Plant-derived products, which have been used in traditional medicine for treating pathological conditions, offer structurally novel therapeutic compounds, including those with anti-viral activity. In addition, plant-derived bioactive substances might serve as the ideal basis for developing sustainable/efficient/cost-effective anti-viral alternatives. Interest in herbal antiviral products has increased. More than 50% of approved drugs originate from herbal sources. Plant-derived compounds offer diverse structures and bioactive molecules that are candidates for new drug development. Combining these therapies with conventional drugs could improve patient outcomes. Epigenetics modifications in the genome can affect gene expression without altering DNA sequences. Host cells can use epigenetic gene regulation as a mechanism to silence incoming viral DNA molecules, while viruses recruit cellular epitranscriptomic (covalent modifications of RNAs) modifiers to increase the translational efficiency and transcript stability of viral transcripts to enhance viral gene expression and replication.
- plant-derived substances
- epigenetic modifications
- virus
- broad-spectrum anti-viral
1. Introduction
2. Plant-Derived Epi-Nutraceuticals as Potential Broad-Spectrum Anti-Viral Agents
2.1. Andrographolide
2.2. Apigenin
Apigenin (4′,5,7-trihydroxyflavone) is a flavone found in a variety of plants, including medicinal plants [28]. Apigenin displayed a potent histone deacetylases (HDAC) inhibitor activity in human prostate cancer PC-3 cells, specifically decreasing HDAC1 and HDAC3 activity [29]. It also increased the global acetylation of histones H3 and H4 and directed histone H3 hyperacetylation to the p21/WAF1 promoter [29]. Furthermore, molecular studies revealed that apigenin enhances acetylated H3, particularly in the p21WAF1/CIP1 promoter region, leading to upregulating p21WAF1/CIP1 transcription [30]. Apigenin exhibited antiviral properties against IAV, human rhinovirus (HRV), HSV, enterovirus, HBV, HCV, EV71, and SARS-CoV-2 [31][32][33][34]. Its anti-viral activity is attributed, in part, to the inhibition of HDAC activity and chromatin remodeling [29][35]. Apigenin, linalool, and ursolic acid showed a strong anti-viral activity towards coxsackievirus B1 (CVB1) [36].2.3. Baicalein
Baicalein is a flavonoid derived from the roots of Scutellaria baicalensis Georgi., a traditional Chinese medicinal herb [37]. It has been investigated for its anti-viral properties against a variety of viruses, including HBV [38], HIV [39], DENV [40], and HSV-1 [41]. Baicalein was reported to inhibit DNA methyltransferases (DNMT) and HDAC and thereby influence epigenetic modifications [42][43]. Baicalein inhibited HDAC-1/8, causing growth suppression and differentiation induction in acute myeloid leukemia (AML) cell lines. Baicalein might activate HDAC-1 degradation mediated by the ubiquitin-proteasome pathway, thereby increasing histone H3 acetylation [42].2.4. Berberine
Berberine (BBR) is a quaternary ammonium salt of the protoberberine group of benzylisoquinoline alkaloids found in plants such as Berberis vulgaris L. [44], which exhibited anticancer activity by affecting epigenetic regulation and AMP-activated protein kinase (AMPK) activation [45]. Berberine has exceptional anticancer effects via affecting the enzyme involved in histone acetylation and methylation in acute myeloid leukemia (AML) cell lines [46] and the suppression of Sirtuin 1 (SIRT1) deacetylases in a p53-dependent manner [47]. Berberine inhibited miR-21 expression and promoted integrin β4 (ITGβ4) and programmed cell death 4 (PDCD4) protein expression in colon cancer cell lines. The overexpression of miR-21 reduced the anti-cancer effects of berberine on cancer cells [48]. BBR was reported to influence multiple biological activities, including anticancer, anti-inflammatory, and anti-viral activities [49]. BBR targets multiple steps of the viral life cycle, rendering it an excellent candidate for use in innovative anti-viral drugs and therapies. BBR was discovered to inhibit viral replication by targeting specific interactions between a virus and its host. BBR binds to DNA, inhibiting DNA synthesis and reverse transcriptase activity. It