A prevalent, well-characterized immobilization design system is the biotin–(strept)avidin interaction. The biotin–(strept)avidin interaction is considered to be one of the most specific and stable non-covalent interaction, whose dissociation constant (KD) is about 103 to 106 times higher than an antigen–antibody interaction. Its high affinity is principally useful for isolating and amplifying the signal, which increases the ability for the detection of very low concentrations of analyte while decreasing the number of steps required for measurement, allowing for a more rapid quantitation of analyte. The biotin–(strept)avidin system offers enormous advantages over other covalent and non-covalent interactions, which include amplification of weak signals, efficient operation, robustness, and astonishing stability against manipulation, proteolytic enzymes, temperature and pH extremes, harsh organic reagents, and other denaturing reagents. Since the biotin–(strept)avidin interaction is one of the strongest known non-covalent interactions in nature, avidin and its analogues have therefore been extensively used as probes and affinity matrices for a wide variety of applications in the field of biotechnology, such as biochemical assays, diagnostics, affinity purification, and drug delivery.
- immunoassays
- interference
- biotin
- avidin
- streptavidin
1. History
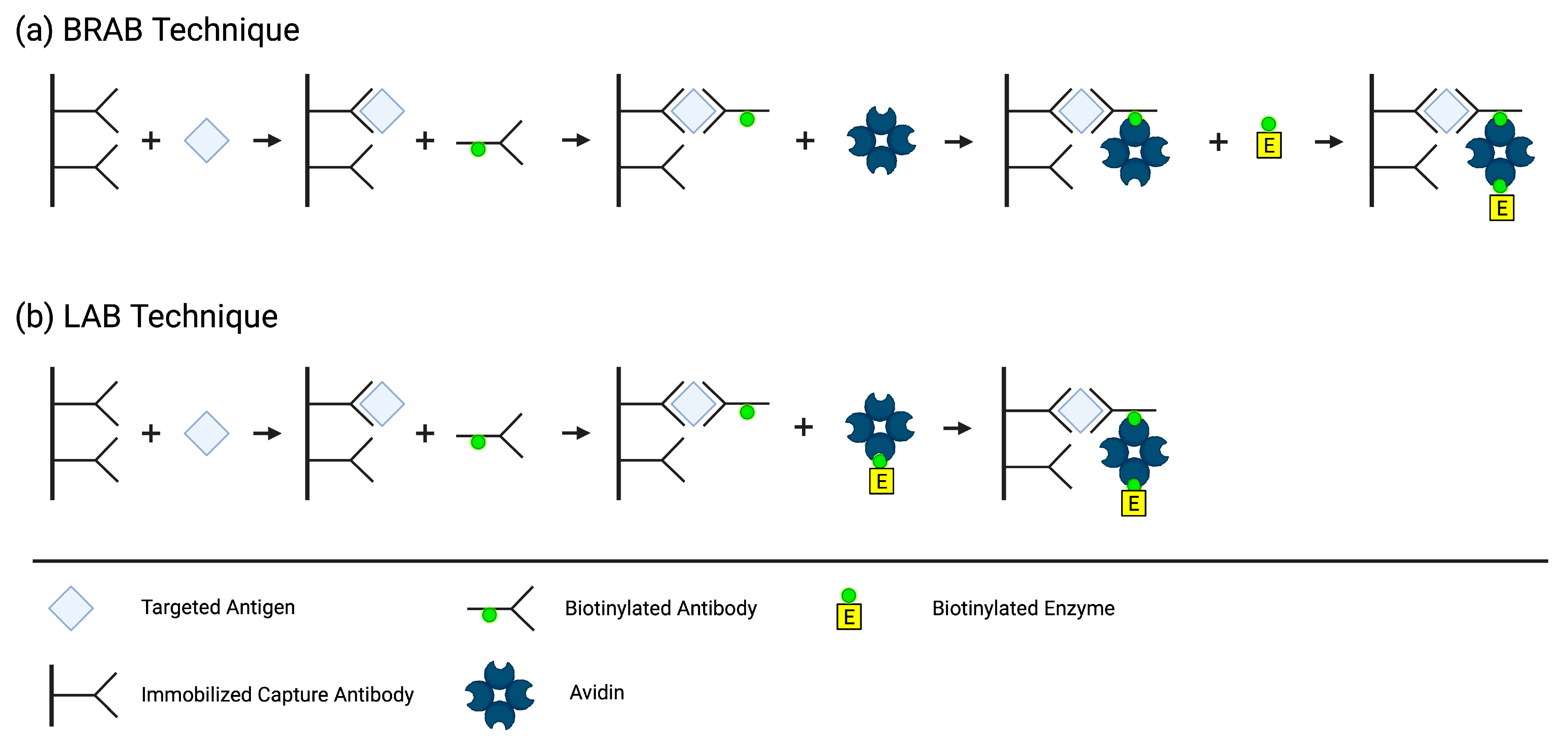
2. Biotin
3. (Strept)avidin
| Origin | MW | pI | Reference | |
|---|---|---|---|---|
| Avidin | Gallus gallus egg white | ~66–69 kDa | ~10 | [27][9] |
| Streptavidin | Bacterium Streptomyces avidinii | ~56 kDa | ~5–6 | [27][9] |
| Neutravidin | Deglycosylated avidin | ~60 kDa | ~6.3 | [27][9] |
| Traptavidin | S52G, R53D mutant of streptavidin | ~56 kDa | ~5.1 | [54][26] |
| Bradavidin II | Bradyrhizobium japonicum | ~58.4 kDa | ~9.6 | [27][9] |
| Tamavidin 2 | Mushroom Pleurotus cornucopiae | ~ 60.9 kDa | ~7.4 | [58][31] |
References
- Wu, A.H. A selected history and future of immunoassay development and applications in clinical chemistry. Clin. Chim. Acta 2006, 369, 119–124.
