in CDs or whether their unique properties are due to other factors, such as surface functionalization or defects. Some researchers may use these alternative names to reflect the particles’ properties more accurately and avoid confusion regarding their true nature [28]. The wide range of synthesized processes and materials is essentially the main cause of the diversity of CDs [29]. Currently, the synthesis of CDs is still in its primary stage [16]. Two major techniques are adapted for the synthesis of CDs. Top-down techniques (breaking down large carbon particles into smaller sizes) employ harsh and powerful processes, including electrochemical oxidation [30], arc discharging [31], chemical oxidation [16], and laser ablation [29]. For bottom-up techniques, CDs are formed from small molecules or polymer precursors such as ethylenediaminetetraacetic acid (EDTA) [32], citric acid, ethylene glycol, etc., [33,34,35][33][34][35] under relatively simple and benign conditions, like microwave-assisted pyrolysis, hydrothermal treatment, and ultrasonic reaction [31,33,34][31][33][34]. The drawbacks of the adapted techniques include low yield, time-consuming, synthetic conditions, treatment processes, high cost, and toxicity [36]. This promotes the requirement of a green synthesis technique for producing superior luminous CDs for practical applications [33,37][33][37] and heteroatom doping for promoting catalytic activity [36].
Natural sources for CDs include materials like vegetables (onion, ginger, and cabbage) [33] and fruits (orange juice, banana juice, and winter melon) [33,36][33][36]. Several other sources include biomass such as betel leaf [37], sugarcane [38], soy milk, bovine serum albumin, gelatin, pomelo peels, bottles, silkworm, chitosan, grape juice, salicin cortex, and papaya powder [33,39][33][39].
2. Carbon Dots as Nanocatalysts in Energy Storage and Conversion
2.1. Biofuel Cells
Biofuel cells (BFCs) have shown great potential as a power source for portable biomedical devices and self-powered biosensor portable electronic devices by transferring electrons between enzymes and electrodes as well as the degradation of organic substrates [18,90,91,92][18][40][41][42]. This is due to their superior efficiency, volumetric power density, biocompatibility, low working temperature, and neutral pH [93,94,95][43][44][45]. Mostly, BFCs’ power supply capacity is in the range of 10 µW–450 mW with a voltage range of 0.5–1 V [93,95][43][45] and a power density of 3.7 mW/cm2 [91][41]. However, the major limitations of the technology include limited mass