was shown to inhibit the replication of HSV [50], HCMV [51], human papillomavirus (HPV) [52], DENV [53], HIV [54], HCV [55], and SARS-CoV-2 [56]. BBR exhibited anti-viral effects on IAV both in vitro and in vivo [57]. BBR possesses the ability to control the Mitogen-activated protein kinase(MEK)/extracellular-signal-regulated kinase (ERK)EK-ERK, AMPK/mammalian target of rapamycin (mTOR), and nuclear factor kappa B (NF-κB)NF-κB signaling pathways, which are all necessary for viral replication. Protein phosphorylation is crucial in the infection cycle of many viruses [58], affecting cellular protein’s stability, activity, interaction with other proteins, and infectivity. Viruses like EBV [59], HCV [59], SARS-CoV-2, DENV [60], and others [61][62][63], rely on MAPK p38 for replication, suggesting that MAPK p38 inhibitors may exhibit broad-spectrum anti-viral activity. Varghese et al. discovered that BBR significantly reduces MAPK activity. The p38 mitogen-activated protein kinases (p38), extracellular signal-regulated kinases (ERK), and JNK signaling pathways are all significantly blocked by BBR, which specifically targets the ERK signaling pathway, resulting in a significant decrease in virion production. The reduction in viral protein expression following BBR treatment is most likely due to a decrease in virus-induced signaling. BBR treatment has no effect on virus entry or viral replicas’ enzymatic activity [64]. Additionally, it has been shown that BBR has the ability to suppress p38 MAPK activity in the context of HBV infection. The virion of HBV comprises a genome consisting of partially double-stranded relaxed circular DNA (rcDNA). Upon infecting a cell, this rcDNA is transformed into covalently closed circular DNA (cccDNA) in the nucleus. MAPK p38 activity plays a crucial role in the preservation of HBV covalently closed circular DNA (cccDNA) within infected cells [65]. The cccDNA functions as a molecular scaffold for the transcription of RNA molecules, such as mRNAs and pregenomic RNAs (pgRNAs).2.5. Betulinic Acid
Betulinic acid (BA) is a naturally occurring pentacyclic triterpenoid found in the bark of various plant species, most notably the white birch (Betula pubescens Ehrh.) [66]. BA is capable of inducing apoptosis in tumor cells by directly activating the mitochondrial apoptosis pathway via a p53- and CD95-independent mechanism [67]. A computational approach demonstrated that BA has the capacity to alter HDAC6 and HDAC10 activities [68]. Furthermore, BA exhibited an anti-cancer activity that is mediated through cannabinoid receptors (CBs). BA functions as both a CB1 antagonist and a CB2 agonist [69]. BA was used for the treatment of various viral diseases [70]. BA has demonstrated activity in inhibiting DENV-2 NS5 polymerase [71]. Furthermore, BA exhibited an inhibitory effect on HBV replication [72]. Interestingly, the C-3 esterification of BA led to the discovery of Bevirimat, an HIV-1 maturation inhibitor patented by Sanofi-Aventis.2.6. Butyric Acid
Butyric acid is a fatty acid derived from multiple vegetable sources that have anticancer activity through several pathways, including its influence on epigenetic machineries. Butyrate, alone or in combination with other drugs, including nicotinamide (NA), was shown to have anticancer activity in vivo [73]. Butyric acid exerts its anti-tumor effect by increasing HDAC expression and activity, which is accompanied by an upregulation of miR-203 promoter methylation [73]. Butyrate inhibited HBV replication and cell proliferation by inhibiting SIRT-1 expression in hepatoma cells. Specifically, butyrate inhibited HBx protein expression, HBV-DNA, and hepatitis B surface antigen (HBsAg) [74].2.7. Cardamonin
Cardamonin (CDN) is a natural chalcone isolated from the seeds of Alpinia katsumadai Hayata [75]. CDN has been shown to have a variety of pharmacological activities, including anticancer and anti-inflammatory properties [76]. It was recently revealed that CDN has anti-viral activity against the human coronavirus HCoV-OC43. CDN exhibits significant efficacy in reducing HCoV-OC43-induced cytopathic effects. CDN suppressed HCoV-OC43 infection by promoting the p38 MAPK signaling pathway and having therapeutic potential against other human coronaviruses [76].2.8. Cordycepin
