Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Rita Xu and Version 1 by Gabriele Carciotto.
Dual antiplatelet therapy (DAPT), comprising aspirin and a P2Y12 receptor inhibitor, is the cornerstone of post-percutaneous coronary intervention treatment to prevent stent thrombosis and reduce the risk of adverse cardiovascular events.
- dual antiplatelet therapy
- percutaneous coronary intervention
- acute coronary syndrome
1. Introduction to the Rationale of Antiplatelet Therapy after Coronary Stenting
Percutaneous coronary intervention (PCI), especially with the availability of new drug-eluting stent (DES) technologies and new devices and drugs, has revolutionized the management of coronary artery disease (CAD) by providing effective revascularization and improving clinical outcomes [1,2,3,4,5,6][1][2][3][4][5][6]. Despite these achievements, CAD still represents the leading cause of mortality in developed countries [7,8][7][8] and the outcome is still unsatisfactory in high-risk patients [9,10,11][9][10][11]. Therefore, great attention has been paid so far to the identification of new risk factors [12,13,14,15][12][13][14][15] and implementation of primary and secondary prevention [16,17,18,19][16][17][18][19]. Antiplatelet therapies represent a keystone in secondary cardiovascular prevention. In fact, dual antiplatelet therapy (DAPT), comprising aspirin and a P2Y12 receptor inhibitor, is a cornerstone therapy in both elective and Acute Coronary Syndrome (ACS) patients treated by PCI and stenting, to prevent both stent thrombosis (ST) and ischemic events of other vascular segments, as it is well known that platelet adhesion, activation, and aggregation play a crucial role in the pathogenesis of vascular thrombosis [16].
Aspirin is still widely considered the “primary agent” for the treatment of acute or chronic coronary syndrome to which additional antiplatelet drugs are added when a more intense antithrombotic effect is needed (Figure 1). Its antithrombotic action is based on the acetylation of the platelet’s cyclooxygenase (COX) [20], inhibiting the thromboxane A2 pathway. The interaction between ADP and the platelet P2Y12 receptor is another essential part of the platelet’s activation process, causing enhanced platelet degranulation and thromboxane production, and prolonged platelet aggregation [21]. The inhibition of the P2Y12 pathway prevents the binding of ADP to the receptor, attenuating platelet aggregation. In patients with acute coronary syndrome (ACS) or undergoing PCI for chronic coronary syndrome (CCS), DAPT has been shown to reduce recurrent major adverse cardiovascular events (MACE) compared to aspirin alone [22,23][22][23].
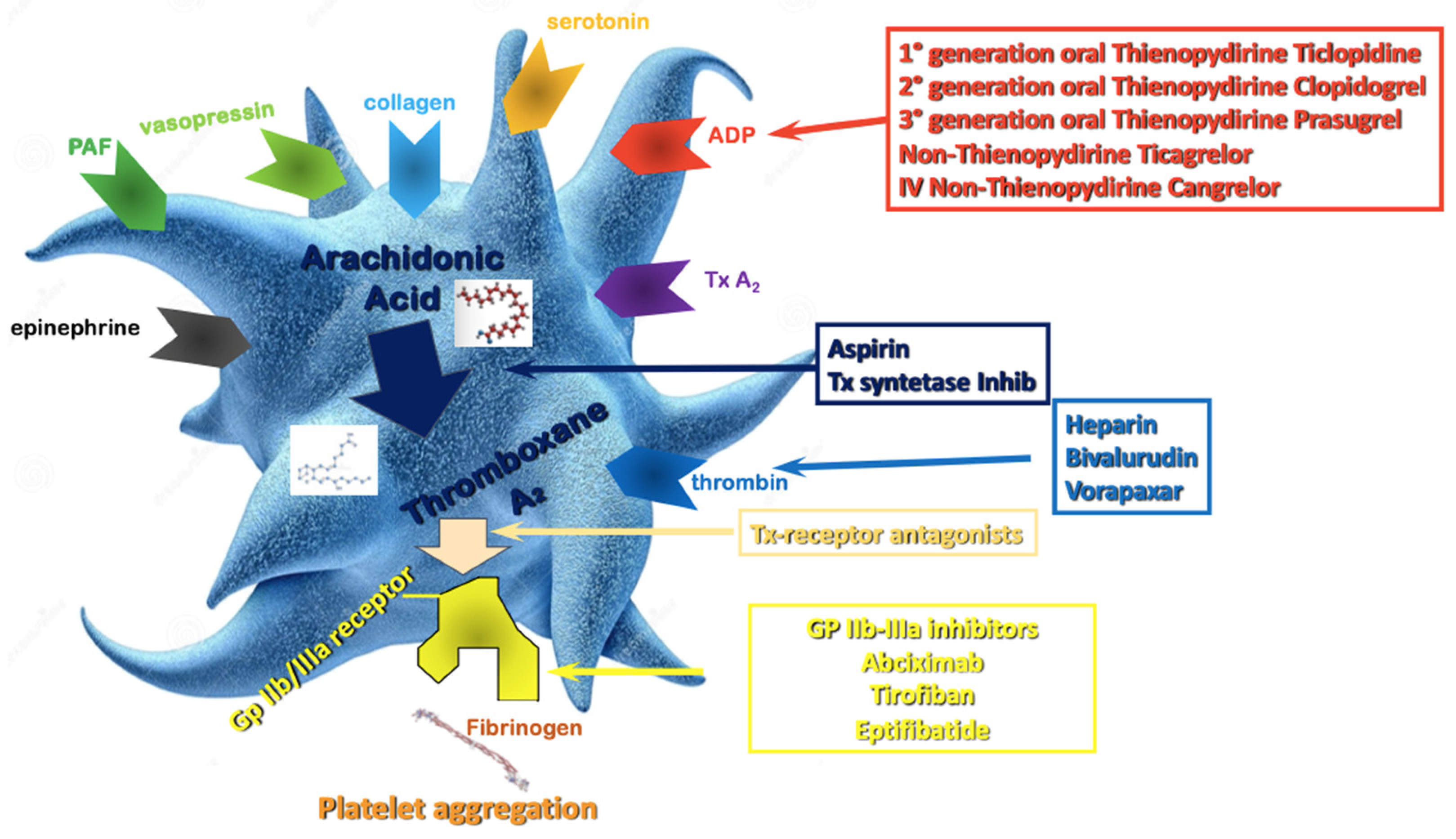
Figure 1.
Antiplatelet therapy treatment pathways. ADP—Adenosine Diphosphate; Tx—Thromboxane; PAF—Platelet Activating Factor; Gp—Glycoprotein.