transportation, the minimal utility of enzymes, low durability and lifetime, and slow electron transfer [91,94,96][41][44][46]. Bioenergy generation through microbial extracellular electron transfer (EET) was reported by Zhang et al. (2022). They reported that by incorporating CDs, the efficiency of electron transfer could be enhanced five times from the pure dissimilatory metal-reducing bacteria (DMRB) due to the enhancement in biofilm immobilization and riboflavin secretion [82][47]. To create advanced BFCs, direct electron transfer (DET) between electrodes and redox enzymes is the best solution. It provides higher stability and better power supply without a redox mediator at an optimal voltage [79,90][40][48]. Since it only relies on the position and direction of the active site within the protein, DET requires an immediate connection between the enzyme and the electrode, which is not always possible [92][42]. Barelli et al. (2019) reported that during successful DET, the tunneling distance should be about 1.5 nm. The effective DET electron transfer process demonstrated that the highly conductive, porous macro or nanofabricated electrode material is the turning point for enhancing the number of wired enzymes per unit volume [18,90][18][40]. Earlier studies have explored important avenues for the efficient utilization of carbon-based materials such as CDs, nanofibers, graphene, nanowires, and carbon black in BFCs for implantable and low-power device applications [90,91,97][40][41][49]. Researchers have developed advanced nanoelectrode materials and immobilization techniques for enhancing the efficiency of BFCs (Figure 1) and biosensors [26]. Recently, nanoscale technologies have addressed the issue of the low electron transfer efficiency between the enzyme and electrode surface, along with permitting the assimilation of a higher enzyme load to improve the efficiency of kinetic processes in BFCs through modified fabrication. Similarly, Wu et al. (2017) prepared CDs from candle soot to design a laccase-based electrode. It was reported that the laccase activity was 220 U/mg, and the CD immobilizing matrixes facilitated high methanol oxidation through direct electron transfer at the anode and oxygen reduction into H2O at the laccase-based cathode. They achieved a better power density of 68.7 ± 0.4 μW/cm2 and an open-circuit voltage (OCV) of 0.71 ± 0.02 V after fabricating the immobilizing matrixes of CDs [18].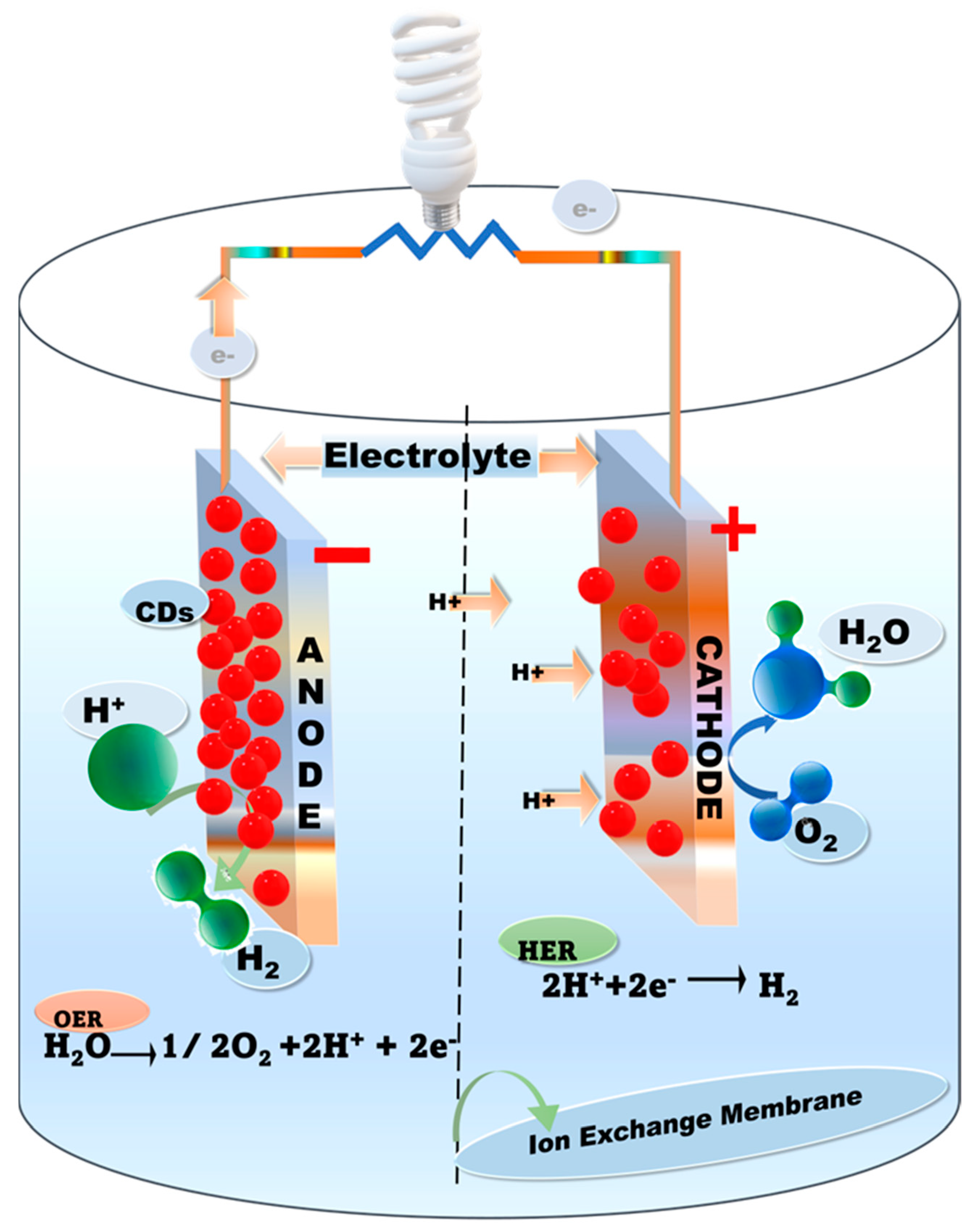
Figure 1. Schematic illustration of the full-cell configuration with CDs as electrocatalysis.