Cordycepin is a nucleotide analog derived from Cordyceps mushrooms [77]. In SNU719 cells, cordycepin treatment enhanced BAF chromatin remodeling complex subunit 7A (BCL7A) methylation while suppressing demethylation [78]. Cordycepin promoted methylation at EBV genomic sites near its Fp/Qp promoters. These findings indicate that cordycepin enhances DNMT3 activation, hence increasing the methylation of both genomic and EBV DNA loci in SNU719 cells [78], causing reduced EBV replication [79]. Cordycepin was also shown to have anti-SARS-CoV-2 replication activity [79]. Cordycepin shows anti-viral activities that are attributable to its ability to inhibit several protein kinases [77]. Cordycepin, an adenosine derivative, differs from adenosine in that its ribose lacks an oxygen atom in the 3′ position [80].2.9. Corosolic Acid
Corosolic acid (CA) is a triterpene acid isolated from Lagerstroemia speciose L. [81]. This bioactive molecule is prevalent in foods such as guava, loquat, and olive, and has anti-inflammatory, anti-metabolic syndrome, and anti-neoplasic properties [82]. CA is implicated in the regulation of DNA methylation and histone H3 methylation. CA modulates CpG methylation sites, resulting in altered gene expression in treated cells [83]. Furthermore, CA inhibits the production and activity of epigenetic modulatory proteins, suggesting its capacity to prevent prostate carcinogenesis [84].2.10. Curcumin
Curcumin, the major bioactive in turmeric, is a polyphenol with anti-inflammatory and anti-cancer activities [85]. Curcumin has been demonstrated to be a powerful epigenetic regulator with multiple effects on HDAC expression and activity. Curcumin decreased the expression of HDAC1, HDAC3, and HDAC8 proteins, as well as histone acetyltransferase p300, while enhancing the acetylation of Ac-histone H4 protein [86]. Curcumin was shown to reduce HAT activity and has been proposed as a potential DNMT and HDAC inhibitor [87]. Curcumin reduced the amount of HBsAg and the number of cccDNA copies, resulting in the inhibition of HBV replication, which was accompanied by a decrease in the acetylation level of cccDNA-bound histone H3 and H4 [88]. An MiRNA array revealed that miR-350, miR-17-2-3p, let 7e-3p, miR-1224, miR-466b-1-3p, miR-18a-5p, and miR-322-5p were downregulated by curcumin while miR-122-5p, miR-3473, miR-182, and miR-344a-3p were upregulated [89]. Overall, the curcumin-modified miRNAs had an impact on a number of signaling pathways, such as Wnt, NK-κB, MAPK, inflammatory response genes, and viral transmission [90].2.11. Ellagic Acid
Ellagic acid (EA) is a ubiquitous phenolic molecule isolated from a variety of fruits and vegetables and is well known for its anti-cancer effect [91]. This bioactive substance has been demonstrated to effectively induce HDAC activity. Human adipogenic stem cells treated with EA showed a substantial increase in HDACs’ gene expression. EA also suppresses adipocyte differentiation through coactivator-associated arginine methyltransferase 1 (CARM1)-mediated chromatin modification. This compound also inhibited adipocyte growth and differentiation by increasing histone arginine methylation [92], resulting in an increase in acetylated histone through epigenetic alterations mediated by coactivator-associated CARM1 inhibition. CARM1 inhibition was shown to limit H3R17 methylation, resulting in decreased H3K9 acetylation and HDAC9 dissociation [92]. Ellagic acid and other plant-derived substances are strongly bound with the multiple targets of the SARS-CoV-2 receptors, inhibiting viral entry, attachment, binding, replication, transcription, maturation, packaging, and spread [93].2.12. Epigallocatechin Gallate
