You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Mona Zou and Version 1 by Marta D. Switlyk.
Accurate preoperative staging and precise outlining of a tumor’s extent are crucial for selecting the most suitable treatment approach and improving outcomes. The current clinical staging of penile cancer is still largely based on physical examination. Multiparametric magnetic resonance imaging (mpMRI) is an important imaging modality that complements physical examination and reduces uncertainties that can easily arise during this examination.
- dynamic contrast-enhanced MRI
- diffusion-weighted imaging
- guidelines
- multiparametric magnetic resonance imaging
- MRI
- penile cancer
- staging
1. MRI in the Assessment of Penile Cancer
1.1. Basic Principles
Magnetic resonance imaging (MRI) is the medical application of nuclear magnetic resonance (NMR), based on the interaction of hydrogen nuclei in the presence of an external magnetic field when exposed to radiofrequency waves of a specific resonance frequency [9][1]. MRI has excellent tissue contrast and does not require the presence of isotopes in the sample or time-consuming calibration, unlike other methods used to characterize the magnetic properties of a tissue, such as the magneto-optic Kerr effect (MOKE) [10][2], Mössbauer spectroscopy [11][3], and magnetic force microscopy [12][4].
MRI offers a noninvasive assessment of both primary and locally recurrent penile malignancies [13][5]. Since its introduction in the early 1980s, MRI has become the reference standard for oncological imaging and has been rapidly evolving, resulting in a variety of sequences, increased quality, and rapid acquisition [14][6]. Morphological MRI sequences provide excellent soft-tissue contrast and anatomical information. Furthermore, functional MRI sequences provide molecular and physiological information using diffusion-weighted imaging (DWI) and dynamic contrast-enhanced MRI (DCE-MRI) perfusion [15][7]. Finally, mpMRI allows for the integrated evaluation of morphological images and at least two functional sequences (DWI and DCE-MRI), and has been successfully performed in several malignancies, such as prostate, breast, and bladder cancer [4,16,17,18,19][8][9][10][11][12]. Gadolinium-based contrast agents are routinely used in contrast-enhanced MRI, both for dynamic and static applications. The indications and use of gadolinium-based contrast agents are well documented; they are generally safe and well tolerated and have powerful paramagnetic properties [20][13]. Gadolinium in standard dosages mainly shortens T1 relaxation times, thereby producing a high T1 signal intensity in MRI [21][14].
DWI provides a noninvasive characterization of biological tissues based on the measurements of the random microscopic motion of water protons (Brownian motion). It is performed by using echo planar imaging (EPI) combined with symmetrical motion probing gradients (MPGs) around the 180° refocusing pulse [22,23][15][16]. The intensity of MPG pulses is represented by the b-value (s/mm2), which measures the strength of the diffusion-sensitizing gradient [22][15]. Using two b-values, one can map and calculate the apparent diffusion coefficient (ADC) [22][15]. Malignant tumors typically display low diffusion because of their hypercellularity; consequently, the contrast is accentuated by high signal intensity in the cellular tumor and low signal intensity in the normal background tissue on high b-value images [24][17]. Low diffusion can also be observed in some benign conditions such as abscesses and hematomas, and DWI findings should not be assessed alone.
DCE-MRI is another functional technique that is used in oncological imaging. DCE-MRI assesses the passage of blood through tissue vessels, providing information on the density, integrity, and leakiness of the tumor vasculature [25][18]. It involves sequentially acquired T1-weighted images collected before, during, and after the intravenous injection of gadolinium-based contrast agent. Thus, each acquired image corresponds to one time point and each pixel in each image set gives rise to its own time course, which can then be analyzed with a mathematical model [25,26][18][19]. To perform such an analysis, the following measurements are required: (1) the measurement of precontrast T1-values in a tissue, (2) dynamic data acquisition after contrast with reasonably high temporal resolution, and (3) an estimate of the arterial input function (AIF) [25][18]. DCE-MRI data can be evaluated qualitatively through a visual analysis of the acquired images, semiquantitatively by analyzing the perfusion curve, or quantitatively by analyzing perfusion parameters such as Ktrans, kep, vp, and ve [27][20]. Tumors can have variable appearances on perfusion; however, high vascular permeability with a rapid rise, strong peak enhancement, and subsequent washout is typical for malignant lesions.
1.2. Clinical Protocols and Technical Considerations
A limited number of reports have assessed the diagnostic potential of MRI in the assessment of penile carcinoma, and inconsistencies in MRI protocols can influence the outcomes and make comparisons difficult [3,4,13,28,29,30,31,32][5][8][21][22][23][24][25][26]. Most of these studies did not incorporate modern MRI protocols or anticipated the high spatial resolution of images. Only one study has combined both DCE-MRI and DWI into high-resolution mpMRI, and the initial findings indicated that non-erectile mpMRI can be implemented as a precise diagnostic tool for the preoperative assessment of primary penile carcinoma [4][8].
Previously, the intracavernosal injection of prostaglandin E1 and artificial erection were suggested for MRI. During erection, penile blood flow increases, resulting in an increase in the image size and signal intensity of the corpora cavernosa. This enhances the penile anatomy, particularly the corpora cavernosa and tunica albuginea, and their anatomical boundaries [33][27]. However, this invasive technique is not routinely used in clinical practice and carries some important contraindications and side effects [28,34][22][28]. Furthermore, the pain associated with this procedure may be an important limitation in patients with invasive penile carcinoma, and several patients may experience embarrassment and discomfort during this procedure [4][8]. The results of a recently published systematic review and meta-analysis showed that MRI with and without artificial erection had similar accuracy for the local staging of penile cancer [28][22]. According to the latest version of the EAU-ASCO Guidelines for Penile Cancer, artificial erection is not mandatory for MRI [6][29].
