Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Gennadii Aleksandrovic Naberezhnii and Version 2 by Fanny Huang.
Sepsis is a life-threatening complication of an infectious process that results from the excessive and uncontrolled activation of the host’s pro-inflammatory immune response to a pathogen. Lipopolysaccharide (LPS), also known as endotoxin, which is a major component of Gram-negative bacteria’s outer membrane, plays a key role in the development of Gram-negative sepsis and septic shock in humans. To date, no specific and effective drug against sepsis has been developed.
- lipopolysaccharide (LPS, endotoxin)
- innate immune system
- marine invertebrates
1. Introduction
Lipopolysaccharide (LPS, endotoxin), the major structural component of the Gram-negative bacteria’s outer membrane, serves as a physical barrier that protects bacteria from the external environment and can be released into the surrounding medium during cell division or death [1]. These molecules are the potent stimulators of the innate immune system, playing an important role in the pathogenesis of Gram-negative infections in animals. When it enters the body of a warm-blooded host, LPS binds and activates the cellular Toll-like receptor 4 (TLR4), which leads to the development of an inflammatory reaction and, ultimately, as a result, to the death and elimination of the pathogen. However, the accumulation of endotoxin in the bloodstream in large quantities (with Gram-negative infection, invasive procedures, etc.) can cause the excessive activation of immunocompetent cells, inducing the overproduction of pro-inflammatory cytokines and a systemic inflammatory response, which leads to sepsis and septic shock within a few days (Figure 1). Lipid A, the predominantly lipophilic and most conserved fragment of the LPS molecule, directly interacts with TLR4 and is responsible for most of the immunobiological and toxic properties of endotoxin [1].
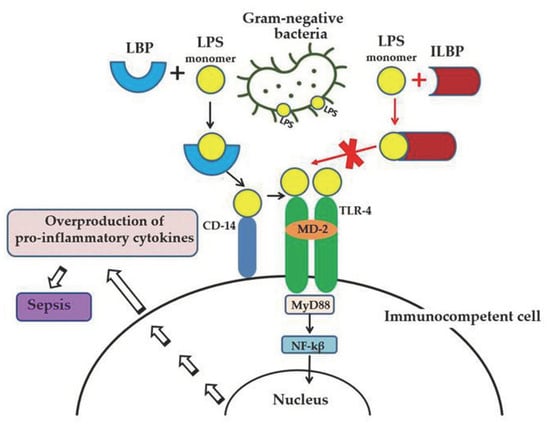
Figure 1. LPS-induced inflammatory response of the innate immune system and the anti-inflammatory effect of LPS-binding peptides/proteins from marine invertebrates. Serum protein LBP (LPS-binding protein) binds the monomer of LPS and delivers it to a CD14 molecule. CD14 transfers LPS to the ectodomain of the TLR4/MD-2 receptor complex, which leads to homodimerization of TLR4. This change in TLR4 conformation provides a binding site for adaptor molecule MyD88 (myeloid differentiation primary-response protein 88). The MyD88-dependent signaling pathway leads to the activation of nuclear factor-κβ (NF-κβ), which regulates the expression of target genes encoding pro-inflammatory mediators. The overproduction of pro-inflammatory cytokines may lead to an uncontrolled inflammatory reaction and eventually to sepsis. The binding of ILBP to LPS blocks CD14–LPS interaction and prevents the transfer of LPS to the TLR4/MD-2 complex, thus interfering with TLR4 dimerization and downstream inflammatory responses.
Gram-negative sepsis remains a serious unresolved problem in clinical medicine. This type (according to the etiology of pathogens) of sepsis is clinically the most severe, often accompanied by septic shock, and has a significantly higher mortality rate than other types. The increasing use of new technologies in medical practice—cytostatic and immunosuppressive therapy and transplantation and prosthetics—as well as the HIV and COVID-19 pandemics and the increasing resistance of pathogens to antibiotics contribute to the growth of septic complications in patients. The aging population, accompanied by an increase in chronic diseases, also leads to an increase in the incidence of sepsis and septic shock. As a result, despite the great advances in antimicrobial chemotherapy, mortality rates from septic shock remain the highest and do not decrease worldwide. The development and introduction of new antibiotics into medical practice does not fundamentally solve the problem; moreover, their use in some cases can lead to a deterioration in the condition of a septic patient.