Epigallocatechin gallate (EGCG) is the most abundant catechin in tea leaves, comprising 50–80% of the total catechins [94]. EGCG was recognized as the primary contributor to the numerous health benefits associated with green tea [94], including a reduction in the symptoms of infectious diseases [95]. EGCG binds to various targets and exerts its influence on the activity of diverse enzymes and signal transduction pathways [96]. Studies with animal models and various cancer cell lines have shown that EGCG and other catechins modulate the activity of DNMTs [97]. Fang et al. suggested that EGCG inhibited DNMT activity, resulting in the reactivation of methylation-silenced genes [98]. In fact, EGCG can reduce DNA methylation through the direct inhibition of the activity of DNMT 1, DNMT 3a, and DNMT 3b, by directly binding to the active site within the enzyme [97]. EGCG also regulates histone modifications by inhibiting the activity of HDACs [99] and consequently inducing changes in gene expression patterns. The inhibition of HDAC activity by EGCG results in a decrease in HDAC enzyme activity and consequently leads to increased levels of acetylation on histone proteins both globally and at specific regions. In human colon cancer cell lines, EGCG inhibited HDAC1, HDAC2, and HDAC3 expression [100]. In addition, EGCG inhibited histone acetyltransferase (HAT) activity [101]. EGCG has been demonstrated to prompt the increased acetylation of lysine 14 and 9 (on histone H3) and lysine 12, 5, and 16 (H3-Lys and H4-Lys) levels [102]. EGCG has also been implicated as a potential modulator of miRNAs by regulating the expression levels of epigenetic modifiers or viral proteins. EGCG has been reported to decrease the levels of let-7e-5p, miR-103a-3p, miR-151a-5p, miR-195-5p, miR-222-3p, miR-23a-3p, miR-23b-3p, miR-26a-5p, miR-27a-3p, miR-29b-3p, miR- 3195, miR-3651, miR-4281, miR-4459, miR-4516, miR-762, and miR-125b-5p [103]. Another study showed that EGCG enhances the expression of miR-3663-3p, miR-1181, miR-3613-3p, miR1281, and miR-1539, while decreasing miR-221-5p, miR-374b, miR-4306, miR-500a-5p, and miR590-5p in human dermal papilla cells [104] and miR-140-3p and miR-221 in melanoma and hepatoma cell lines, respectively [105][106]. The anti-viral properties of EGCG have been demonstrated for a wide range of virus families, including Retroviridae, Orthomyxoviridae, and Flaviviridae. EGCG also exerts anti-viral activity by modulating miRNA expression, such as upregulating miR-548m expression. Reports found miR-548m binding sites in the 3′UTR of CD81 mRNA′. This suggests that miR-548m may lower the expression of CD81, which would make HCV less likely to infect cells. These results suggest that EGCG may act as an anti-HCV drug by increasing the expression of miR-548m while decreasing the expression of the CD81 receptor required for HCV infection [107]. The liver-specific miR-122 [108] is the most abundant miRNA in the liver, accounting for 60–70% of the total miRNA in hepatocytes. Many investigations have found that miR-122 is required for HCV replication in infected cells [109][110][111]. EGCG (and also resveratrol) modulates the expression levels of miR-122 and thus might exert an anti-HCV effect via this mechanism. IAV infection caused a significant decrease in micro-RNA let-7 expression in host cells that normally regulate the expression of type I interferon required for the host cells’ anti-viral activity. The overexpression of let-7 increased the expression of the interferon and effectively inhibited the IAV infection. EGCG upregulates the expression of let-7 and thereby has the potential to exhibit anti-influenza activity [112] (Figure 1).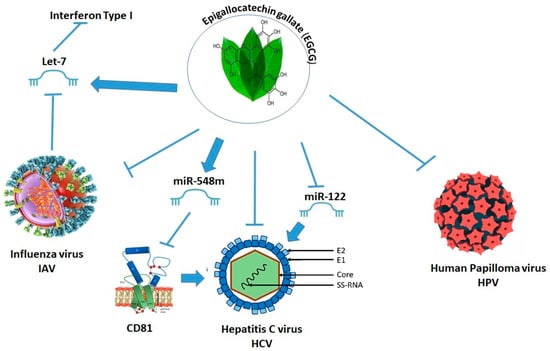
2.13. Galangin
Galangin is a naturally occurring flavonoid found in honey that is also an active ingredient in galangal, a spice used in traditional Chinese medicine [113]. This natural compound appears to effectively inhibit HDAC activity. In SH-SY5Y human neuroblastoma cells, treatment with galangin increased endogenous HDAC1-mediated deacetylation independently of DNA methylation status and subsequently decreased histone H3 acetylation in BACE1 promoter regions [114]. Galangin upregulates miR-455-5p to modulate the regulatory subunit 12A of protein phosphatase 1 (PPP1R12A). This effect suppresses the activation of the MAPK and the phosphoinositide 3-kinases/protein kinase B (PI3K/AKT) pathways, controlling cancer cell survival and metastasis [115]. Galangin showed significant antiviral activity against HSV-1 and Coxsackie B1 (CoxB1) [116].2.14. Garcinol