However, the anti-thrombotic benefits of DAPT are counterbalanced by an increase in bleeding, which is directly related to the duration and intensity of the antiplatelet regimen, and significantly impact mortality with similar time-dependency of myocardial infarction [24]. Hence, determining the optimal duration and selection of antiplatelet agents for DAPT is of the utmost importance to optimize clinical outcomes and remains a subject of ongoing investigation and clinical debate [25,26][25][26]. Recent studies have challenged the traditional notion of a one-size-fits-all approach, suggesting that individualized treatment strategies based on patient-specific factors and procedural characteristics may be necessary to select treatment [27]. Nowadays, the number of options to personalize DAPT treatment is vast, and those options are increasingly endorsed by international guidelines, leveraging on the modulation of DAPT duration (i.e., short vs. long treatment courses) [28], DAPT components in term of P2Y12 inhibitor type (i.e., potent vs. non-potent P2Y12 inhibitor), and dosage [29].
2. Optimal DAPT Duration
2.1. Twelve Months DAPT
In the last ten years, there has been a great number of studies exploring optimal duration of DAPT followed by a single antiplatelet agent. Initially the recommended duration for DAPT after coronary stenting was 1–6 months [36][30]. This treatment window was non-randomly tested in the studies that compared drug-eluting stents (DES) with bare metal stents (BMS). The mean follow-up time for clopidogrel-based DAPT in the PCI CURE trial was 8 months [16]. The evidence of an increased risk of late and very late ST after first-generation DES led to a more cautious approach with extension of DAPT well beyond 12 months after implantation [37,38,39,40][31][32][33][34]. Based on the available evidence, consensus-based guidelines recommended a minimum of 12 months of DAPT in this setting [41][35]. However, the introduction of safer stent platforms [42,43][36][37] progressively showed that DAPT duration could be safely shortened [44][38]. The main reason to strike an optimal duration of DAPT is to reasonably balance the risk of ischemic and bleeding events, both impacting prognosis, and both opposingly affected by the duration of antithrombotic therapy. In this context, trials comparing three or six months of DAPT to one-year DAPT found no difference in ischemic events and showed that a short-term DAPT was safer in terms of bleeding [45][39]. For patients presenting with CCS, 2019 ESC guidelines for the management of chronic coronary syndromes recommend a 6-month DAPT (ASA and clopidogrel) regimen after PCI (Class I, level A). Prasugrel or ticagrelor may be considered in high-risk situations, such as suboptimal stent deployment or complex PCI [46][40]. In patients with ACS, 2023 ESC guidelines for the management of ACS recommend a default strategy of 12 months of DAPT with a potent P2Y12 inhibitor (Class I); however, if the patient undergoes PCI, prasugrel should be considered as the P2Y12 inhibitor of choice, considering the results of the ISAR-REACT 5 RCT. Clopidgrel should only be used when ticagrelor and prasugrel are contraindicated, not available or in HBR patients [34][41].2.2. Prolonged (>12 Months) DAPT
Compared to bare-metal stents, DESs reduced the rate of restenosis; however, there was an initial concern that first-generation DESs may be associated with an additional risk of late and very-late ST [47][42]. In addition, beyond stent-related events, ischemic events unrelated to the treated plaque may also occur, which supports the potential for prolonged antiplatelet therapy as a secondary prevention strategy [48,49][43][44]. Patients with MI have heightened platelet activation and aggregation compared with patients with CCS, leading to a higher predisposition to atherothrombosis [50,51[45][46][47],52], which may persist for years afterwards [53,54,55][48][49][50]. Hence, these patients may benefit from more intensive antiplatelet therapies following PCI. The DAPT study was a multicenter randomized trial that enrolled patients treated with DAPT after PCI with DES. At 12 months, patients who had no MACCE, another revascularization, bleedings (moderate to severe) and had been adherent to the therapy were randomized to continue it or to placebo for another 18 months, to compare the 12 month-strategy to the 30-month strategy. The efficacy end points (cumulative incidence of definite or probable ST and the composite of death, MI, or stroke) were significantly lower in the group that continued thienopyridine (0.4% vs. 1.4% for stent thrombosis; 4.3% vs. 5.9% for MACCE) and was consistent across stent type and thienopyridine drug used. However, prolonged DAPT led to a significantly higher rate of bleeding, and all-cause mortality was increased by 36% in the prolonged DAPT group [56][51]. Importantly, most of the benefit observed with a prolonged DAPT treatment was observed among patients presenting with a MI [57,58][52][53]. Ticagrelor, when added to aspirin after an ACS, compared with clopidogrel, reduces the rate of major adverse CV events. Therefore, patients that suffered a MI, who are at higher risk for recurrent ischemic events, could benefit from prolonged DAPT with ticagrelor. The Prevention of Cardiovascular Events in Patients with Prior Heart Attack Using Ticagrelor Compared to Placebo on a Background of Aspirin–Thrombolysis in Myocardial Infarction 54 (PEGASUS-TIMI 54) trial, a randomized, double-blind, placebo-controlled clinical trial, tested long-term DAPT with ticagrelor, evaluating two different doses: 90 mg twice daily and 60 mg twice daily. The patients were considered eligible if they had a spontaneous MI 1 to 3 years before, were 