2.2. Electrocatalysts for Energy Conversion
The performance of CDs as carbon nanomaterials for electrocatalysts has been advocated due to their high dispersibility in polar solvents, strong coordination, and distinctive electron transfer ability [98][50]. Additionally, CDs can be combined with other nanomaterials or metal to create 2D and 3D nanostructures along with −COOH, −NH2, −OH, or other similar functional groups via self-crosslinking or splicing, hydrophilic terminals, or covalent bonds with rich edge structures. Thus, it accelerates the hydrogen evolution reaction (HER) by significantly expanding the three-phase boundary where reactants, electrolytes, and electrons converge [87][51]. Moreover, the flexible carbon structure and the surface chemistry of the CDs allow optimization of the electrocatalysts based on CDs while also promoting the development of advanced biofuel cells [98,99][50][52]. The CD-based electrocatalysts have used several synthetic techniques, including hydrothermal treatment, calcination, electrodeposition, and reflux. These catalysts can be classified as metallic or non-metallic based on the availability of the metal components [42,98,99][50][52][53]. HER is an extensively researched topic in the electrocatalyst field and involves an uncomplicated proton–electron transfer process without any accompanying reactions. A three-electrode system (working, reference, and counter electrode) is used to measure HER. The reference electrode’s potential is measured via the reference electrode [56][54]. According to Volmer’s equation, the intermediate step entails the adsorption of hydrogen onto the electrode’s surface. Electronic interactions and thermodynamics with catalyst materials have a significant impact on hydrogen generation. The binding energies are determined by the kinetics and thermodynamics of the reactions occurring on the electrode surface, as well as other factors such as solvent contribution. In the case of transition metal catalysts, the catalyst performs according to the Sabatier principle [100][55]. Once absorbed, the HER proceeds through the Heyrovsky or Tafel equation, depending on the pathway. The following Volmer–Heyrovský mechanism involves the reaction of the HER electrode system [56,100][54][55].H+ + e− → H (Proton-coupled electron transfer step (PCET))
H+ + e− + H → H2 (Heyrovsky reaction)
H + H → H2 (Tafel reaction)
Table 1. Reported data on the utilization of various CD composites and their application in energy conversion and storage.
| Catalyst Name | Synthesis Method | Size (nm) | Current Density (mA/cm2) | Applications | References |
|---|---|---|---|---|---|
| Graphitic N | Hydrogel | 3–5 | 1.62 | ORR, HER, photocatalysis | [47][58] |
| Nitrogen-deficient g-C3N4 | Hydrothermal | <10 | 10 | HER | [102][57] |
| C3N4/(Co(OH)2/Cu(OH)2 | Microwave-assisted | 0.27 | 8.9 | CO2 reduction | [103][59] |
| Ni-CDs | Electrochemical | 2–5 | 0.23 | HER | [101][56] |
| Polyaniline Carrot derived-CDs | Hydrothermal | 6–8 | 5 A/g | Window supercapacitor | [52][60] |
2.3. Supercapacitor