Garcinol is a polyisoprenylated benzophenone isolated from the peel of the Garcinia indica Choisy fruit [117]. Garcinol has anti-cancer, anti-inflammatory, and antioxidant properties [118]. In tumor cells, it primarily inhibits the NF-κB and Janus kinase (JAK)/STAT3 transcription factors [117]. Garcinol has been shown to decrease the HAT activity of p300 and pCAF in vitro and in vivo [119]. As a result, garcinol was discovered to be a potent inducer of apoptosis and to affect global gene expression in HeLa cells [119]. The chemical structure of garcinol shows some similarities with curcumin (β-diketone, phenol). Garcinol has shown significant anticancer activity by targeting NF-κB, 5-lipoxygenase (5- LOX), and STAT proteins [120][121].2.15. Genistein
Genistein is a naturally occurring isoflavone isolated from the plant Genista tinctoria [122] and is well known for its potential chemotherapeutic action against a variety of cancer cells. Studies on HAT and HDAC activity revealed that genistein reduces HDAC while increasing HAT activity [122]. In prostate cancer cell lines, a chromatin immunoprecipitation analysis with multiple antibodies revealed the enrichment of acetylated histones H3, H4, and H3 di- and tri-methylated lysine 4 after incubation with genistein [123]. Furthermore, genistein inhibited the phosphorylation of serine 10 and the methylation of lysine 9 in the promoter regions of several genes, including wingless-related integration site (Wnt5a), as well as induced the secretion of frizzled-related protein 5 (Sfrp5), and frizzled-related protein 2 (Sfrp2) [124].2.16. Ginkgolic Acid
Ginkgolic acid (GIA) is a phenolic acid found in Ginkgo biloba L. with neuroprotective, antimicrobial, and antitumor properties [125]. Ginkgo biloba has been used in traditional Chinese medicine since at least the 11th century BC to treat various ailments, such as dementia, asthma, bronchitis, and kidney and bladder diseases. Ginkgolic acid is a potent multitarget inhibitor of key enzymes in the biosynthesis of proinflammatory substances [125]. Ginkgolic acid impairs SUMOylation by blocking the formation of an E1-SUMO thioester complex by binding directly to E1 [126]. SUMOylation is a process by which small ubiquitin-related modifier proteins (SUMO) covalently bind to specific lysine residues in target proteins, thereby regulating various aspects of protein function, including transcription, subcellular localization, DNA repair, and the cell cycle [127]. JMJD2A, a member of the Jumonji domain 2 (JMJD2) family, is the histone demethylase responsible for the accumulation of SUMO-2/3. JMJD2A is SUMOylated at lysine 471 by Kaposi’s sarcoma-associated herpesvirus (KSHV) K-bZIP, a viral SUMO-2/3-specific E3 ligase, in a SUMO-interacting motif (SIM)-dependent manner. SUMOylation is required for the stabilization of chromatin association and gene transactivation by JMJD2A [128]. Recently, ginkgolic acid was reported to inhibit HSV-1 by disrupting the virus’ structure, blocking fusion, and inhibiting viral protein synthesis [129].2.17. Glycyrrhizic Acid
Glycyrrhizic acid (GA) is a triterpene isolated from the roots and rhizomes of licorice (Glycyrrhiza glabra L.)) [130]. GA is the principal bioactive ingredient of licorice with anti-viral [131], anti-inflammatory, and hepatoprotective effects [132]. The licorice plant is native to Europe and Asia and has been used for centuries in traditional medicine. Ancient documentations from China, India, and Greece stated that it was traditionally used to alleviate the symptoms of viral respiratory tract infections and hepatitis [130]. Licorice is known for its ability to inhibit the viral replication of various viruses including HBV, HCV, IAV H1N1, and HIV, as reviewed by Zhong et al. [133]. Licorice extract containing glycyrrhiza inhibited the replication of Newcastle disease virus (NDV) and was non-toxic in an embryonic egg assay [134]. Glycyrrhizin exhibited antiviral activity by affecting cellular signaling pathways and increasing the expression of nitrous oxide synthase (NOS) [135]. Presumably by controlling the expression of the NF-κB and PI3K signaling pathways, glycyrrhizin’s anti-inflammatory impact may be obtained [136]. Glabridin licorice (Glycyrrhiza glabra) contains significant amounts of the isoflavan glabridin, which demonstrated anti-inflammatory and neuro- and cardioprotective activities in addition to distinct anti-cancer properties (growth inhibition as well as anti-angiogenic and anti-metastatic effects [137][138][139]. Glabridin suppressed cancer stem-cell-like features in hepatocellular carcinoma cells by the upregulation of miR-148a that targets SMAD2 (mothers against decapentaplegic homolog 2) associated with the inhibition of TGF (transforming growth factor)-β/SMAD2 signaling [140][141].2.18. Grifolin