A biopsy of penile lesions and histopathological evaluation are important for the diagnosis of penile cancer. Performing MRI prior to biopsy avoids disturbing post-biopsy changes such as hemorrhage and inflammation, which can make the evaluation of smaller, superficial lesions difficult. Moreover, mpMRI can be used to guide biopsies, targeting the most suitable tumor components and avoiding ulcerations or necrosis.
MpMRI allows for the integrated assessment of tumor morphology, cellularity, and vascularization. Although the data are limited, the use of DCE-MRI and DWI in the evaluation of penile cancers can help to identify small lesions more precisely, allowing to accurately determine the infiltration depth, outline the tumor extension, and detect tumor satellites [4,28,34][8][22][28]. Morphological sequences can over- or underestimate the tumor extent because peritumoral edema and hyperemia are difficult to differentiate from tumor tissue using these sequences [4,35][8][30]. Functional sequences seem to depict tumor tissue more selectively and, despite lower spatial resolution, delineate the tumor extent more precisely. DWI visualizes the high cellularity of the tumor as opposed to the surrounding tissue, and the early phases of DCE-MRI visualize the enhancement in the intra-tumoral vessels before the contrast medium reaches the peritumoral vessels [4,24,36][8][17][31]. A summary of institutional imaging protocol for the assessment of penile carcinoma, which incorporates high-resolution morphological and functional sequences, is presented in Table 1 and Figure 1.
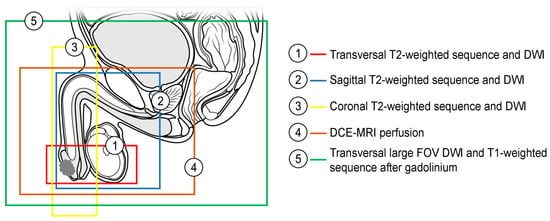
Figure 1. Recommended coverage of the anatomical area by morphological and functional sequences in penile MRI (DWI—diffusion-weighted imaging, DCE-MRI—dynamic contrast-enhanced MRI, FOV—field of view).
|
DWI—diffusion-weighted imaging; DCE-MRI—dynamic contrast-enhanced magnetic resonance imaging; FOV—field of view; GE—gradient echo.
MpMRI is considered safe and well tolerated by most patients, with only a few disadvantages. MRI scans can be contraindicated in a few cases of devices and implants. Moreover, patients with strong claustrophobia may be unable to complete the examination. However, using wide-bore MRI systems, good patient information and sedation when needed can address this issue. Some patients may not cooperate and will not be able to lie motionless during the MRI scan. However, the functional sequences are frequently robust to motion artifacts. Finally, group II gadolinium-based contrast agents (Clariscan) in standard dosages are considered very safe, although caution should be exercised when administering to patients with severe renal impairment [37][32]. In summary, non-erectile mpMRI is a noninvasive examination performed without artificial erection (without intracavernosal administration of prostaglandin E1) and is well tolerated by the majority of patients in different age groups and with varying health statuses. In most cases, mpMRI provides sufficient clinical information, even when part of the examination is hampered by artifacts.
DWI of the penis can be challenging because of geometric distortion and susceptibility artifacts from the inhomogeneous magnetic field due to air–tissue boundaries frequently affecting this region. In addition, high-resolution images are difficult to achieve with standard single-shot EPI DWI. T2* decay and EPI blurring (T2* blurring) limit the image quality of the standard single-shot EPI DWI techniques [40][33].
Several DWI techniques have been developed to reduce distortion caused by magnetic susceptibility. These include reduced FOV techniques utilizing spatially selective excitation, such as FOCUS delivered by GE Healthcare, ZOOMit delivered by Siemens, and ZOOM delivered by Philips [41][34]; readout-segmented multi-shot EPI, such as RESOLVE delivered by Siemens [42][35]; and phase-segmented multi-shot EPI, such as MUSE delivered by GE Healthcare and IRIS delivered by Philips [43,44][36][37]. Sequences that combine these techniques and/or DL are available and help to further improve the image quality.
These advanced EPI techniques minimize susceptibility and blurring artifacts and are particularly useful for the evaluation of smaller lesions and areas where the magnetic field is inhomogeneous, such as the penis. However, it is important to optimize DWI with parallel imaging to shorten the readout window and minimize the T2* blurring, and with a high sampling bandwidth to shorten echo spacing and reduce the image distortion. Multi-shot EPI techniques seem to be necessary for 3-T MRI scanners, while reduced FOV techniques are sufficient for 1.5-T MRI scanners. High b-value images are useful for lesion detection and characterization; however, they should be calculated rather than acquired using one of the several available extrapolation models, allowing for a reduced scan time and improved image quality, owing to a higher SNR [45,46][38][39]. It is important to avoid using too-high b-values when calculating ADC maps to prevent reaching the noise floor, preferably b0 and b800 [47][40].
Finally, contrast-enhanced imaging, both for dynamic and static applications, can be performed using the Dixon technique, which allows for homogeneous, fat-free imaging, even in the presence of magnetic susceptibility [48][41]. High-resolution DCE-MRI has previously been shown to detect superficial, tumor-like skin lesions as small as 1–2 mm, and can be beneficial in the work-up of penile cancers [49][42]. Because of the curved anatomy of the glans, DCE-MRI should be as isotropic as possible, allowing for the multiplanar interpretation of the images. An approximately 1 mm isotropic resolution is achievable using slice interpolation within a dynamic timeframe of 10–15 s.