Supercapacitors or ultracapacitors are based on redox reactions to store energy (Figure 2). There are three types of supercapacitors based on the type of electrochemical reactions occurring: pseudo-capacitors (PCs)/Faraday capacitors, electrostatic double-layer capacitors (EDLCs), and hybrid ion capacitors (HICs) that combine the two capacitors [40][61]. When conducting polymers, metal oxides, or metal nitrides are used as electrode materials in a pseudo-capacitor, a reduction–oxidation reaction takes place, resulting in a higher transfer of electron charges between the electrode and the electrolyte, resulting in a higher electrochemical pseudo-capacitance. Therefore, compared to electric double-layer capacitances (EDLCs), PCs and HICs perform better [40,104][61][62].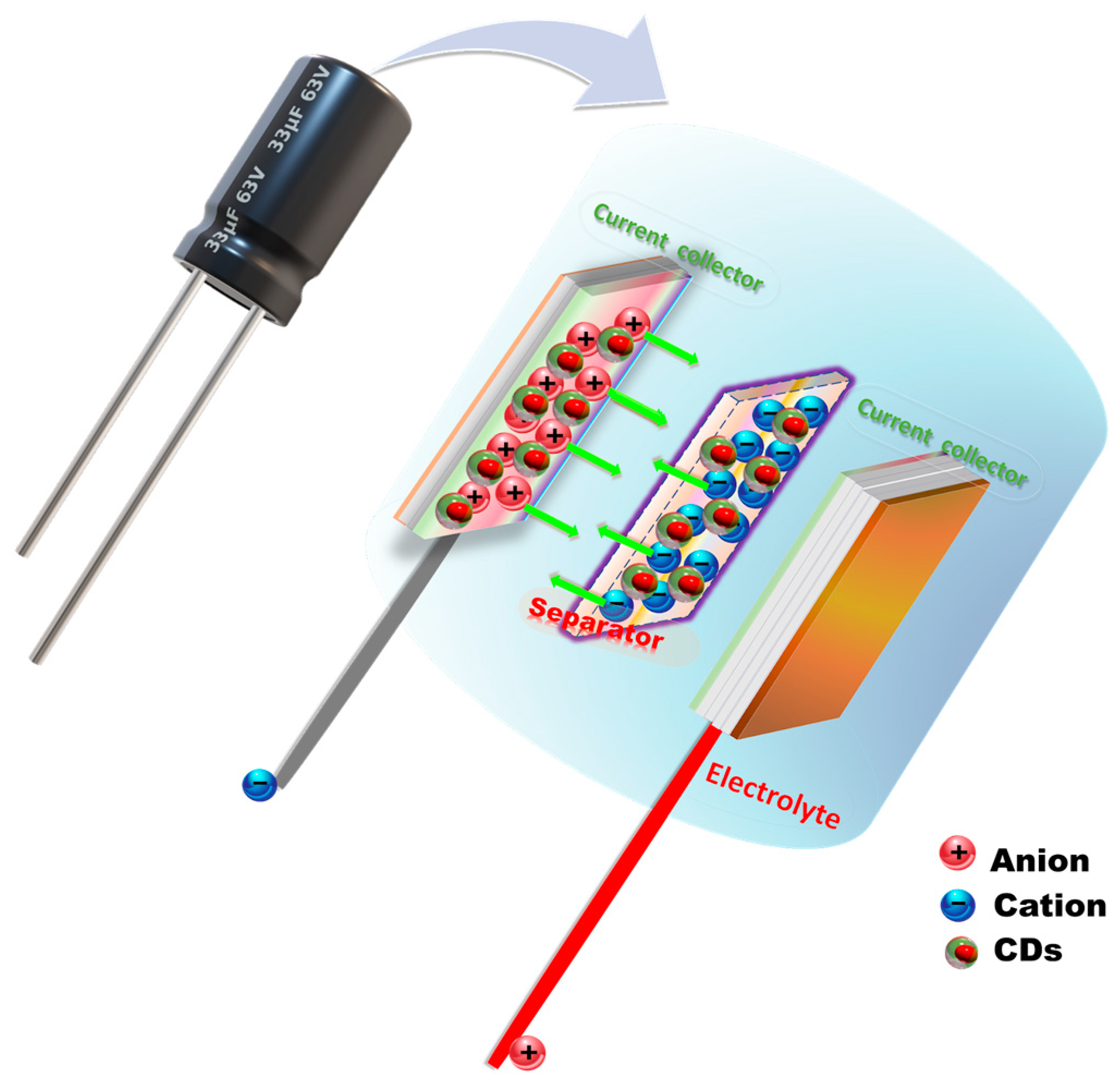
Figure 2. The schematic mechanism illustration of fabricated asymmetric supercapacitor devices based on CDs.
2.4. Photocatalysts
In photocatalysis, CDs have proven to be adaptable materials with a variety of uses. They are extremely useful for capturing solar energy in a variety of catalytic processes due to their special qualities. The wide absorption spectrum extending into the visible range is one of the main advantages of being able to use sunlight effectively. This characteristic distinguishes CDs from traditional semiconductor photocatalysts such as titanium dioxide (TiO2) and enables them to effectively catalyze environmentally important reactions. CDs are used in environmental remediation to degrade colors and medicines and to break down organic contaminants in wastewater [110][69]. CDs are also essential for hydrogen generation via water splitting, which helps produce hydrogen in a greener manner. In the process of reducing carbon dioxide, CDs help to convert CO2 into organic molecules or valuable hydrocarbons, facilitating the capture and usage of CO2, and are used to increase solar cell efficiency, which helps convert sunlight into power. Furthermore, their antimicrobial qualities make them useful for sterilizing and purifying water. When exposed to light, they help break down organic pollutants and are integrated into self-cleaning surfaces [111][70]. Additionally, CDs participate in selective photoredox reactions, providing excellent selectivity in the production of different compounds and medications. Researchers continue to find ways to modify CDs to further improve photocatalytic efficiency, which would increase the number of sustainable and energy-efficient processes in which they can be used [110,111][69][70].References
- Bu, Y.; Kim, H.K.; Lee, J.S.; Jang, H.G.; Jeong, J.H.; Chun, S.W.; Sharma, M.; Kim, B.S. Improvement of biodegradable polymer film properties by incorporating functionalized few-layer graphene. Food Packag. Shelf Life 2023, 40, 101205.
- Sharma, M.; Das, P.P.; Purkait, M.K. Chapter 16—Energy storage properties of nanomaterials. In Advances in Smart Nanomaterials and Their Applications; Husen, A., Siddiqi, K.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 337–350.