50 years old or older, and had one of these additional high-risk features: age of 65 years or older, diabetes mellitus (DM), a second prior spontaneous MI, multivessel coronary artery disease, or chronic renal dysfunction. They were randomly assigned in a 1:1:1 ratio to receive either ticagrelor at a dose of 90 mg twice daily (b.i.d.), ticagrelor at a dose of 60 mg b.i.d., or a placebo. The median time from the occurrence of MI, of which 53.6% were STEMI, to the point of randomization was 1.7 years. Both doses of ticagrelor reduced the incidence of cardiovascular death, MI, or stroke but led to a higher risk of bleeding, including TIMI major bleeding, bleeding requiring a transfusion, and bleeding leading to discontinuation of the study drug. Ultimately, the overall risk to benefit trade-off was improved with the ticagrelor 60 mg b.i.d. dose, which led to the final approval of this drug dose with the new indication for extended DAPT [59][54]. Udell et al. conducted a meta-analysis of six RCTs on secondary prevention, including 33,435 patients with prior-MI randomized to extended DAPT beyond 1 year or standard DAPT for 12 months. A DAPT regimen longer than 12 months led to a 22% reduction in the relative risk and a 1.1% reduction in the absolute risk of MACE during an average follow-up period of 31 months. There was a slight 0.8% absolute increase in the risk of major bleeding, but this did not result in a significant excess of ICH or fatal bleeding, and there were no significant differences in non-cardiovascular deaths. Importantly, extended DAPT was associated with a significant reduction in CV mortality [60][55]. Taking this into consideration, extended DAPT appears to be an attractive approach in patients with prior MI and a low risk for bleeding. Whether the type of P2Y12i may be associated with a different impact on the overall long-term outcomes is not well established [61][56]. According to current ESC guidelines, a prolonged DAPT duration should be considered in patients with high ischemic risk and without HBR (Class IIa) and may be considered in patients with moderate ischemic risk without HBR (Class IIb) [34][41].2.3. Short (1 or 3 or 6-Months) DAPT
A short DAPT strategy of less than 12 months after implanting a coronary stent has been compared to one-year DAPT in several RCTs, generally testing non-inferiority for ischemic events and superiority for bleeding. The EXCELLENT trial (A Comparison of Xience/Promus Versus Cypher in Reducing Late Loss After Stenting) aimed to assess the effectiveness of a short-term DAPT strategy (6 months). A total of 1443 patients were randomized according to DAPT duration (6 vs. 12 months) and the type of stent (everolimus-eluting stent vs. sirolimus-eluting stent). One of the points of the study was to determine if the short-term DAPT strategy was not inferior to the standard care concerning the occurrence of cardiac death, MI, or ischemia-driven target vessel revascularization (TVR). The primary endpoint of the study was in-stent late loss at 9 months as the primary endpoint when comparing stent types, while the co-primary endpoint was the occurrence of target vessel failure (TVF) at 12 months, in relation to the duration of DAPT. The findings revealed that the six-month DAPT strategy was as effective as the standard care. Furthermore, while there was a numerical rise in TIMI minor and major bleeding events in the one-year DAPT group, this difference did not reach statistical significance (HR 0.40; 95% CI: 0.13–1.27; p = 0.12) [62][57]. The PRODIGY (PROlonging Dual antIplatelet treatment after Grading stent-induced intimal hYperplasia study) trial was a 4:2 randomized open-label clinical trial that evaluated the efficacy and the safety of prolonged DAPT with clopidogrel as P2Y12 inhibitor, randomly allocating patients (predominantly presenting with ACS) to four coronary stents (BMS, paclitaxel-eluting stent, E-ZES, everolimus-eluting stent) and two DAPT duration regimens (6 vs. 24 months of DAPT). The study showed no differences in the primary efficacy endpoint (death, MI, and stroke between 6 and 24 months of DAPT), and, as expected, among the patients receiving 24-month DAPT, there was a risk of type 2, 3 or bleeding events two-fold greater than in the group receiving 6-month DAPT (HR 2.17, 95% CI 1.44–3.22; p = 0.00018) [63][58]. Results remained largely consistent in multiple subgroups [64,65,66][59][60][61]. The REDUCE trial randomized 1496 ACS patients treated with COMBO stent to 3 or 12 months of DAPT. The composite primary study endpoint of all-cause mortality, MI, ST, stroke, target vessel revascularization and bleeding at 12 months was similar in the two groups, reaching prespecified non-inferiority (8.2% vs. 8.4%, p-value non-inferiority < 0.001). However, numerically higher rates of mortality and ST in the three-month DAPT group were observed [67][62]. The non-inferiority of 3 vs. 12 months DAPT was confirmed in several high-risk subgroups [68,69,70][63][64][65]. After 3–6 months of DAPT, in patients who are event-free and who are not at high ischemic risk, SAPT with a P2Y12 inhibitor or aspirin should be considered according to the current ESC guidelines (Class IIa) [34][41].2.4. Individualization of DAPT Duration Based on Ischemic and Bleeding Risk
The optimal DAPT strategy, that maximizes the efficacy and the safety of the treatment, balancing ischemic and bleeding risk, should be individual and selected based on patient and procedural characteristics (Figure 2) [71,72,73][66][67][68].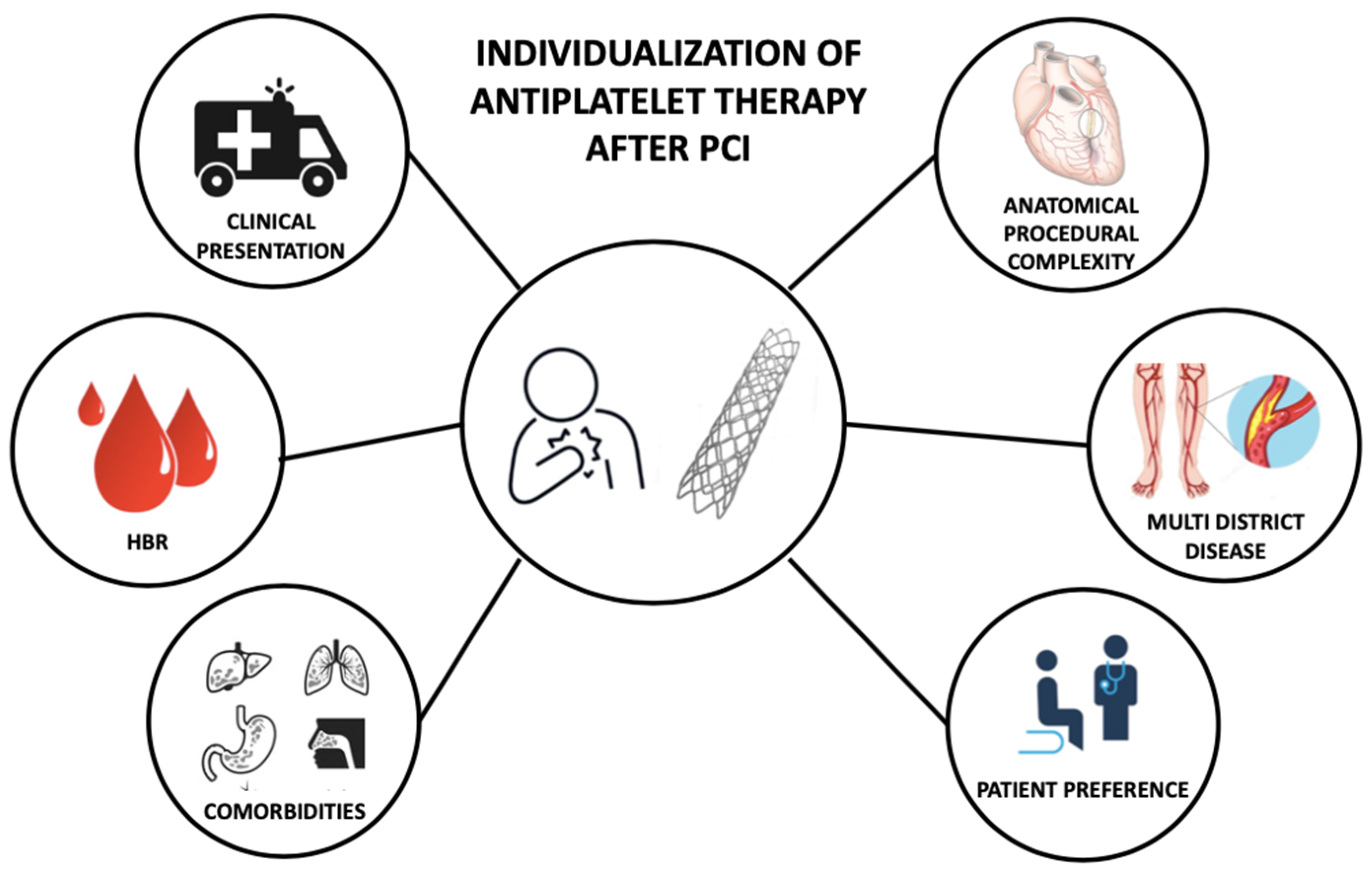
Figure 2. Features informing individualization of antiplatelet therapy after percutaneous coronary intervention. PCI—Percutaneous Coronary Intervention; HBR—High Bleeding Risk.