2. Classification and Staging of Penile Cancer
2.1. Classification
The latest 2022 World Health Organization (WHO) classification reinforces the 2016 classification, and subclassifies precursor lesions and invasive tumors into HPV-associated and HPV-independent types [1][43].
There are no established prognostic or treatment differences between HPV-associated and HPV-independent lesions; however, some evidence suggests better prognostic and therapeutic outcomes in HPV-associated malignancies [1,50,51,52][43][44][45][46].
The prognostic role of histology was investigated in previous studies, which showed that the basaloid, sarcomatoid, and adenosquamous subtypes correlated with poorly differentiated tumors and deep tissue infiltration, whereas the verrucous, papillary, and condylomatous (warty) subtypes were associated with low-grade tumors and superficial invasion [53,54][47][48].
2.2. Staging of Penile Cancer
Penile cancer staging models are useful for planning treatment strategies and predicting prognosis [55][49]. The TNM staging system is mainly based on stratified anatomical routes of penile cancer spread [55][49]. The eighth edition of the Union for International Cancer Control (UICC)/American Joint Committee on Cancer (AJCC) TNM classification is currently used to stage penile cancer [6,55,56][29][49][50]. This version was last updated in 2017, and one of the major changes concerned the tumor involvement of the corpus cavernosum, corpus spongiosum, and urethra [34,56][28][50]. In the eighth edition, T-stage T2 is defined as the involvement of the corpus spongiosum, while the involvement of the corpus cavernosum (including the tunica albuginea) is defined as stage T3. Urethral involvement is no longer relevant to local staging (Table 3 and Figure 2).
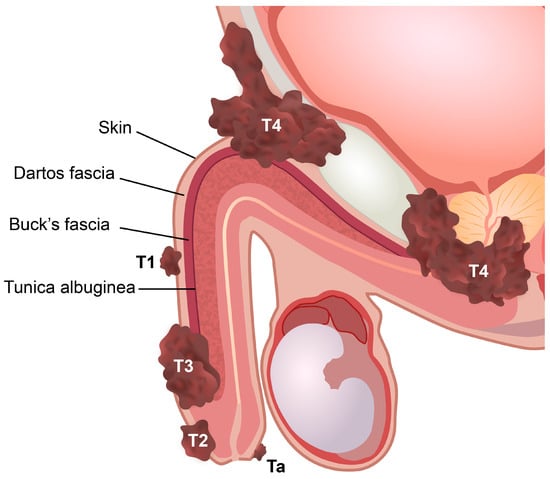

Figure 2. Stages of penile cancer according to the eighth edition of the Union for International Cancer Control (UICC)/American Joint Committee on Cancer (AJCC) TNM classification. Ta, noninvasive localized squamous cell carcinoma. T1, invasion of subepithelial connective tissue. T2, invasion of corpus spongiosum with or without invasion of urethra. T3, invasion of corpus cavernosum with or without invasion of urethra. T4, invasion of adjacent structures.
Table 3. The eighth edition of Union for International Cancer Control (UICC)/American Joint Committee on Cancer (AJCC) TNM classification for the pathological staging of penile cancer. Adapted with permission from [56][50].
| pT-Primary Tumor | TX | Primary tumor cannot be assessed |
| T0 | No evidence of primary tumor | |
| Tis | PeIN | |
| Ta | Noninvasive localized squamous cell carcinoma | |
| T1 | Invasion of subepithelial connective tissue:
|
|
| T2 | Invasion of corpus spongiosum with or without invasion of urethra | |
| T3 | Invasion of corpus cavernosum with or without invasion of urethra | |
| T4 | Invasion of adjacent structures | |
| pN-Regional Lymph Nodes | pNX | Regional lymph nodes cannot be assessed |
| pN0 | No regional lymph node metastases | |
| pN1 | Metastasis in ≤ 2 unilateral inguinal lymph nodes | |
| pN2 | Metastasis in ≥ 3 unilateral inguinal lymph nodes or bilateral inguinal lymph nodes | |
| pN3 | Metastasis in pelvic lymph node(s) or ENE | |
| pM-Distant Metastasis | pM0 | No distant metastasis |
| pM1 | Distant metastasis microscopically confirmed |
PeIN—penile intraepithelial neoplasia; ENE—extranodal extension.
Reliable preoperative staging is crucial for decision making and decreasing the recurrence rate after surgery. Penile amputation is associated with significant functional, sexual, and psychological deficits despite high local control rates [57,58][51][52]. A majority of early penile carcinomas are amenable to organ-sparing surgery [13][5], which may improve not only the quality of life but also the quality of sexual function [3,58,59,60][21][52][53][54]. However, there is a potential for an increased risk of local recurrence after organ-sparing surgery compared with the amputation of the penis [61][55]. Thus, accurate preoperative staging, together with the precise outlining of the tumor extension, infiltration depth, and tumor satellites, is important before selecting a surgical method, particularly prior to organ-sparing surgery.
Published evidence suggests that tumors restricted to the corpus spongiosum have a better prognosis than those that invade the corpus cavernosum [55][49]. However, this view has been challenged in some studies that found a prognostic overlap between T2 and T3 patients or no significant differences in lymph node status in these patients [55,62,63,64,65,66][49][56][57][58][59][60]. Furthermore, some of these studies have proposed lymphovascular invasion as a significant separator of both stages [63,66][57][60]. Although tumor extent and infiltration depth are not relevant to TNM staging, it has been shown that tumors invading superficially in the corpus spongiosum (no more than 5 mm) rarely metastasize, whereas tumors that are deeply invasive in the corpus spongiosum have a higher rate of metastasis (Figure 3) [55,67][49][61].