- Sharma, M.; Das, P.P.; Chakraborty, A.; Purkait, M.K. 29—Extraction of clean energy from industrial wastewater using bioelectrochemical process. In Resource Recovery in Industrial Waste Waters; Sillanpää, M., Khadir, A., Gurung, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 601–620.
- Sharma, M.; Das, P.P.; Sood, T.; Chakraborty, A.; Purkait, M.K. Reduced graphene oxide incorporated polyvinylidene fluoride/cellulose acetate proton exchange membrane for energy extraction using microbial fuel cells. J. Electroanal. Chem. 2022, 907, 115890.
- Singh, A.; Kushwaha, A.; Goswami, S.; Tripathi, A.; Bhasney, S.M.; Goswami, L.; Hussain, C.M. Roadmap from microalgae to biorefinery: A circular bioeconomy approach. In Emerging Trends to Approaching Zero Waste: Environmental and Social Perspectives; Elsevier: Amsterdam, The Netherlands, 2021.
- Perumal, S.; Atchudan, R.; Edison, T.N.J.I.; Lee, Y.R. Sustainable synthesis of multifunctional carbon dots using biomass and their applications: A mini-review. J. Environ. Chem. Eng. 2021, 9, 105802.
- Sharma, M.; Chakraborty, A.; Kuttippurath, J.; Yadav, A.K. Potential Power Production from Salinity Gradient at the Hooghly Estuary System. Innov. Energy Res. 2018, 7, 210.
- Zuo, P.; Lu, X.; Sun, Z.; Guo, Y.; He, H. A review on syntheses, properties, characterization and bioanalytical applications of fluorescent carbon dots. Microchim. Acta 2015, 183, 519–542.
- Wu, S.; Li, W.; Zhou, W.; Zhan, Y.; Hu, C.; Zhuang, J.; Zhang, H.; Zhang, X.; Lei, B.; Liu, Y. Large-Scale One-Step Synthesis of Carbon Dots from Yeast Extract Powder and Construction of Carbon Dots/PVA Fluorescent Shape Memory Material. Adv. Opt. Mater. 2018, 6, 1701150.
- Pham, P.V.; Bodepudi, S.C.; Shehzad, K.; Liu, Y.; Xu, Y.; Yu, B.; Duan, X. 2D Heterostructures for Ubiquitous Electronics and Optoelectronics: Principles, Opportunities, and Challenges. Chem. Rev. 2022, 122, 6514–6613.
- He, Z.; Zhang, S.; Zheng, L.; Liu, Z.; Zhang, G.; Wu, H.; Wang, B.; Liu, Z.; Jin, Z.; Wang, G. Si-Based NIR Tunneling Heterojunction Photodetector With Interfacial Engineering and 3D-Graphene Integration. IEEE Electron Device Lett. 2022, 43, 1818–1821.
- Zeng, L.; Li, X.; Fan, S.; Li, J.; Mu, J.; Qin, M.; Wang, L.; Gan, G.; Tadé, M.; Liu, S. The bioelectrochemical synthesis of high-quality carbon dots with strengthened electricity output and excellent catalytic performance. Nanoscale 2019, 11, 4428–4437.
- Mocci, F.; Engelbrecht, L.d.V.; Olla, C.; Cappai, A.; Casula, M.F.; Melis, C.; Stagi, L.; Laaksonen, A.; Carbonaro, C.M. Carbon Nanodots from an In Silico Perspective. Chem. Rev. 2022, 122, 13709–13799.
- Langer, M.; Paloncýová, M.; Medveď, M.; Pykal, M.; Nachtigallová, D.; Shi, B.; Aquino, A.J.A.; Lischka, H.; Otyepka, M. Progress and challenges in understanding of photoluminescence properties of carbon dots based on theoretical computations. Appl. Mater. Today 2021, 22, 100924–100951.
- Kang, Z.; Lee, S.T. Carbon dots: Advances in nanocarbon applications. Nanoscale 2019, 11, 19214–19224.
- Kurian, M.; Paul, A. Recent trends in the use of green sources for carbon dot synthesis-A short review. Carbon Trends 2021, 3, 32.
- Cui, L.; Ren, X.; Sun, M.; Liu, H.; Xia, L. Carbon dots: Synthesis, properties and applications. Nanomaterials 2021, 11, 3419.
- Wu, G.; Gao, Y.; Zhao, D.; Ling, P.; Gao, F. Methanol/Oxygen Enzymatic Biofuel Cell Using Laccase and NAD+-Dependent Dehydrogenase Cascades as Biocatalysts on Carbon Nanodots Electrodes. ACS Appl. Mater. Interfaces 2017, 9, 40978–40986.