Grifolin is an adenosine derivative isolated from the fresh fruiting bodies of the fungus Albatrellus confluens (Alb. & Schwein.) Kotl. & Pouz. [142]. Grifolin was shown to suppress tumor cell lines’ proliferation. Grifolin inhibited Bcl-2 expression while increasing Bax expression [142]. Grifolin reduced ETS Like-1 protein (Elk1) transcription as well as its binding to the DNMT1 promoter region. The mRNA levels of pTEN and tissue inhibitor of metalloproteinases 2 (Timp2) Timp2 are likewise increased by griforolin. Grifolin’s anti-tumor effects may be exerted by ERK1/2-Elk1-DNMT1 signaling’s epigenetic activation of metastasis-inhibitory genes [143].2.19. Oleacein
Oleacein, a secoiridoid [144], is the most prominent phenolic compound in Olea europaea L. (olive). This substance exhibited anti-cancer activity against multiple myeloma cell lines (NCI-H929, RPMI-8226, U266, MM1s, and JJN3) and was found to be an effective epigenetic modulator. Oleacein was reported to downregulate several class I/II HDACs both at the mRNA and protein level; conversely, no effect on global DNA methylation was associated with this compound [145][146]. Oleacein inhibited the proliferation of numerous melanoma cell lines [147]. It has been shown that oleacein can stop HIV-1 infection, replication, and the production of the viral core antigen p24 [148].2.20. Organosulfur Chemicals
Organosulfur chemicals (OSC) are a group of compounds found in garlic (Allium sativum L.). More than thirty sulfur-containing compounds have been identified so far [149]. Garlic extracts were found to have broad-spectrum anti-viral activity [150]. Conversely, the mechanism by which these extracts or their purified constituents exert anti-viral activity may differ depending on the virus strains and viral lifecycle, which includes viral entry, fusion, replication, assembly, and virus–host-specific interactions [151]. Garlic has been used as an ethnomedicinal herb to cure infectious diseases for ages [152]. It has been utilized to treat a variety of illnesses in African traditional medicine, including sexually transmitted diseases, Mycobacterium tuberculosis (TB), respiratory tract infections, and wounds [153][154]. Garlic was shown to have effects against viral infections in humans, animals, and plants. In addition to garlic extracts or powders, purified bioactive components from garlic also exhibited anti-viral activity. As an example, alliin (S-allyl-L-cysteine sulfoxide), which is the most abundant sulfur compound found in fresh and dry garlic [155], is rapidly converted into allicin (diallyl thiosulfinate) by alliinase enzymes when fresh garlic is chopped, minced, crushed, or chewed [155][156]. Allicin is the primary component responsible for its anti-viral activity [157][158], immunomodulatory characteristics [159], anti-inflammatory [160] and antioxidant [161] activities, and other pharmacological properties. Allicin is very unstable and breaks down into other OSCs, such as andajoen, vinyl dithiins, diallyl disulfide (also known as garlicin or DADS), diallyl trisulfide (also known as allitridin or DATS), and diallyl disulfide (also known as DAS). In vivo, allicin can interact with cellular thiols such as glutathione and L-cysteine to form S-allyl mercapto glutathione (SAMG) and S-allyl mercaptocysteine (SAMC) [162][163]. These compounds may be responsible for structural changes in pathogen proteins [156]. Preclinical in vitro and in vivo studies have shown that allicin-derived OSCs such as ajoene, allitridine, garlicin, and DAS have antiviral [164][165][166][167][168], immunostrengthening [169][170][171], and other therapeutic activities [162][172][173].2.21. Orobol 7-O-d-Glucoside