References
- Secco, G.G.; Ghione, M.; Mattesini, A.; Dall’Ara, G.; Ghilencea, L.; Kilickesmez, K.; De Luca, G.; Fattori, R.; Parisi, R.; Marino, P.N.; et al. Very High-Pressure Dilatation for Undilatable Coronary Lesions: Indications and Results with a New Dedicated Balloon. EuroIntervention 2016, 12, 359–365.
- Van Zandvoort, L.J.C.; Ali, Z.; Kern, M.; Van Mieghem, N.M.; Mintz, G.S.; Daemen, J. Improving PCI Outcomes Using Postprocedural Physiology and Intravascular Imaging. JACC Cardiovasc. Interv. 2021, 14, 2415–2430.
- Neumann, F.-J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.-P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on Myocardial Revascularization. Eur. Heart J. 2019, 40, 87–165.
- Shiomi, H.; Kozuma, K.; Morimoto, T.; Kadota, K.; Tanabe, K.; Morino, Y.; Tamura, T.; Abe, M.; Suwa, S.; Ito, Y.; et al. Ten-year Clinical Outcomes from a Randomized Trial Comparing New-generation Everolimus-eluting Stent versus First-generation Sirolimus-eluting Stent: Results from the RESET Extended Study. Catheter. Cardiovasc. Interv. 2023, 102, 594–607.
- De Luca, G.; Smits, P.; Hofma, S.H.; Di Lorenzo, E.; Vlachojannis, G.J.; Van’T Hof, A.W.J.; Van Boven, A.J.; Kedhi, E.; Stone, G.W.; Suryapranata, H. Everolimus Eluting Stent vs First Generation Drug-Eluting Stent in Primary Angioplasty: A Pooled Patient-Level Meta-Analysis of Randomized Trials. Int. J. Cardiol. 2017, 244, 121–127.
- De Luca, G.; Suryapranata, H.; Stone, G.W.; Antoniucci, D.; Tcheng, J.E.; Neumann, F.-J.; Bonizzoni, E.; Topol, E.J.; Chiariello, M. Relationship Between Patient’s Risk Profile and Benefits in Mortality from Adjunctive Abciximab to Mechanical Revascularization for ST-Segment Elevation Myocardial Infarction: A Meta-Regression Analysis of Randomized Trials. J. Am. Coll. Cardiol. 2006, 47, 685–686.
- Silverio, A.; Di Maio, M.; Citro, R.; Esposito, L.; Iuliano, G.; Bellino, M.; Baldi, C.; De Luca, G.; Ciccarelli, M.; Vecchione, C.; et al. Cardiovascular Risk Factors and Mortality in Hospitalized Patients with COVID-19: Systematic Review and Meta-Analysis of 45 Studies and 18,300 Patients. BMC Cardiovasc. Disord. 2021, 21, 23.
- Roth, G.A.; Forouzanfar, M.H.; Moran, A.E.; Barber, R.; Nguyen, G.; Feigin, V.L.; Naghavi, M.; Mensah, G.A.; Murray, C.J.L. Demographic and Epidemiologic Drivers of Global Cardiovascular Mortality. N. Engl. J. Med. 2015, 372, 1333–1341.
- De Luca, G.; Dirksen, M.T.; Spaulding, C.; Kelbæk, H.; Schalij, M.; Thuesen, L.; Van Der Hoeven, B.; Vink, M.A.; Kaiser, C.; Musto, C.; et al. Impact of Diabetes on Long-Term Outcome After Primary Angioplasty. Diabetes Care 2013, 36, 1020–1025.
- Serruys, P.W.; Revaiah, P.C.; Ninomiya, K.; Masuda, S.; Kotoku, N.; Kageyama, S.; Onuma, Y.; Morel, M.A.; Garg, S.; Feldman, T.; et al. 10 Years of SYNTAX. JACC Asia 2023, 3, 409–430.
- Sterling, L.H.; Fernando, S.M.; Talarico, R.; Qureshi, D.; Van Diepen, S.; Herridge, M.S.; Price, S.; Brodie, D.; Fan, E.; Di Santo, P.; et al. Long-Term Outcomes of Cardiogenic Shock Complicating Myocardial Infarction. J. Am. Coll. Cardiol. 2023, 82, 985–995.
- Makris, A.; Barkas, F.; Sfikakis, P.P.; Liberopoulos, E.; Filippatos, T.D.; Ray, K.K.; Agouridis, A.P. Lipoprotein(a), Interleukin-6 Inhibitors, and Atherosclerotic Cardiovascular Disease: Is There an Association? Atheroscler. Plus 2023, 54, 1–6.
- Rizzacasa, B.; Amati, F.; Romeo, F.; Novelli, G.; Mehta, J.L. Epigenetic Modification in Coronary Atherosclerosis. J. Am. Coll. Cardiol. 2019, 74, 1352–1365.
- Novara Atherosclerosis Study Group (NAS); De Luca, G.; Verdoia, M.; Cassetti, E.; Schaffer, A.; Cavallino, C.; Bolzani, V.; Marino, P. High Fibrinogen Level Is an Independent Predictor of Presence and Extent of Coronary Artery Disease among Italian Population. J. Thromb. Thrombolysis 2011, 31, 458–463.
- Verdoia, M.; Schaffer, A.; Barbieri, L.; Aimaretti, G.; Marino, P.; Sinigaglia, F.; Suryapranata, H.; De Luca, G. Impact of Diabetes on Neutrophil-to-Lymphocyte Ratio and Its Relationship to Coronary Artery Disease. Diabetes Metab. 2015, 41, 304–311.
- Mehta, S.R.; Yusuf, S.; Peters, R.J.; Bertrand, M.E.; Lewis, B.S.; Natarajan, M.K.; Malmberg, K.; Rupprecht, H.-J.; Zhao, F.; Chrolavicius, S.; et al. Effects of Pretreatment with Clopidogrel and Aspirin Followed by Long-Term Therapy in Patients Undergoing Percutaneous Coronary Intervention: The PCI-CURE Study. Lancet 2001, 358, 527–533.