Modern medicine does not have specific and effective anti-sepsis drugs whose molecular target is LPS. Molecules that can selectively block TLR4, preventing endotoxin from binding to receptor and the development of a systemic inflammatory response, may have therapeutic potential for the treatment of sepsis. In particular, TLR-4 receptor antagonists are structural analogs of lipid A with low toxicity, including native lipid A from a number of marine proteobacteria (Proteobacteria) [2][3][2,3]. Another approach to the endotoxin-neutralizing drug design is based on the use of synthetic or natural substances that can suppress the biological activity of LPS due to the formation of strong complexes with it (Figure 1) [4]. Searching for such compounds among the host defense proteins of marine invertebrates—which represent one of the most extensive and diverse groups of animals, numbering 153,434 species—seems very promising (UN data for 2019).
Marine invertebrates only have an innate immune system, including a huge set of defense proteins, which was formed during a long evolution and allowed them to survive in the environment enriched with pathogenic microorganisms [5]. Host defense proteins are traditionally referred to as antimicrobial peptides, although they have been shown to be polyfunctional compounds. These proteins are constitutively expressed and rapidly induced in various cells and tissues, interact directly with infectious agents, and/or activate immune responses to eliminate pathogens. Defense proteins usually recognize and bind to the surface of the pathogen the most conservative biopolymers that are common and vital for a large group of microorganisms but not present in the host. These molecules, known as pathogen-associated molecular patterns (PAMPs), trigger innate immune responses in the host [6]. In Gram-negative bacteria, this PAMP is LPS.
Host defense proteins and peptides with lipopolysaccharide-binding capacity from marine invertebrates (ILBPs, invertebrate lipopolysaccharide-binding proteins) may possess different biological properties depending on the structure and nature of the interaction with endotoxins. With a high affinity for LPS, these proteins may have antimicrobial or endotoxin-neutralizing activity, or both [4]. ILBPs that combine both of these properties are considered today as the most effective potential drugs for the treatment of human sepsis.
2. Anti-Lipopolysaccharide Factor (ALF)
A cationic protein that inhibited the LPS-induced activation of the crab hemolymph coagulation system has been found in hemocyte lysates of Japanese (Tachypleus tridentatus) and American (Limulus polyphemus) horseshoe crabs [7][8][9][7,8,9]. This protein, called the anti-lipopolysaccharide factor (ALF), was able to bind LPS, neutralize its biological activity (in vitro and in vivo), and inhibit the growth of R-type Gram-negative bacteria. The natural and recombinant anti-LPS factor Limulus (ALF-L) also suppressed endotoxin-mediated activation of cultured endothelial and B cells, reduced the concentration of endotoxin and TNF-α in the blood serum of experimental animals, and protected them from death in the late stages of endotoxemia and sepsis [10][11][12][13][14][10,11,12,13,14].
Numerous ALF homologues have been identified and characterized in different types of crustaceans: shrimp, lobster, crabs, and crayfish [15][16][17][18][19][20][21][22][15,16,17,18,19,20,21,22]. The most extensively studied ALFs are isolated from shrimp of the Penaeidae family, which includes many economically important species that are of interest as objects of industrial fishing and breeding [23].
ALFs show a broad spectrum of antimicrobial activity against Gram-negative and Gram-positive bacteria, fungi, human enveloped viruses (herpesvirus type 1, adenovirus), and white spot syndrome virus (WSSV), which is widely distributed throughout the world and considered as one of the most destructive and pathogenic viruses in shrimp farms [24][25][26][24,25,26].
In crustaceans, one organism usually contains several isoforms of ALF, which are either encoded by different genes or formed as a result of alternative mRNA splicing [27]. Thus, six isoforms were identified in the tiger shrimp Penaeus monodon, and seven isoforms were found in the Chinese shrimp Fenneropenaeus chinensis and the South Korean blue crab Portunus trituberculatus [15][28][29][30][31][32][15,28,29,30,31,32]. The isoforms differed in tissue distribution and antimicrobial properties. The wide diversity of ALF sequences within a species may provide a synergistic enhancement of their protective action against bacterial infection.