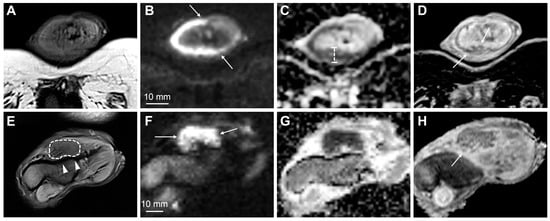
Figure 3. MpMRI findings of superficial (A–D) and deeply invasive (E–H) T2 penile cancer. The superficial tumor is difficult to outline on T2-weighted imaging (A); however, the high b-value DWI increases the conspicuity of the lesion, showing high signal intensity in tumor tissue (B, arrows). The tumor has a moderately low ADC (950 μmm2/s) (C). Subsequent surgery presented a good correlation between tumor thickness on mpMRI (C) and histopathology. The contrast-enhanced T1-weighted imaging shows tumor infiltration into the dorsal part of the glans (D, arrows). The lower row (E–H) shows a deeply invasive tumor in the glans (E—T2-weighted imaging, dashed line), located close but not infiltrating into the adjacent tunica albuginea (E, arrowheads). The conspicuity of the tumor and its boundaries increase on high b-value DWI (F, arrows), ADC (G) and contrast-enhanced T1-weighted imaging (H, arrow).
From the penis, the tumor disseminates to the inguinal superficial lymph nodes and then to the deep and pelvic lymph nodes [55][49]. Skip metastasis from the superficial or deep inguinal nodes to the pelvic or other retroperitoneal nodes is extremely unusual [55][49]. Nodal involvement is one of the best predictors of penile cancer outcomes [31,68,69,70][25][62][63][64]. Major changes in N-staging in the eighth edition of the TNM classification include an increased number of inguinal lymph node metastases in N1 and N2 disease (Table 3).
2.3. Role of MRI in Staging of Penile Cancer
Current EAU-ASCO guidelines for the diagnosis and staging of penile cancer recommend the use of MRI in cases intended for organ-sparing surgery or when an invasion of the corpus cavernosum is suspected [6][29]. However, this recommendation is rated as weak, and the supporting evidence is sparse and limited to a few older reports [3,13][5][21]. According to these guidelines, physical examination is a reliable method for estimating penile tumor size and stage, and MRI does not outperform physical examination, at least in the ability to differentiate between T-stages T1 and T2 [6][29]. This rationale is supported by an older study with anticipated low-resolution images and principal technical differences from the modern mpMRI protocols currently used [71][65]. In general, there are some important pitfalls of physical examination that can be easily avoided using mpMRI. It is difficult to precisely estimate tumor infiltration depth through clinical examination alone; however, a strong and significant correlation between mpMRI and histopathology has been shown for both tumor size and infiltration depth [4][8]. Furthermore, the researchers experienced that mpMRI can precisely identify small, deeply located tumor satellites that are difficult to detect through physical examination alone (Figure 4). The identification of tumor satellites and accurate delineation of the proximal tumor extent are important for surgical planning. Superimposed infections and body habitus can also make physical examinations difficult [31][25].
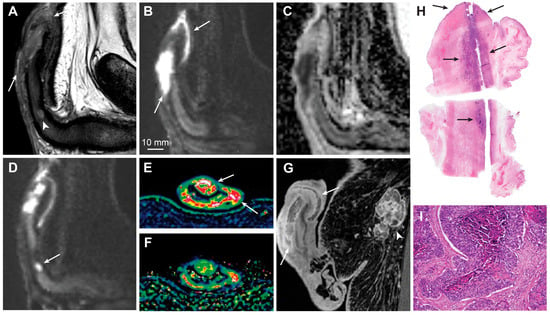
Figure 4. Sagittal T2-weighted imaging (A), high b-value DWI (B) and ADC (C) showing a large T3 penile cancer (arrows). The tumor infiltrates the corpus spongiosum and cavernosum and there is massive infiltration in the penile urethra. The small, deeply located tumor satellite in the corpus cavernosum is shown (A, arrowhead). This finding is crucial for surgery planning since the satellite defines the proximal tumor extent and is almost impossible to detect clinically because of its small size. The tumor satellite has a high signal intensity in DWI (D, arrow). The tumor has high vascular permeability, as shown on the wash-in (Ktrans) (E, arrows) and washout (kep) (F) perfusion maps and contrast-enhanced T1-weighted imaging (G, arrows). A large inguinal lymph node metastasis is also shown (G, arrowhead). Photomicrograph of a whole mount hematoxylin and eosin-stained section shows a large tumor in the corpus spongiosum, cavernosum, and urethra (H, arrows). Photomicrograph of histologic specimen shows infiltration of basaloid squamous cell carcinoma (I, magnification ×10).
A recently published systematic review and meta-analysis showed that MRI has 86% sensitivity and 89% specificity for identifying T1 versus T2 disease, 80% and 96% for identifying T3 disease, and 86% and 93% for identifying urethral involvement [28][22]. The data were extracted from eight studies involving 481 patients [3,13,29,30,31,32,71,72][5][21][23][24][25][26][65][66]. The performances of MRI with and without artificial erection were comparable [28][22]. A recent study comparing high-resolution mpMRI and histopathological findings in the assessment of penile cancer showed very good agreement between mpMRI and histopathology for identifying T1 versus T2 disease (κ = 0.834) (Figure 3 and Figure 5), good agreement for identifying T2 versus T3 disease (κ = 0.702) (Figure 3 and Figure 4), and good agreement for identifying urethral involvement (κ = 0.746) [4][8]. Urethral involvement is no longer relevant for local staging; however, it is still important for surgical planning.