- Castellano, J.M.; Pocock, S.J.; Bhatt, D.L.; Quesada, A.J.; Owen, R.; Fernandez-Ortiz, A.; Sanchez, P.L.; Marin Ortuño, F.; Vazquez Rodriguez, J.M.; Domingo-Fernández, A.; et al. Polypill Strategy in Secondary Cardiovascular Prevention. N. Engl. J. Med. 2022, 387, 967–977.
- Bhatt, D.L.; Steg, P.G.; Miller, M.; Brinton, E.A.; Jacobson, T.A.; Ketchum, S.B.; Doyle, R.T.; Juliano, R.A.; Jiao, L.; Granowitz, C.; et al. Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia. N. Engl. J. Med. 2019, 380, 11–22.
- Wiviott, S.D.; Raz, I.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Silverman, M.G.; Zelniker, T.A.; Kuder, J.F.; Murphy, S.A.; et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2019, 380, 347–357.
- Patrono, C.; García Rodríguez, L.A.; Landolfi, R.; Baigent, C. Low-Dose Aspirin for the Prevention of Atherothrombosis. N. Engl. J. Med. 2005, 353, 2373–2383.
- Fontana, P.; Dupont, A.; Gandrille, S.; Bachelot-Loza, C.; Reny, J.-L.; Aiach, M.; Gaussem, P. Adenosine Diphosphate–Induced Platelet Aggregation Is Associated with P2Y 12 Gene Sequence Variations in Healthy Subjects. Circulation 2003, 108, 989–995.
- Giustino, G.; Mehran, R.; Dangas, G.D.; Kirtane, A.J.; Redfors, B.; Généreux, P.; Brener, S.J.; Prats, J.; Pocock, S.J.; Deliargyris, E.N.; et al. Characterization of the Average Daily Ischemic and Bleeding Risk After Primary PCI for STEMI. J. Am. Coll. Cardiol. 2017, 70, 1846–1857.
- Patrono, C.; Morais, J.; Baigent, C.; Collet, J.-P.; Fitzgerald, D.; Halvorsen, S.; Rocca, B.; Siegbahn, A.; Storey, R.F.; Vilahur, G. Antiplatelet Agents for the Treatment and Prevention of Coronary Atherothrombosis. J. Am. Coll. Cardiol. 2017, 70, 1760–1776.
- Valgimigli, M.; Costa, F.; Lokhnygina, Y.; Clare, R.M.; Wallentin, L.; Moliterno, D.J.; Armstrong, P.W.; White, H.D.; Held, C.; Aylward, P.E.; et al. Trade-off of Myocardial Infarction vs. Bleeding Types on Mortality after Acute Coronary Syndrome: Lessons from the Thrombin Receptor Antagonist for Clinical Event Reduction in Acute Coronary Syndrome (TRACER) Randomized Trial. Eur. Heart J. 2016, 38, 804–810.
- Collet, J.-P.; Roffi, M.; Byrne, R.A.; Costa, F.; Valgimigli, M.; Task Force for the Management of Dual Antiplatelet Therapy in Coronary Artery Disease of the European Society of Cardiology (ESC); Valgimigli, M.; Bueno, H.; Byrne, R.A.; Collet, J.-P.; et al. Case-Based Implementation of the 2017 ESC Focused Update on Dual Antiplatelet Therapy in Coronary Artery Disease. Eur. Heart J. 2018, 39, e1–e33.
- Valgimigli, M.; Costa, F.; Byrne, R.; Haude, M.; Baumbach, A.; Windecker, S. Dual Antiplatelet Therapy Duration after Coronary Stenting in Clinical Practice: Results of an EAPCI Survey. EuroIntervention 2015, 11, 68–74.
- Costa, F.; Windecker, S.; Valgimigli, M. Dual Antiplatelet Therapy Duration: Reconciling the Inconsistencies. Drugs 2017, 77, 1733–1754.
- Costa, F.; Valgimigli, M. The Optimal Duration of Dual Antiplatelet Therapy after Coronary Stent Implantation: To Go Too Far Is as Bad as to Fall Short. Cardiovasc. Diagn. Ther. 2018, 8, 630–646.
- Capodanno, D.; Mehran, R.; Krucoff, M.W.; Baber, U.; Bhatt, D.L.; Capranzano, P.; Collet, J.-P.; Cuisset, T.; De Luca, G.; De Luca, L.; et al. Defining Strategies of Modulation of Antiplatelet Therapy in Patients with Coronary Artery Disease: A Consensus Document from the Academic Research Consortium. Circulation 2023, 147, 1933–1944.
- Kikkert, W.J.; Damman, P. Optimal Duration of Dual Antiplatelet Therapy for Coronary Artery Disease. Neth. Heart J. 2018, 26, 321–333.
- De Luca, G.; Dirksen, M.T.; Spaulding, C.; Kelbæk, H.; Schalij, M.; Thuesen, L.; Van Der Hoeven, B.; Vink, M.A.; Kaiser, C.; Musto, C.; et al. Drug-Eluting vs Bare-Metal Stents in Primary Angioplasty: A Pooled Patient-Level Meta-Analysis of Randomized Trials. Arch. Intern. Med. 2012, 172, 611–621.
- McFadden, E.P.; Stabile, E.; Regar, E.; Cheneau, E.; Ong, A.T.; Kinnaird, T.; Suddath, W.O.; Weissman, N.J.; Torguson, R.; Kent, K.M.; et al. Late Thrombosis in Drug-Eluting Coronary Stents after Discontinuation of Antiplatelet Therapy. Lancet 2004, 364, 1519–1521.
- Joner, M.; Finn, A.V.; Farb, A.; Mont, E.K.; Kolodgie, F.D.; Ladich, E.; Kutys, R.; Skorija, K.; Gold, H.K.; Virmani, R. Pathology of Drug-Eluting Stents in Humans. J. Am. Coll. Cardiol. 2006, 48, 193–202.
- Camenzind, E.; Steg, P.G.; Wijns, W. A Cause for Concern. Circulation 2007, 115, 1440–1455.
- Kolh, P.; Wijns, W.; Danchin, N.; Di Mario, C.; Falk, V.; Folliguet, T.; Garg, S.; Huber, K.; James, S.; Knuuti, J. Guidelines on Myocardial Revascularization. Eur. J. Cardiothorac. Surg. 2010, 38, S1–S52.
- Valgimigli, M.; Sabate, M.; Kaiser, C.; Brugaletta, S.; De La Torre Hernandez, J.M.; Galatius, S.; Cequier, A.; Eberli, F.; De Belder, A.; Serruys, P.W.; et al. Effects of Cobalt-Chromium Everolimus Eluting Stents or Bare Metal Stent on Fatal and Non-Fatal Cardiovascular Events: Patient Level Meta-Analysis. BMJ 2014, 349, g6427.