ALFs are a group of small single-domain antimicrobial proteins consisting of 97–124 amino acid residues with a relatively short 16–28 residue signal sequence. The molecular weight of the mature protein is about 11 kDa: ALFs from L. polyphemus and shrimp of various species have masses of 11.8 and 10.74 to 12.23 kDa, respectively [9][33][9,33]. According to the values of the isoelectric points (pI), ALFs were classified as cationic peptides, but more and more data are emerging on the existence of anionic proteins among them [33][34][35][33,34,35]. The theoretical pI values of mature shrimp ALFs range from 5.02 to 10.29 [33]. Typically, ALF molecules have a highly hydrophobic N-terminal region and conserved cluster of positively charged and hydrophobic amino acid residues within a loop fixed by a disulfide bond between two conserved cysteine residues, which is commonly referred to as the LPS-binding domain [36]. This amphipathic loop is an important functional molecule moiety, which is responsible for the biological activity of ALFs. Indeed, synthetic peptides corresponding to this fragment from various ALFs have shown antimicrobial activity, the ability to inhibit WSSV virus replication, and a protective effect in sepsis [20][37][38][39][20,37,38,39].
Despite the large number of registered ALFs (more than 300 proteins of this class from crustaceans were isolated and characterized until 2021), only one crystal structure of them has been established to date. The X-ray structure of recombinant ALF-L consists of three α-helices (one at the N-terminus and two at the C-terminus) packed against a four-stranded β-sheet, giving rise to a wedge-shaped molecule [36]. The potential LPS-binding domain includes an amphipathic β-hairpin formed by the longest β-strands S2 and S3 of the β-sheet and stabilized by the single disulfide bond (Cys31–Cys52). The positively charged residues within the β-hairpin of ALF-L are supposed to interact with the negatively charged phosphate groups of lipid A. However, the lipid A binding site on ALF remains poorly understood to date. Later, the spatial ALF structure from the shrimp P. monodon, expressed in yeast cells, rALFPm3, was determined using NMR (Figure 2) [40]. The structure of rALF-Pm3, like ALF-L, is composed of three α-helices, a four-stranded β-sheet, and contains a β-hairpin formed by S2 and S3 β-strands closely linked via a Cys34-Cys55 disulfide bond. A Comparison of the 3D structures of these proteins revealed highly similar clusters of positively charged and hydrophobic residues on the β-sheet surface. This suggests that ALF-L and ALFPm3 have a similar LPS binding site, which is located on the β-sheet and mainly consists of 5–6 positively charged and several hydrophobic residues capable of binding lipid A through electrostatic and hydrophobic interactions.
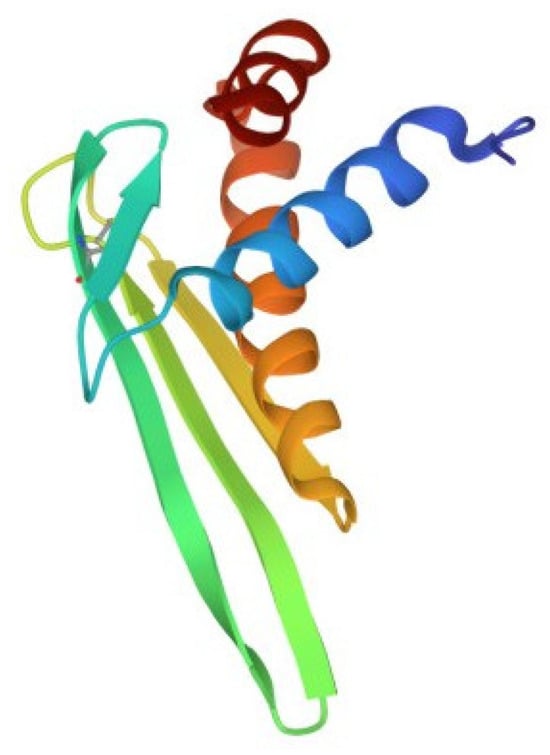
Figure 2. Structure of the anti-lipopolysaccharide factor from Penaeus monodon (pdb, 2job).
A series of peptides of various lengths, including cyclic ones, derived from the ALF-L sequence, were synthesized [41]. These peptides demonstrated high endotoxin-binding and neutralizing activities, comparable with those of the parent recombinant protein, and were non-toxic for erythrocytes or cultured human monocytes. A new class of peptides based on the LPS-binding domain of ALF-L or part of it with significant changes in length and primary sequence calculated for optimal lipid A binding was also designed [42]. A preclinical study revealed that these peptides have high selectivity for LPS, as well as high LPS-neutralizing activity in vitro and the ability to protect against sepsis in vivo. An analysis of the obtained data showed that the endotoxin-neutralizing activity of the peptides is closely related to their affinity for LPS and the ability to incorporate into LPS aggregates with changes in their structure. The authors highly appreciate the potential of synthetic peptides as drugs for the treatment of endotoxemia and sepsis.