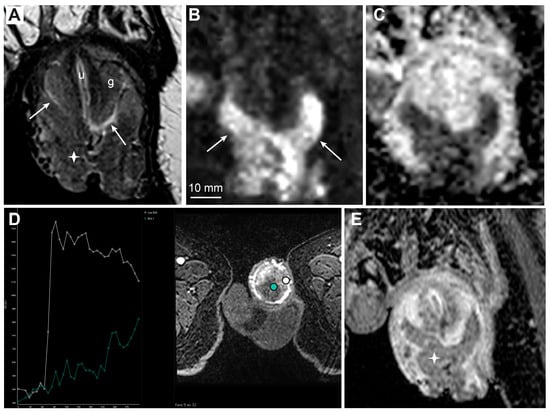
Figure 5. MRI findings of large T1 penile cancer. T2-weighted imaging (A, asterisk) and high b-value DWI (B, arrows) show a large prepuce tumor. The tumor lies close to but does not infiltrate the glans, with a visible fluid layer between the glans surface and tumor (A, arrows, g—glans, u—urethra). The ADC map displays low diffusion in the tumor (C). DCE-MRI (D) shows high permeability in the tumor with subsequent washout (D, white curve), in contrast to homogenously enhancing the glans with lower vascular permeability and no washout (D, green curve). The contrast-enhanced T1-weighted imaging shows reduced contrast enhancement in the tumor compared to surrounding structures, due to washout (E, asterisk).
The cost-effectiveness of penile mpMRI is difficult to assess due to the scarce data available. However, the incidence of penile cancer is low and there are several potential benefits arising from MRI-based assessment, such as the precise staging and delineation of the tumor extent, resulting in proper preoperative patient selection, particularly for organ-sparing procedures, with subsequent improving quality of life and sexual function and a potentially reduced number of local recurrences after surgery.
Penile Doppler ultrasound is an alternative imaging modality recommended by EAU-ASCO for the assessment of primary tumors [6][29]. Bozzini et al. reported that penile ultrasound has higher sensitivity for detecting corpus cavernosum invasion than MRI with artificial erection [32][26]. However, the MRI protocols used in their study implemented only morphological sequences, and the technical parameters were unknown. Other studies have proven that penile ultrasound is unreliable, especially in the presence of microscopic invasion, and has important limitations, such as operator dependency and practical difficulty in assessing ulcerative tumors [3,13,73][5][21][67]. Several patients may also experience embarrassment and discomfort during the procedure.
References
- Chan, R.W.; Lau, J.Y.C.; Lam, W.W.; Lau, A.Z. Encyclopedia of Biomedical Engineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 574–587.
- Yamamoto, S.; Matsuda, I. Measurement of the Resonant Magneto-Optical Kerr Effect Using a Free Electron Laser. Appl. Sci. 2017, 7, 662.
- Kuzmann, E.; Homonnay, Z.; Klencsár, Z.; Szalay, R. 57Fe Mössbauer Spectroscopy as a Tool for Study of Spin States and Magnetic Interactions in Inorganic Chemistry. Molecules 2021, 26, 1062.
- Winkler, R.; Ciria, M.; Ahmad, M.; Plank, H.; Marcuello, C. A Review of the Current State of Magnetic Force Microscopy to Unravel the Magnetic Properties of Nanomaterials Applied in Biological Systems and Future Directions for Quantum Technologies. Nanomaterials 2023, 13, 2585.
- Kayes, O.; Minhas, S.; Allen, C.; Hare, C.; Freeman, A.; Ralph, D.; Pizzocaro, G. The Role of Magnetic Resonance Imaging in the Local Staging of Penile Cancer. Eur. Urol. 2007, 51, 1313–1318, discussion 1318–1319.
- Becker, M.; Zaidi, H. Imaging in head and neck squamous cell carcinoma: The potential role of PET/MRI. Br. J. Radiol. 2014, 87, 20130677.
- Dai, Y.L.; King, A.D. State of the art MRI in head and neck cancer. Clin. Radiol. 2018, 73, 45–59.
- Switlyk, M.D.; Hopland, A.; Sivanesan, S.; Brennhovd, B.; Ottosson, F.; Berner, K.; Axcrona, U.; Hole, K.H. Multi-parametric MRI without artificial erection for preoperative assessment of primary penile carcinoma: A pilot study on the correlation between imaging and histopathological findings. Eur. J. Radiol. Open 2023, 10, 100478.
- Hoeks, C.M.A.; Barentsz, J.O.; Hambrock, T.; Yakar, D.; Somford, D.M.; Heijmink, S.W.T.P.J.; Scheenen, T.W.J.; Vos, P.C.; Huisman, H.; van Oort, I.M.; et al. Prostate cancer: Multiparametric MR imaging for detection, localization, and staging. Radiology 2011, 261, 46–66.
- Turkbey, B.; Rosenkrantz, A.B.; Haider, M.A.; Padhani, A.R.; Villeirs, G.; Macura, K.J.; Tempany, C.M.; Choyke, P.L.; Cornud, F.; Margolis, D.J.; et al. Prostate Imaging Reporting and Data System Version 2.1, 2019 Update of Prostate Imaging Reporting and Data System Version 2. Eur. Urol. 2019, 76, 340–351.
- Mann, R.M.; Cho, N.; Moy, L. Breast MRI: State of the Art. Radiology 2019, 292, 520–536.
- Panebianco, V.; Narumi, Y.; Altun, E.; Bochner, B.H.; Efstathiou, J.A.; Hafeez, S.; Huddart, R.; Kennish, S.; Lerner, S.; Montironi, R.; et al. Multiparametric Magnetic Resonance Imaging for Bladder Cancer: Development of VI-RADS (Vesical Imaging-Reporting and Data System). Eur. Urol. 2018, 74, 294–306.