- Wijns, W.; Steg, P.G.; Mauri, L.; Kurowski, V.; Parikh, K.; Gao, R.; Bode, C.; Greenwood, J.P.; Lipsic, E.; Alamgir, F.; et al. Endeavour Zotarolimus-Eluting Stent Reduces Stent Thrombosis and Improves Clinical Outcomes Compared with Cypher Sirolimus-Eluting Stent: 4-Year Results of the PROTECT Randomized Trial. Eur. Heart J. 2014, 35, 2812–2820.
- Capodanno, D.; Bhatt, D.L.; Gibson, C.M.; James, S.; Kimura, T.; Mehran, R.; Rao, S.V.; Steg, P.G.; Urban, P.; Valgimigli, M.; et al. Bleeding Avoidance Strategies in Percutaneous Coronary Intervention. Nat. Rev. Cardiol. 2022, 19, 117–132.
- Benenati, S.; Galli, M.; De Marzo, V.; Pescetelli, F.; Toma, M.; Andreotti, F.; Bona, R.D.; Canepa, M.; Ameri, P.; Crea, F.; et al. Very Short vs. Long Dual Antiplatelet Therapy after Second Generation Drug-Eluting Stents in 35 785 Patients Undergoing Percutaneous Coronary Interventions: A Meta-Analysis of Randomized Controlled Trials. Eur. Heart J.-Cardiovasc. Pharmacother. 2021, 7, 86–93.
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the Diagnosis and Management of Chronic Coronary Syndromes. Eur. Heart J. 2020, 41, 407–477.
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.-A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the Management of Acute Coronary Syndromes. Eur. Heart J. 2023, 44, 3720–3826.
- Mauri, L.; Hsieh, W.; Massaro, J.M.; Ho, K.K.L.; D’Agostino, R.; Cutlip, D.E. Stent Thrombosis in Randomized Clinical Trials of Drug-Eluting Stents. N. Engl. J. Med. 2007, 356, 1020–1029.
- Cutlip, D.E.; Chhabra, A.G.; Baim, D.S.; Chauhan, M.S.; Marulkar, S.; Massaro, J.; Bakhai, A.; Cohen, D.J.; Kuntz, R.E.; Ho, K.K.L. Beyond Restenosis: Five-Year Clinical Outcomes from Second-Generation Coronary Stent Trials. Circulation 2004, 110, 1226–1230.
- Stone, G.W.; Maehara, A.; Lansky, A.J.; De Bruyne, B.; Cristea, E.; Mintz, G.S.; Mehran, R.; McPherson, J.; Farhat, N.; Marso, S.P.; et al. A Prospective Natural-History Study of Coronary Atherosclerosis. N. Engl. J. Med. 2011, 364, 226–235.
- Trip, M.D.; Cats, V.M.; Van Capelle, F.J.L.; Vreeken, J. Platelet Hyperreactivity and Prognosis in Survivors of Myocardial Infarction. N. Engl. J. Med. 1990, 322, 1549–1554.
- Gawaz, M.; Neumann, F.-J.; Ott, I.; Schiessler, A.; Schömig, A. Platelet Function in Acute Myocardial Infarction Treated with Direct Angioplasty. Circulation 1996, 93, 229–237.
- Brandt, J.T.; Payne, C.D.; Wiviott, S.D.; Weerakkody, G.; Farid, N.A.; Small, D.S.; Jakubowski, J.A.; Naganuma, H.; Winters, K.J. A Comparison of Prasugrel and Clopidogrel Loading Doses on Platelet Function: Magnitude of Platelet Inhibition Is Related to Active Metabolite Formation. Am. Heart J. 2007, 153, 66.e9–66.e16.
- Alnasser, S.M.A.; Huang, W.; Gore, J.M.; Steg, P.G.; Eagle, K.A.; Anderson, F.A.; Fox, K.A.A.; Gurfinkel, E.; Brieger, D.; Klein, W.; et al. Late Consequences of Acute Coronary Syndromes: Global Registry of Acute Coronary Events (GRACE) Follow-Up. Am. J. Med. 2015, 128, 766–775.
- Jernberg, T.; Hasvold, P.; Henriksson, M.; Hjelm, H.; Thuresson, M.; Janzon, M. Cardiovascular Risk in Post-Myocardial Infarction Patients: Nationwide Real World Data Demonstrate the Importance of a Long-Term Perspective. Eur. Heart J. 2015, 36, 1163–1170.
- Crimi, G.; Leonardi, S.; Costa, F.; Adamo, M.; Ariotti, S.; Valgimigli, M. Role of Stent Type and of Duration of Dual Antiplatelet Therapy in Patients with Chronic Kidney Disease Undergoing Percutaneous Coronary Interventions. Is Bare Metal Stent Implantation Still a Justifiable Choice? A Post-Hoc Analysis of the All Comer PRODIGY Trial. Int. J. Cardiol. 2016, 212, 110–117.
- Mauri, L.; Kereiakes, D.J.; Yeh, R.W.; Driscoll-Shempp, P.; Cutlip, D.E.; Steg, P.G.; Normand, S.-L.T.; Braunwald, E.; Wiviott, S.D.; Cohen, D.J.; et al. Twelve or 30 Months of Dual Antiplatelet Therapy after Drug-Eluting Stents. N. Engl. J. Med. 2014, 371, 2155–2166.
- Yeh, R.W.; Kereiakes, D.J.; Steg, P.G.; Windecker, S.; Rinaldi, M.J.; Gershlick, A.H.; Cutlip, D.E.; Cohen, D.J.; Tanguay, J.-F.; Jacobs, A.; et al. Benefits and Risks of Extended Duration Dual Antiplatelet Therapy After PCI in Patients with and without Acute Myocardial Infarction. J. Am. Coll. Cardiol. 2015, 65, 2211–2221.
- Costa, F.; Valgimigli, M. Impact of Clinical Presentation on Dual Antiplatelet Therapy Duration. J. Am. Coll. Cardiol. 2015, 66, 1203–1204.
- Bonaca, M.P.; Bhatt, D.L.; Cohen, M.; Steg, P.G.; Storey, R.F.; Jensen, E.C.; Magnani, G.; Bansilal, S.; Fish, M.P.; Im, K.; et al. Long-Term Use of Ticagrelor in Patients with Prior Myocardial Infarction. N. Engl. J. Med. 2015, 372, 1791–1800.
- Udell, J.A.; Bonaca, M.P.; Collet, J.-P.; Lincoff, A.M.; Kereiakes, D.J.; Costa, F.; Lee, C.W.; Mauri, L.; Valgimigli, M.; Park, S.-J.; et al. Long-Term Dual Antiplatelet Therapy for Secondary Prevention of Cardiovascular Events in the Subgroup of Patients with Previous Myocardial Infarction: A Collaborative Meta-Analysis of Randomized Trials. Eur. Heart J. 2015, 37, 390–399.