- Darinel, S.; Landa, T.; Kumar, N.; Bogireddy, R.; Kaur, I.; Batra, V.; Agarwal, V. Heavy metal ion detection using green precursor derived carbon dots. iScience 2022, 25, 103816.
- Devadas, B.; Imae, T. Effect of Carbon Dots on Conducting Polymers for Energy Storage Applications. ACS Sustain. Chem. Eng. 2018, 6, 127–134.
- Duarah, P.; Das, P.P.; Sharma, M.; Purkait, M.K. Recent Advances in Dye Removal Technologies by Designer Biochar. In Designer Biochar Assisted Bioremediation of Industrial Effluents; CRC Press: Boca Raton, FL, USA, 2022; pp. 223–239.
- Essner, J.B.; Kist, J.A.; Polo-Parada, L.; Baker, G.A. Artifacts and Errors Associated with the Ubiquitous Presence of Fluorescent Impurities in Carbon Nanodots. Chem. Mater. 2018, 30, 1878–1887.
- Rizvi, S.; Singh, A.; Gupta, S.K. A parametric study using Box-Behnken design for melanoidin removal via Cu-impregnated activated carbon prepared from waste leaves biomass. Appl. Water Sci. 2022, 12, 81.
- Rizvi, S.; Goswami, L.; Gupta, S.K. A holistic approach for melanoidin removal via Fe-impregnated activated carbon prepared from Mangifera indica leaves biomass. Bioresour. Technol. Rep. 2020, 12, 100591.
- Sharma, M.; Mondal, P.; Sontakke, A.D.; Chakraborty, A.; Purkait, M.K. High performance graphene-oxide doped cellulose acetate based ion exchange membrane for environmental remediation applications. Int. J. Environ. Anal. Chem. 2021, 1–22.
- Neto, S.A.; de Andrade, A.R. New energy sources: The enzymatic biofuel cell. J. Braz. Chem. Soc. 2013, 24, 1891–1912.
- Liu, H.; Zheng, S.M.; Xiong, H.F.; Alwahibi, M.S.; Niu, X. Biosynthesis of copperoxide nanoparticles using Abies spectabilis plant extract and analyzing its antinociceptive and anti-inflammatory potency in various mice models. Arab. J. Chem. 2020, 13, 6995–7006.
- Lecroy, G.E.; Messina, F.; Sciortino, A.; Bunker, C.E.; Wang, P.; Fernando KA, S.; Sun, Y.P. Characteristic Excitation Wavelength Dependence of Fluorescence Emissions in Carbon “quantum” Dots. J. Phys. Chem. C 2017, 121, 28180–28186.
- Hu, C.; Li, M.; Qiu, J.; Sun, Y.P. Design and fabrication of carbon dots for energy conversion and storage. Chem. Soc. Rev. 2019, 48, 2315–2337.
- Arul, V.; Edison TN, J.I.; Lee, Y.R.; Sethuraman, M.G. Biological and catalytic applications of green synthesized fluorescent N-doped carbon dots using Hylocereus undatus. J. Photochem. Photobiol. B Biol. 2017, 168, 142–148.
- Wang, Y.; Hu, A. Carbon quantum dots: Synthesis, properties and applications. J. Mater. Chem. C 2014, 2, 6921–6939.
- Ortega-Liebana, M.C.; Encabo-Berzosa, M.M.; Casanova, A.; Pereboom, M.D.; Alda, J.O.; Hueso, J.L.; Santamaria, J. Upconverting Carbon Nanodots from Ethylenediaminetetraacetic Acid (EDTA) as Near-Infrared Activated Phototheranostic Agents. Chem.—A Eur. J. 2019, 25, 5539–5546.
- Ventrella, A.; Camisasca, A.; Fontana, A.; Giordani, S. Synthesis of green fluorescent carbon dots from carbon nano-onions and graphene oxide. RSC Adv. 2020, 10, 36404–36412.
- Wareing, T.C.; Gentile, P.; Phan, A.N. Biomass-Based Carbon Dots: Current Development and Future Perspectives. ACS Nano 2021, 15, 15471–15501.
- Sharker, S.M.d.; Do, M. Nanoscale Carbon-Polymer Dots for Theranostics and Biomedical Exploration. J. Nanotheranostics 2021, 2, 118–130.
- Hoang, V.C.; Dave, K.; Gomes, V.G. Carbon quantum dot-based composites for energy storage and electrocatalysis: Mechanism, applications and future prospects. Nano Energy 2019, 66, 104093.