- Bellin, M.F. MR contrast agents, the old and the new. Eur. J. Radiol. 2006, 60, 314–323.
- Costelloe, C.M.; Amini, B.; Madewell, J.E. Risks and Benefits of Gadolinium-Based Contrast-Enhanced MRI. Semin. Ultrasound CT MR 2020, 41, 170–182.
- Coutinho, A.C., Jr.; Krishnaraj, A.; Pires, C.E.; Bittencourt, L.K.; Guimaraes, A.R. Pelvic applications of diffusion magnetic resonance images. Magn. Reson. Imaging Clin. N. Am. 2011, 19, 133–157.
- Messina, C.; Bignone, R.; Bruno, A.; Bruno, A.; Bruno, F.; Calandri, M.; Caruso, D.; Coppolino, P.; De Robertis, R.; Gentili, F.; et al. Diffusion-Weighted Imaging in Oncology: An Update. Cancers 2020, 12, 1493.
- Qayyum, A. Diffusion-weighted imaging in the abdomen and pelvis: Concepts and applications. Radiographics 2009, 29, 1797–1810.
- Yankeelov, T.E.; Gore, J.C. Dynamic Contrast Enhanced Magnetic Resonance Imaging in Oncology: Theory, Data Acquisition, Analysis, and Examples. Curr. Med. Imaging Rev. 2009, 3, 91–107.
- Tofts, P.S.; Brix, G.; Buckley, D.L.; Evelhoch, J.L.; Henderson, E.; Knopp, M.V.; Larsson, H.B.; Lee, T.-Y.; Mayr, N.A.; Parker, G.J.; et al. Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: Standardized quantities and symbols. J. Magn. Reson. Imaging 1999, 10, 223–232.
- Verma, S.; Turkbey, B.; Muradyan, N.; Rajesh, A.; Cornud, F.; Haider, M.A.; Choyke, P.L.; Harisinghani, M. Overview of dynamic contrast-enhanced MRI in prostate cancer diagnosis and management. AJR Am. J. Roentgenol. 2012, 198, 1277–1288.
- Hanchanale, V.; Yeo, L.; Subedi, N.; Smith, J.; Wah, T.; Harnden, P.; Bhattarai, S.; Chilka, S.; Eardley, I. The accuracy of magnetic resonance imaging (MRI) in predicting the invasion of the tunica albuginea and the urethra during the primary staging of penile cancer. BJU Int. 2016, 117, 439–443.
- Krishna, S.; Schieda, N.; Kulkarni, G.S.; Shanbhogue, K.; Baroni, R.H.; Woo, S. Diagnostic Accuracy of MRI in Local Staging (T Category) of Penile Cancer and the Value of Artificial Erection: A Systematic Review and Meta-Analysis. AJR Am. J. Roentgenol. 2022, 219, 28–36.
- Petralia, G.; Villa, G.; Scardino, E.; Zoffoli, E.; Renne, G.; de Cobelli, O.; Bellomi, M. Local staging of penile cancer using magnetic resonance imaging with pharmacologically induced penile erection. Radiol. Med. 2008, 113, 517–528.
- Ghosh, P.; Chandra, A.; Mukhopadhyay, S.; Chatterjee, A.; Lingegowda, D.; Gehani, A.; Gupta, B.; Gupta, S.; Midha, D.; Sen, S. Accuracy of MRI without intracavernosal prostaglandin E1 injection in staging, preoperative evaluation, and operative planning of penile cancer. Abdom. Radiol. 2021, 46, 4984–4994.
- Lucchesi, F.R.; Reis, R.B.; Faria, E.F.; Machado, R.D.; Rossini, R.R.; Borregales, L.D.; Silva, G.E.B.; Muglia, V.F. Incremental value of MRI for preoperative penile cancer staging. J. Magn. Reson. Imaging 2017, 45, 118–124.
- Bozzini, G.; Provenzano, M.; Otero, J.R.; Margreiter, M.; Cruz, E.G.; Osmolorskij, B.; Verze, P.; Pavan, N.; Sanguedolce, F.; Buffi, N.; et al. Role of Penile Doppler US in the Preoperative Assessment of Penile Squamous Cell Carcinoma Patients: Results from a Large Prospective Multicenter European Study. Urology 2016, 90, 131–135.
- Scardino, E.; Villa, G.; Bonomo, G.; Matei, D.; Verweij, F.; Rocco, B.; Varela, R.; de Cobelli, O. Magnetic resonance imaging combined with artificial erection for local staging of penile cancer. Urology 2004, 63, 1158–1162.
- Krishna, S.; Shanbhogue, K.; Schieda, N.; Morbeck, F.; Hadas, B.; Kulkarni, G.; McInnes, M.D.; Baroni, R.H. Role of MRI in Staging of Penile Cancer. J. Magn. Reson. Imaging 2020, 51, 1612–1629.
- Brouwer, O.R.; Albersen, M.; Parnham, A.; Protzel, C.; Pettaway, C.A.; Ayres, B.; Antunes-Lopes, T.; Barreto, L.; Campi, R.; Crook, J.; et al. European Association of Urology-American Society of Clinical Oncology Collaborative Guideline on Penile Cancer: 2023 Update. Eur. Urol. 2023, 83, 548–560.
- Thoeny, H.C.; De Keyzer, F.; King, A.D. Diffusion-weighted MR imaging in the head and neck. Radiology 2012, 263, 19–32.
- Takamura, M.; Kobayashi, T.; Nikkuni, Y.; Katsura, K.; Yamazaki, M.; Maruyama, S.; Tanuma, J.I.; Hayashi, T. A comparative study between CT, MRI, and intraoral US for the evaluation of the depth of invasion in early stage (T1/T2) tongue squamous cell carcinoma. Oral. Radiol. 2022, 38, 114–125.