- Costa, F.; Adamo, M.; Ariotti, S.; Navarese, E.P.; Biondi-Zoccai, G.; Valgimigli, M. Impact of Greater than 12-Month Dual Antiplatelet Therapy Duration on Mortality: Drug-Specific or a Class-Effect? A Meta-Analysis. Int. J. Cardiol. 2015, 201, 179–181.
- Park, K.W.; Yoon, J.-H.; Kim, J.-S.; Hahn, J.-Y.; Cho, Y.-S.; Chae, I.-H.; Gwon, H.-C.; Ahn, T.; Oh, B.-H.; Park, J.-E.; et al. Efficacy of Xience/Promus versus Cypher in rEducing Late Loss after stENTing (EXCELLENT) Trial: Study Design and Rationale of a Korean Multicenter Prospective Randomized Trial. Am. Heart J. 2009, 157, 811–817.e1.
- Valgimigli, M.; Campo, G.; Monti, M.; Vranckx, P.; Percoco, G.; Tumscitz, C.; Castriota, F.; Colombo, F.; Tebaldi, M.; Fucà, G.; et al. Short- Versus Long-Term Duration of Dual-Antiplatelet Therapy After Coronary Stenting: A Randomized Multicenter Trial. Circulation 2012, 125, 2015–2026.
- Gargiulo, G.; Costa, F.; Ariotti, S.; Biscaglia, S.; Campo, G.; Esposito, G.; Leonardi, S.; Vranckx, P.; Windecker, S.; Valgimigli, M. Impact of Proton Pump Inhibitors on Clinical Outcomes in Patients Treated with a 6- or 24-Month Dual-Antiplatelet Therapy Duration: Insights from the PROlonging Dual-Antiplatelet Treatment after Grading Stent-Induced Intimal Hyperplasia study Trial. Am. Heart J. 2016, 174, 95–102.
- Adamo, M.; Costa, F.; Vranckx, P.; Leonardi, S.; Navarese, E.P.; Garcia-Garcia, H.M.; Valgimigli, M. Does Smoking Habit Affect the Randomized Comparison of 6 versus 24-Month Dual Antiplatelet Therapy Duration? Insights from the PRODIGY Trial. Int. J. Cardiol. 2015, 190, 242–245.
- Costa, F.; Vranckx, P.; Leonardi, S.; Moscarella, E.; Ando, G.; Calabro, P.; Oreto, G.; Zijlstra, F.; Valgimigli, M. Impact of Clinical Presentation on Ischaemic and Bleeding Outcomes in Patients Receiving 6- or 24-Month Duration of Dual-Antiplatelet Therapy after Stent Implantation: A Pre-Specified Analysis from the PRODIGY (Prolonging Dual-Antiplatelet Treatment after Grading Stent-Induced Intimal Hyperplasia) Trial. Eur. Heart J. 2015, 36, 1242–1251.
- De Luca, G.; Damen, S.A.; Camaro, C.; Benit, E.; Verdoia, M.; Rasoul, S.; Liew, H.B.; Polad, J.; Ahmad, W.A.; Zambahari, R.; et al. Final Results of the Randomised Evaluation of Short-Term Dual Antiplatelet Therapy in Patients with Acute Coronary Syndrome Treated with a New-Generation Stent (REDUCE Trial). EuroIntervention 2019, 15, e990–e998.
- Vranken, N.P.A.; Rasoul, S.; Luijkx, J.J.P.; Pustjens, T.F.S.; Postma, S.; Kolkman, E.J.; Kedhi, E.; Rifqi, S.; Lee, M.K.Y.; Ebelt, H.; et al. Short-term Dual Antiplatelet Therapy in Diabetic Patients Admitted for Acute Coronary Syndrome Treated with a New-generation Drug-eluting Stent. Diabetes Metab. Res. Rev. 2022, 38, e3530.
- Verdoia, M.; Suryapranata, H.; Damen, S.; Camaro, C.; Benit, E.; Barbieri, L.; Rasoul, S.; Liew, H.B.; Polad, J.; Ahmad, W.A.W.; et al. Gender Differences with Short-Term vs 12 Months Dual Antiplatelet Therapy in Patients with Acute Coronary Syndrome Treated with the COMBO Dual Therapy Stent: 2-Years Follow-up Results of the REDUCE Trial. J. Thromb. Thrombolysis 2021, 52, 797–807.
- Kedhi, E.; Verdoia, M.; Suryapranata, H.; Damen, S.; Camaro, C.; Benit, E.; Barbieri, L.; Rasoul, S.; Liew, H.B.; Polad, J.; et al. Impact of Age on the Comparison between Short-Term vs 12-Month Dual Antiplatelet Therapy in Patients with Acute Coronary Syndrome Treated with the COMBO Dual Therapy Stent: 2-Year Follow-up Results of the REDUCE Trial. Atherosclerosis 2021, 321, 39–44.
- Valgimigli, M.; Ariotti, S.; Costa, F. Duration of Dual Antiplatelet Therapy after Drug-Eluting Stent Implantation: Will We Ever Reach a Consensus? Eur. Heart J. 2015, 36, 1219–1222.
- Morici, N.; De Servi, S.; De Luca, L.; Crimi, G.; Montalto, C.; De Rosa, R.; De Luca, G.; Rubboli, A.; Valgimigli, M.; Savonitto, S. Management of Acute Coronary Syndromes in Older Adults. Eur. Heart J. 2022, 43, 1542–1553.
- Montalto, C.; Crimi, G.; Morici, N.; Piatti, L.; Grosseto, D.; Sganzerla, P.; Tortorella, G.; De Rosa, R.; De Luca, L.; De Luca, G.; et al. Bleeding Risk Prediction in Elderly Patients Managed Invasively for Acute Coronary Syndromes: External Validation of the PRECISE-DAPT and PARIS Scores. Int. J. Cardiol. 2021, 328, 22–28.
- Campos, C.M.; Costa, F.; Garcia-Garcia, H.M.; Bourantas, C.; Suwannasom, P.; Valgimigli, M.; Morel, M.-A.; Windecker, S.; Serruys, P.W. Anatomic Characteristics and Clinical Implications of Angiographic Coronary Thrombus: Insights from a Patient-Level Pooled Analysis of SYNTAX, RESOLUTE, and LEADERS Trials. Circ. Cardiovasc. Interv. 2015, 8, e002279.
- McAllister, K.S.L.; Ludman, P.F.; Hulme, W.; De Belder, M.A.; Stables, R.; Chowdhary, S.; Mamas, M.A.; Sperrin, M.; Buchan, I.E. A Contemporary Risk Model for Predicting 30-Day Mortality Following Percutaneous Coronary Intervention in England and Wales. Int. J. Cardiol. 2016, 210, 125–132.