- Kalanidhi, K.; Nagaraaj, P. Facile and Green synthesis of fluorescent N-doped carbon dots from betel leaves for sensitive detection of Picric acid and Iron ion. J. Photochem. Photobiol. A Chem. 2021, 418, 113369.
- Huang, G.; Chen, X.; Wang, C.; Zheng, H.; Huang, Z.; Chen, D.; Xie, H. Photoluminescent carbon dots derived from sugarcane molasses: Synthesis, properties, and applications. RSC Adv. 2017, 7, 47840–47847.
- Venkatesan, G.; Rajagopalan, V.; Chakravarthula, S.N. Boswellia ovalifoliolata bark extract derived carbon dots for selective fluorescent sensing of Fe3+. J. Environ. Chem. Eng. 2019, 7, 103013.
- Barelli, L.; Bidini, G.; Calzoni, E.; Cesaretti, A.; di Michele, A.; Emiliani, C.; Gammaitoni, L.; Sisani, E. Enzymatic fuel cell technology for energy production from bio-sources. AIP Conf. Proc. 2019, 2191, 020014.
- Kwon, C.H.; Ko, Y.; Shin, D.; Kwon, M.; Park, J.; Bae, W.K.; Lee, S.W.; Cho, J. High-power hybrid biofuel cells using layer-by-layer assembled glucose oxidase-coated metallic cotton fibers. Nat. Commun. 2018, 9, 4479.
- Zhao, M.; Gao, Y.; Sun, J.; Gao, F. Mediatorless glucose biosensor and direct electron transfer type glucose/air biofuel cell enabled with carbon nanodots. Anal. Chem. 2015, 87, 2615–2622.
- De Poulpiquet, A.; Ciaccafava, A.; Lojou, E. New trends in enzyme immobilization at nanostructured interfaces for efficient electrocatalysis in biofuel cells. Electrochim. Acta 2014, 126, 104–114.
- Jayaramulu, K.; Mukherjee, S.; Morales, D.M.; Dubal, D.P.; Nanjundan, A.K.; Schneemann, A.; Masa, J.; Kment, S.; Schuhmann, W.; Otyepka, M.; et al. Graphene-Based Metal-Organic Framework Hybrids for Applications in Catalysis, Environmental, and Energy Technologies. Chem. Rev. 2022, 122, 17241–17338.
- Yang, Y.; Liu, T.; Tao, K.; Chang, H. Generating Electricity on Chips: Microfluidic Biofuel Cells in Perspective. Ind. Eng. Chem. Res. 2018, 57, 2746–2758.
- Haque, S.U.; Nasar, A.; Duteanu, N.; Pandey, S. Carbon based-nanomaterials used in biofuel cells—A review. Fuel 2022, 331, 125634.
- Zhang, P.; Yang, C.; Li, Z.; Liu, J.; Xiao, X.; Li, D.; Chen, C.; Yu, M.; Feng, Y. Accelerating the extracellular electron transfer of Shewanella oneidensis MR-1 by carbon dots: The role of carbon dots concentration. Electrochim. Acta 2022, 421, 140490.
- Zhu, S.; Song, Y.; Zhao, X.; Shao, J.; Zhang, J.; Yang, B. The photoluminescence mechanism in carbon dots (graphene quantum dots, carbon nanodots, and polymer dots): Current state and future perspective. Nano Res. 2015, 8, 355–381.
- Cosnier, S.; Holzinger, M.; Goff, A. Recent advances in carbon nanotube-based enzymatic fuel cells. Front. Bioeng. Biotechnol. 2014, 2, 45.
- Wu, H.; Lu, S.; Yang, B. Carbon-Dot-Enhanced Electrocatalytic Hydrogen Evolution. Acc. Mater. Res. 2022, 3, 319–330.
- Sharma, M.; Pramanik, A.; Bhowmick, G.D.; Tripathi, A.; Ghangrekar, M.M.; Pandey, C.; Kim, B.-S. Premier, Progress and Prospects in Renewable Hydrogen Generation: A Review. Fermentation 2023, 9, 537.
- Sharma, A.; Singh, G.; Arya, S.K. Biofuel cell nanodevices. Int. J. Hydrogen Energy 2021, 46, 3270–3288.
- Tian, L.; Li, Z.; Wang, P.; Zhai, X.; Wang, X.; Li, T. Carbon quantum dots for advanced electrocatalysis. J. Energy Chem. 2021, 55, 279–294.
- Jana, J.; Ngo, Y.L.T.; Chung, J.S.; Hur, S.H. Contribution of carbon dot nanoparticles in electrocatalysis: Development in energy conversion process. J. Electrochem. Sci. Technol. 2020, 11, 220–237.