- ESUR Guidelines on Contrast Agents. Available online: https://www.esur.org/esur-guidelines-on-contrast-agents/ (accessed on 25 October 2023).
- Wu, W.; Miller, K.L. Image formation in diffusion MRI: A review of recent technical developments. J. Magn. Reson. Imaging 2017, 46, 646–662.
- Warndahl, B.A.; Borisch, E.A.; Kawashima, A.; Riederer, S.J.; Froemming, A.T. Conventional vs. reduced field of view diffusion weighted imaging of the prostate: Comparison of image quality, correlation with histology, and inter-reader agreement. Magn. Reson. Imaging 2018, 47, 67–76.
- Hosseiny, M.; Sung, K.H.; Felker, E.; Suvannarerg, V.; Tubtawee, T.; Shafa, A.; Arora, K.R.; Ching, J.; Gulati, A.; Azadikhah, A.; et al. Read-out Segmented Echo Planar Imaging with Two-Dimensional Navigator Correction (RESOLVE): An Alternative Sequence to Improve Image Quality on Diffusion-Weighted Imaging of Prostate. Br. J. Radiol. 2022, 95, 20211165.
- Lawrence, E.M.; Zhang, Y.; Starekova, J.; Wang, Z.; Pirasteh, A.; Wells, S.A.; Hernando, D. Reduced field-of-view and multi-shot DWI acquisition techniques: Prospective evaluation of image quality and distortion reduction in prostate cancer imaging. Magn. Reson. Imaging 2022, 93, 108–114.
- Tamada, T.; Kido, A.; Ueda, Y.; Takeuchi, M.; Kanki, A.; Neelavalli, J.; Yamamoto, A. Comparison of single-shot EPI and multi-shot EPI in prostate DWI at 3.0 T. Sci. Rep. 2022, 12, 16070.
- Grant, K.B.; Agarwal, H.K.; Shih, J.H.; Bernardo, M.; Pang, Y.; Daar, D.; Merino, M.J.; Wood, B.J.; Pinto, P.A.; Choyke, P.L.; et al. Comparison of calculated and acquired high b value diffusion-weighted imaging in prostate cancer. Abdom. Imaging 2015, 40, 578–586.
- Jendoubi, S.; Wagner, M.; Montagne, S.; Ezziane, M.; Mespoulet, J.; Comperat, E.; Estellat, C.; Baptiste, A.; Renard-Penna, R. MRI for prostate cancer: Can computed high b-value DWI replace native acquisitions? Eur. Radiol. 2019, 29, 5197–5204.
- Baltzer, P.; Mann, R.M.; Iima, M.; Sigmund, E.E.; Clauser, P.; Gilbert, F.J.; Martincich, L.; Partridge, S.C.; Patterson, A.; Pinker, K.; et al. Diffusion-weighted imaging of the breast-a consensus and mission statement from the EUSOBI International Breast Diffusion-Weighted Imaging working group. Eur. Radiol. 2020, 30, 1436–1450.
- Switlyk, M.D. Magnetic resonance imaging for assessing treatment response in bone marrow metastases. Acta Radiol. 2021, 62, 483–499.
- Tang, M.; Huang, R.; Chen, J.; Sheng, M.; Zhang, Z.; Xing, J.; Guo, L.; Li, Y. Clinical value of high-resolution dynamic contrast-enhanced (DCE) MRI in diagnosis of cutaneous squamous cell carcinoma. Ski. Res. Technol. 2021, 27, 511–520.
- Menon, S.; Moch, H.; Berney, D.; Cree, I.; Srigley, J.; Tsuzuki, T.; Compérat, E.; Hartmann, A.; Netto, G.; Rubin, M.; et al. WHO 2022 classification of penile and scrotal cancers: Updates and evolution. Histopathology 2023, 82, 508–520.
- Chahoud, J.; Zacharias, N.M.; Pham, R.; Qiao, W.; Guo, M.; Lu, X.; Alaniz, A.; Segarra, L.; Martinez-Ferrer, M.; Gleber-Netto, F.O.; et al. Prognostic Significance of p16 and Its Relationship with Human Papillomavirus Status in Patients with Penile Squamous Cell Carcinoma: Results of 5 Years Follow-Up. Cancers 2022, 14, 6024.
- Steinestel, J.; Al Ghazal, A.; Arndt, A.; Schnoeller, T.J.; Schrader, A.J.; Moeller, P.; Steinestel, K. The role of histologic subtype, p16(INK4a) expression, and presence of human papillomavirus DNA in penile squamous cell carcinoma. BMC Cancer 2015, 15, 220.
- Mentrikoski, M.J.; Stelow, E.B.; Culp, S.; Frierson, H.F., Jr.; Cathro, H.P. Histologic and immunohistochemical assessment of penile carcinomas in a North American population. Am. J. Surg. Pathol. 2014, 38, 1340–1348.
- D’Aniello, C.; Cavaliere, C.; A Facchini, B.; D’Errico, D.; Capasso, M.; Iovane, G.; Romis, L.; Mordente, S.; Liguori, C.; Cicala, S.; et al. Penile cancer: Prognostic and predictive factors in clinical decision-making. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 12093–12108.
- Cubilla, A.L. The role of pathologic prognostic factors in squamous cell carcinoma of the penis. World J. Urol. 2009, 27, 169–177.