- Kahn, C.R.; Roth, J. Berson, Yalow, and the JCI: The agony and the ecstasy. J. Clin. Investig. 2004, 114, 1051–1054.
- Yalow, R.S.; Berson, S.A. Immunoassay of endogenous plasma insulin in man. J. Clin. Investig. 1960, 39, 1157–1175.
- Grange, R.D.; Thompson, J.P.; Lambert, D.G. Radioimmunoassay, enzyme and non-enzyme-based immunoassays. Br. J. Anaesth. 2014, 112, 213–216.
- Alhajj, M.; Zubair, M.; Farhana, A. Enzyme Linked Immunosorbent Assay; StatPearls: Treasure Island, FL, USA, 2023.
- Guesdon, J.L.; Ternynck, T.; Avrameas, S. The use of avidin-biotin interaction in immunoenzymatic techniques. J. Histochem. Cytochem. 1979, 27, 1131–1139.
- Wilchek, M. My life with affinity. Protein Sci. 2004, 13, 3066–3070.
- McConnell, D.B. Biotin’s Lessons in Drug Design. J. Med. Chem. 2021, 64, 16319–16327.
- Jain, A.; Cheng, K. The principles and applications of avidin-based nanoparticles in drug delivery and diagnosis. J. Control. Release 2017, 245, 27–40.
- Bowen, R.; Benavides, R.; Colon-Franco, J.M.; Katzman, B.M.; Muthukumar, A.; Sadrzadeh, H.; Straseski, J.; Klause, U.; Tran, N. Best practices in mitigating the risk of biotin interference with laboratory testing. Clin. Biochem. 2019, 74, 1–11.
- Waldrop, G.L.; Holden, H.M.; St. Maurice, M. The enzymes of biotin dependent CO2 metabolism: What structures reveal about their reaction mechanisms. Protein Sci. 2012, 21, 1597–1619.
- Woodward, J.D. Biotin. Sci. Am. 1961, 204, 139–150.
- Patel, D.P.; Swink, S.M.; Castelo-Soccio, L. A Review of the Use of Biotin for Hair Loss. Ski. Appendage Disord. 2017, 3, 166–169.
- Luong, J.H.T.; Vashist, S.K. Chemistry of Biotin-Streptavidin and the Growing Concern of an Emerging Biotin Interference in Clinical Immunoassays. ACS Omega 2020, 5, 10–18.
- Erbach, J.; Bonn, F.; Diesner, M.; Arnold, A.; Stein, J.; Schroder, O.; Aksan, A. Relevance of Biotin Deficiency in Patients with Inflammatory Bowel Disease and Utility of Serum 3 Hydroxyisovaleryl Carnitine as a Practical Everyday Marker. J. Clin. Med. 2022, 11, 1118.