- Voiry, D.; Shin, H.S.; Loh, K.P.; Chhowalla, M. Low-dimensional catalysts for hydrogen evolution and CO2 reduction. Nat. Rev. Chem. 2018, 2, 0105.
- Yang, Y.; Liu, J.; Guo, S.; Liu, Y.; Kang, Z. A nickel nanoparticle/carbon quantum dot hybrid as an efficient electrocatalyst for hydrogen evolution under alkaline conditions. J. Mater. Chem. A 2015, 3, 18598–18604.
- Li, X.; Zhang, K.; Zhou, M.; Yang, K.; Yang, S.; Ma, X.; Yu, C.; Xie, Y.; Huang, W.; Fan, Q. A Novel Approach to Synthesize Nitrogen-Deficient g-C3N4 for the Enhanced Photocatalytic Contaminant Degradation and Electrocatalytic Hydrogen Evolution. Nano 2020, 15, 2050006.
- Zhang, J.; Zhang, G.; Jin, S.; Zhou, Y.; Ji, Q.; Lan, H.; Liu, H.; Qu, J. Graphitic N in nitrogen-Doped carbon promotes hydrogen peroxide synthesis from electrocatalytic oxygen reduction. Carbon 2020, 163, 154–161.
- Nazir, R.; Kumar, A.; Saleh Saad, M.A.; Ashok, A. Synthesis of hydroxide nanoparticles of Co/Cu on carbon nitride surface via galvanic exchange method for electrocatalytic CO2 reduction into formate. Colloids Surf. A Physicochem. Eng. Asp. 2020, 598, 124835.
- Oskueyan, G.; Mansour Lakouraj, M.; Mahyari, M. Fabrication of polyaniline–carrot derived carbon dots/polypyrrole–graphene nanocomposite for wide potential window supercapacitor. Carbon Lett. 2021, 31, 269–276.
- Jiang, Z.; Guan, L.; Xu, X.; Wang, E.; Wang, C. Applications of Carbon Dots in Electrochemical Energy Storage. ACS Appl. Electron. Mater. 2022, 4, 5144–5164.
- Xiao, J.; Momen, R.; Liu, C. Application of carbon quantum dots in supercapacitors: A mini review. Electrochem. Commun. 2021, 132, 107143.
- Wang, L.; Zhang, X.; Yang, K.; Wang, L.; Lee, C.S. Oxygen/nitrogen-related surface states controlled carbon nanodots with tunable full-color luminescence: Mechanism and bio-imaging. Carbon 2020, 160, 298–306.
- Permatasari, F.A.; Irham, M.A.; Bisri, S.Z.; Iskandar, F. Carbon-based quantum dots for supercapacitors: Recent advances and future challenges. Nanomaterials 2021, 11, 91.
- Shen, D.; Zhu, L.; Wu, C.; Gu, S. State-of-the-art on the preparation, modification, and application of biomass-derived carbon quantum dots. Ind. Eng. Chem. Res. 2020, 59, 22017–22039.
- Xu, L.; Dun, X.; Zou, J.; Li, Y.; Jia, M.; Cui, L.; Gao, J.; Jin, X. Graphene Hydrogel Decorated with N, O Co-Doped Carbon Dots for Flexible Supercapacitor Electrodes. J. Electrochem. Soc. 2018, 165, A2217–A2224.
- Acerce, M.; Voiry, D.; Chhowalla, M. Metallic 1T phase MoS2 nanosheets as supercapacitor electrode materials. Nat. Nanotechnol. 2015, 10, 313–318.
- Li, X.; Zhang, C.; Xin, S.; Yang, Z.; Li, Y.; Zhang, D.; Yao, P. Facile Synthesis of MoS2/Reduced Graphene Oxide@Polyaniline for High-Performance Supercapacitors. ACS Appl. Mater. Interfaces 2016, 8, 21373–21380.
- Gan, Z.; Wu, X.; Meng, M.; Zhu, X.; Yang, L.; Chu, P.K. Photothermal Contribution to Enhanced Photocatalytic Performance of Graphene-Based Nanocomposites. ACS Nano 2014, 8, 9304–9310.
- Lu, J.; Shi, Y.; Chen, Z.; Sun, X.; Yuan, H.; Guo, F.; Shi, W. Photothermal effect of carbon dots for boosted photothermal-assisted photocatalytic water/seawater splitting into hydrogen. Chem. Eng. J. 2023, 453, 139834.
More