- Sanchez, D.F.; Fernandez-Nestosa, M.J.; Canete-Portillo, S.; Rodriguez, I.; Cubilla, A.L. What Is New in the Pathologic Staging of Penile Carcinoma in the 8th Edition of AJCC TNM Model: Rationale for Changes with Practical Stage-by-stage Category Diagnostic Considerations. Adv. Anat. Pathol. 2021, 28, 209–227.
- Brierley, J.D.; Gospodarowicz, M.K.; Wittekind, C. (Eds.) TNM Classification of Malignant Tumors, 8th ed.; Wiley: West Sussex, UK, 2016.
- Lindner, A.K.; Schachtner, G.; Steiner, E.; Kroiss, A.; Uprimny, C.; Steinkohl, F.; Horninger, W.; Heidegger, I.; Madersbacher, S.; Pichler, R. Organ-sparing surgery of penile cancer: Higher rate of local recurrence yet no impact on overall survival. World J. Urol. 2020, 38, 417–424.
- Kieffer, J.M.; Djajadiningrat, R.S.; van Muilekom, E.A.; Graafland, N.M.; Horenblas, S.; Aaronson, N.K. Quality of life for patients treated for penile cancer. J. Urol. 2014, 192, 1105–1110.
- Philippou, P.; Shabbir, M.; Malone, P.; Nigam, R.; Muneer, A.; Ralph, D.J.; Minhas, S. Conservative surgery for squamous cell carcinoma of the penis: Resection margins and long-term oncological control. J. Urol. 2012, 188, 803–808.
- Romero, F.R.; Romero, K.R.; Mattos, M.A.; Garcia, C.R.; Fernandes Rde, C.; Perez, M.D. Sexual function after partial penectomy for penile cancer. Urology 2005, 66, 1292–1295.
- Veeratterapillay, R.; Teo, L.; Asterling, S.; Greene, D. Oncologic Outcomes of Penile Cancer Treatment at a UK Supraregional Center. Urology 2015, 85, 1097–1103.
- Kearns, J.T.; Winters, B.D.; Holt, S.K.; Mossanen, M.; Lin, D.W.; Wright, J.L. Pathologic Nodal Involvement in Patients With Penile Cancer With Cavernosal Versus Spongiosal Involvement. Clin. Genitourin. Cancer 2019, 17, e156–e161.
- Li, Z.; Ornellas, A.A.; Schwentner, C.; Li, X.; Chaux, A.; Netto, G.; Burnett, A.L.; Tang, Y.; Geng, J.; Yao, K.; et al. A modified clinicopathological tumor staging system for survival prediction of patients with penile cancer. Cancer Commun. 2018, 38, 68.
- Menon, H.; Patel, R.R.; Ludmir, E.B.; Muralidhar, V.; Cushman, T.R.; Amini, A.; Seyedin, S.N.; Nguyen, P.L.; Verma, V. Local management of preinvasive and clinical T1-3 penile cancer: Utilization of diverse treatment modalities. Future Oncol. 2020, 16, 955–960.
- Sali, A.P.; Menon, S.; Murthy, V.; Prakash, G.D.; Bakshi, G.M.; Joshi, A.D.; Desai, S.B. A Modified Histopathologic Staging in Penile Squamous Cell Carcinoma Predicts Nodal Metastasis and Outcome Better Than the Current AJCC Staging. Am. J. Surg. Pathol. 2020, 44, 1112–1117.
- Ornellas, A.A.; Nobrega, B.L.; Wei Kin Chin, E.; Wisnescky, A.; da Silva, P.C.; de Santos Schwindt, A.B. Prognostic factors in invasive squamous cell carcinoma of the penis: Analysis of 196 patients treated at the Brazilian National Cancer Institute. J. Urol. 2008, 180, 1354–1359.
- Velazquez, E.F.; Ayala, G.; Liu, H.; Chaux, A.; Zanotti, M.; Torres, J.; Cho, S.I.; Barreto, J.E.; Soares, F.; Cubilla, A.L. Histologic grade and perineural invasion are more important than tumor thickness as predictor of nodal metastasis in penile squamous cell carcinoma invading 5 to 10 mm. Am. J. Surg. Pathol. 2008, 32, 974–979.
- Ravi, R. Correlation between the extent of nodal involvement and survival following groin dissection for carcinoma of the penis. Br. J. Urol. 1993, 72, 817–819.
- Svatek, R.S.; Munsell, M.; Kincaid, J.M.; Hegarty, P.; Slaton, J.W.; Busby, J.E.; Gaston, K.E.; Spiess, P.E.; Pagliaro, L.C.; Tamboli, P.; et al. Association between lymph node density and disease specific survival in patients with penile cancer. J. Urol. 2009, 182, 2721–2727.
- Bloom, J.B.; Stern, M.; Patel, N.H.; Zhang, M.; Phillips, J.L. Detection of lymph node metastases in penile cancer. Transl. Androl. Urol. 2018, 7, 879–886.
- Lont, A.P.; Besnard, A.P.; Gallee, M.P.; van Tinteren, H.; Horenblas, S. A comparison of physical examination and imaging in determining the extent of primary penile carcinoma. BJU Int. 2003, 91, 493–495.
- de Kerviler, E.; Ollier, P.; Desgrandchamps, F.; Zagdanski, A.M.; Attal, P.; Teillac, P.; Frija, J.; Le Duc, A.; Laval-Jeantet, M. Magnetic resonance imaging in patients with penile carcinoma. Br. J. Radiol. 1995, 68, 704–711.
- Horenblas, S.; Kroger, R.; Gallee, M.P.; Newling, D.W.; van Tinteren, H. Ultrasound in squamous cell carcinoma of the penis; a useful addition to clinical staging? A comparison of ultrasound with histopathology. Urology 1994, 43, 702–707.
More