- Zimarino, M.; Angiolillo, D.J.; Dangas, G.; Capodanno, D.; Barbato, E.; Hahn, J.-Y.; Giustino, G.; Watanabe, H.; Costa, F.; Cuisset, T.; et al. Antithrombotic Therapy after Percutaneous Coronary Intervention of Bifurcation Lesions. EuroIntervention 2021, 17, 59–66.
- Giustino, G.; Costa, F. Characterization of the Individual Patient Risk After Percutaneous Coronary Intervention. JACC Cardiovasc. Interv. 2019, 12, 831–834.
- Caracciolo, A.; Mazzone, P.; Laterra, G.; Garcia-Ruiz, V.; Polimeni, A.; Galasso, S.; Saporito, F.; Carerj, S.; D’Ascenzo, F.; Marquis-Gravel, G.; et al. Antithrombotic Therapy for Percutaneous Cardiovascular Interventions: From Coronary Artery Disease to Structural Heart Interventions. J. Clin. Med. 2019, 8, 2016.
- Costa, F.; Adamo, M.; Ariotti, S.; Ferrante, G.; Navarese, E.P.; Leonardi, S.; Garcia-Garcia, H.; Vranckx, P.; Valgimigli, M. Left Main or Proximal Left Anterior Descending Coronary Artery Disease Location Identifies High-Risk Patients Deriving Potentially Greater Benefit from Prolonged Dual Antiplatelet Therapy Duration. EuroIntervention 2016, 11, e1222–e1230.
- Giustino, G.; Chieffo, A.; Palmerini, T.; Valgimigli, M.; Feres, F.; Abizaid, A.; Costa, R.A.; Hong, M.-K.; Kim, B.-K.; Jang, Y.; et al. Efficacy and Safety of Dual Antiplatelet Therapy After Complex PCI. J. Am. Coll. Cardiol. 2016, 68, 1851–1864.
- Costa, F.; Van Klaveren, D.; Feres, F.; James, S.; Räber, L.; Pilgrim, T.; Hong, M.-K.; Kim, H.-S.; Colombo, A.; Steg, P.G.; et al. Dual Antiplatelet Therapy Duration Based on Ischemic and Bleeding Risks After Coronary Stenting. J. Am. Coll. Cardiol. 2019, 73, 741–754.
- Andreotti, F.; Rocca, B.; Husted, S.; Ajjan, R.A.; Ten Berg, J.; Cattaneo, M.; Collet, J.-P.; De Caterina, R.; Fox, K.A.A.; Halvorsen, S.; et al. Antithrombotic Therapy in the Elderly: Expert Position Paper of the European Society of Cardiology Working Group on Thrombosis. Eur. Heart J. 2015, 36, 3238–3249.
- Andreotti, F.; Geisler, T.; Collet, J.-P.; Gigante, B.; Gorog, D.A.; Halvorsen, S.; Lip, G.Y.H.; Morais, J.; Navarese, E.P.; Patrono, C.; et al. Acute, Periprocedural and Longterm Antithrombotic Therapy in Older Adults. Eur. Heart J. 2023, 44, 262–279.
- De Servi, S.; Landi, A.; Savonitto, S.; Morici, N.; De Luca, L.; Montalto, C.; Crimi, G.; De Rosa, R.; De Luca, G. Antiplatelet Strategies for Older Patients with Acute Coronary Syndromes: Finding Directions in a Low-Evidence Field. J. Clin. Med. 2023, 12, 2082.
- Valgimigli, M.; Bueno, H.; Byrne, R.A.; Collet, J.-P.; Costa, F.; Jeppsson, A.; Jüni, P.; Kastrati, A.; Kolh, P.; Mauri, L.; et al. 2017 ESC Focused Update on Dual Antiplatelet Therapy in Coronary Artery Disease Developed in Collaboration with EACTS. Eur. Heart J. 2018, 39, 213–260.
- Wiviott, S.D.; Braunwald, E.; McCabe, C.H.; Montalescot, G.; Ruzyllo, W.; Gottlieb, S.; Neumann, F.-J.; Ardissino, D.; De Servi, S.; Murphy, S.A.; et al. Prasugrel versus Clopidogrel in Patients with Acute Coronary Syndromes. N. Engl. J. Med. 2007, 357, 2001–2015.
- Wallentin, L.; Becker, R.C.; Budaj, A.; Cannon, C.P.; Emanuelsson, H.; Held, C.; Horrow, J.; Husted, S.; James, S.; Katus, H.; et al. Ticagrelor versus Clopidogrel in Patients with Acute Coronary Syndromes. N. Engl. J. Med. 2009, 361, 1045–1057.
- Meredith, I.T.; Tanguay, J.-F.; Kereiakes, D.J.; Cutlip, D.E.; Yeh, R.W.; Garratt, K.N.; Lee, D.P.; Steg, P.G.; Weaver, W.D.; Holmes, D.R.; et al. Diabetes Mellitus and Prevention of Late Myocardial Infarction After Coronary Stenting in the Randomized Dual Antiplatelet Therapy Study. Circulation 2016, 133, 1772–1782.
- Bhatt, D.L.; Bonaca, M.P.; Bansilal, S.; Angiolillo, D.J.; Cohen, M.; Storey, R.F.; Im, K.; Murphy, S.A.; Held, P.; Braunwald, E.; et al. Reduction in Ischemic Events with Ticagrelor in Diabetic Patients With Prior Myocardial Infarction in PEGASUS–TIMI 54. J. Am. Coll. Cardiol. 2016, 67, 2732–2740.
- Bhatt, D.L.; Fox, K.A.A.; Hacke, W.; Berger, P.B.; Black, H.R.; Boden, W.E.; Cacoub, P.; Cohen, E.A.; Creager, M.A.; Easton, J.D.; et al. Clopidogrel and Aspirin versus Aspirin Alone for the Prevention of Atherothrombotic Events. N. Engl. J. Med. 2006, 354, 1706–1717.
- Bonaca, M.P.; Bhatt, D.L.; Storey, R.F.; Steg, P.G.; Cohen, M.; Kuder, J.; Goodrich, E.; Nicolau, J.C.; Parkhomenko, A.; López-Sendón, J.; et al. Ticagrelor for Prevention of Ischemic Events After Myocardial Infarction in Patients with Peripheral Artery Disease. J. Am. Coll. Cardiol. 2016, 67, 2719–2728.
- Franzone, A.; Piccolo, R.; Gargiulo, G.; Ariotti, S.; Marino, M.; Santucci, A.; Baldo, A.; Magnani, G.; Moschovitis, A.; Windecker, S.; et al. Prolonged vs Short Duration of Dual Antiplatelet Therapy after Percutaneous Coronary Intervention in Patients with or Without Peripheral Arterial Disease: A Subgroup Analysis of the PRODIGY Randomized Clinical Trial. JAMA Cardiol. 2016, 1, 795.
More