- Sardo, A. Genetic optimization of biotin-binding proteins: Artificial metalloenzymes and beyond. In Philosophy and Natural Sciences; University of Basel: Basel, Switerland, 2010; p. 166.
- Laitinen, O.H.; Kuusela, T.P.; Kukkurainen, S.; Nurminen, A.; Sinkkonen, A.; Hytönen, V.P. Bacterial avidins are a widely distributed protein family in Actinobacteria, Proteobacteria and Bacteroidetes. BMC Ecol. Evol. 2021, 21, 53.
- Eakin, R.E.; Snell, E.E.; Williams, R.J. A constituent of raw egg white capable of inactivating biotin in vitro. J. Biol. Chem. 1940, 136, 801–802.
- György, P.; Rose, C.S.; Eakin, R.E.; Snell, E.E.; Williams, R.J. Egg-White Injury as the Result of Nonabsorption or Inactivation of Biotin. Science 1941, 93, 477–478.
- Eakin, R.E.; Snell, E.E.; Williams, R.J. The concentration and assay of avidin, the injury-producing protein in raw egg white. J. Biol. Chem. 1941, 140, 535–543.
- Kresge, N.; Simoni, R.D.; Hill, R.L. The Discovery of Avidin by Esmond E. Snell. J. Biol. Chem. 2004, 279, e5–e6.
- Jain, A.; Barve, A.; Zhao, Z.; Jin, W.; Cheng, K. Comparison of Avidin, Neutravidin, and Streptavidin as Nanocarriers for Efficient siRNA Delivery. Mol. Pharm. 2017, 14, 1517–1527.
- Le Trong, I.; Wang, Z.; Hyre, D.E.; Lybrand, T.P.; Stayton, P.S.; Stenkamp, R.E. Streptavidin and its biotin complex at atomic resolution. Acta Crystallogr. D Biol. Crystallogr. 2011, 67, 813–821.
- Delgadillo, R.F.; Mueser, T.C.; Zaleta-Rivera, K.; Carnes, K.A.; Gonzalez-Valdez, J.; Parkhurst, L.J. Detailed characterization of the solution kinetics and thermodynamics of biotin, biocytin and HABA binding to avidin and streptavidin. PLoS ONE 2019, 14, e0204194.
- Helppolainen, S.H.; Määttä, J.A.E.; Halling, K.K.; Slotte, J.P.; Hytönen, V.P.; Jänis, J.; Vainiotalo, P.; Kulomaa, M.S.; Nordlund, H.R. Bradavidin II from Bradyrhizobium japonicum: A new avidin-like biotin-binding protein. Biochim. Biophys. Acta (BBA) Proteins Proteom. 2008, 1784, 1002–1010.
- Chivers, C.E.; Crozat, E.; Chu, C.; Moy, V.T.; Sherratt, D.J.; Howarth, M. A streptavidin variant with slower biotin dissociation and increased mechanostability. Nat. Methods 2010, 7, 391–393.
- Chivers, C.E.; Koner, A.L.; Lowe, E.D.; Howarth, M. How the biotin-streptavidin interaction was made even stronger: Investigation via crystallography and a chimaeric tetramer. Biochem. J. 2011, 435, 55–63.
- Nguyen, T.T.; Sly, K.L.; Conboy, J.C. Comparison of the energetics of avidin, streptavidin, neutrAvidin, and anti-biotin antibody binding to biotinylated lipid bilayer examined by second-harmonic generation. Anal. Chem. 2012, 84, 201–208.
- Sut, T.N.; Park, H.; Koo, D.J.; Yoon, B.K.; Jackman, J.A. Distinct Binding Properties of Neutravidin and Streptavidin Proteins to Biotinylated Supported Lipid Bilayers: Implications for Sensor Functionalization. Sensors 2022, 22, 5185.
- Berg Luecke, L.; Gundry, R.L. Assessment of Streptavidin Bead Binding Capacity to Improve Quality of Streptavidin-based Enrichment Studies. J. Proteome Res. 2021, 20, 1153–1164.
- Takakura, Y.; Suzuki, J.; Oka, N.; Kakuta, Y. Tamavidin 2-HOT, a highly thermostable biotin-binding protein. J. Biotechnol. 2014, 169, 1–8.