Orobol 7-O-d-glucoside (O7G) isolated from banaba Lagerstroemia speciosa L. (Lythraceae) [174] was tested for its anti-viral efficacy against eight different strains of HRV, a cause of common viral respiratory tract disease [174]. O7G displayed anti-viral activity against HRV A and B, as well as species resistance to pleconaril, a potent capsid inhibitor of HRVs [175].2.22. Orsaponin
Orsaponin (OSW-1) is a natural substance derived from the bulbs of the plant Ornithogalum saundersiae which has anti-proliferative and anti-cancer properties [176]. Enteroviruses (EV) use oxysterol-binding protein (OSBP) as a host lipid transport protein [177]. Several studies have shown that OSW-1 binds to one of the two identified OSBP ligand-binding sites and exerts prophylactic antiviral activity against all enteroviruses tested, including EV71, coxsackievirus A21 (CVA21), and HRV-B [178][179].2.23. Plitidepsin
Plitidepsin is a cyclic depsipeptide isolated from the Mediterranean marine tunicate Aplidium albicans [180]. Plitidepsin is made and sold as alpidine, a drug that has been approved for a limited number of uses to treat multiple myeloma. Its target is eukaryotic translation elongation factor 1A (eEF1A) [181]. This cellular component is necessary for enzymes to move aminoacyl-tRNAs to the ribosome. It has also been found to be an important host factor in the replication of many viruses, such as RSV and gastroenteritis coronavirus [182].2.24. Pterostilbene
Pterostilbene (3,5-dimethoxy-4-hydroxystilbene) is a bioactive chemical found in grapes and several berries, mainly blueberries [183]. Pterostilbene alters gene expression in breast cancer cells, which are mediated by epigenetic mechanisms such as HDAC modifications [184]. It inhibits SIRT1 and regulates cell proliferation, apoptosis, stress response, metabolism, cellular senescence, and cancer [184][185].2.25. Quercetin
Quercetin is a flavonoid found in many medicinal plants and food products [186]. This compound has a variety of biological properties, including anticancer activity, through several modes of action. Quercetin, alone or in combination with other drugs, promotes epigenetic modifications. It enhances histone H3 acetylation via FasL overexpression, the activation of HAT, and the inhibition of HDAC activities [187]. Furthermore, quercetin reduced HMT activities, particularly HMT-H3K9 activity [46].2.26. Raoulic Acid
Raoulic acid isolated from Raoulia australis [12] has shown possible anti-viral activity against coxsackievirus B3 (CVB3) and coxsackievirus B4 (CVB4), as well as HRV types A and B [12].2.27. Resveratrol
Resveratrol (3,5,4′-trihydroxystilbene) is a bioactive molecule isolated by Saiko et al. [188] from the roots of white hellebore (Veratrum grandiflorum Loes.). More than 50 plant species contain this bioactive substance, including grapes, apples, blueberries, plums, and peanuts. It has been intensively researched for its health benefits against a variety of diseases, including cancer [189]. Resveratrol treatment increased p21 expression in Caski cells via the inhibition of HDAC [190]. HDAC activity is decreased by resveratrol in a dose-dependent manner [191]. Pterostilbene is a phytoalexin dimethyl ether molecule that is a dimethoxylated derivative of resveratrol [192].2.28. Silibinin
Silibinin is a flavonolignan derived from milk thistle [193] and has powerful anticancer effects, targeting multiple checkpoints, including epigenetic processes such as HDAC activity. Silibinin was shown to inhibit the expression of HDAC2 and HDAC3 proteins, as well as HDAC1, HDAC6, SET domain proteins (SETD1A, D4, D6), and lysine-specific demethylases (KDM 5B, 5C, and 4A) are some of these [124]. Silibinin also inhibited the expression of HDAC1-2 in DU145 and PC3 human prostate cancer cell lines [194].2.29. Silvestrol
Silvestrol, isolated from Aglaia plants [195], has been shown to target eukaryotic initiation factor-4A (eIF4A), an RNA helicase whose activity is required to unravel RNA secondary structures in the 5’-untranslated region (5′-UTRs) and facilitate translation initiation [196]. Silvestrol showed activity against EBOV, ZIKV, CHIKV, and coronaviruses [197][198][199].2.30. Sulforaphane
Sulforaphane (1-isothiocyanato-4(methylsulfinyl)butane) (SFN) is an isothiocyanate present mostly in cruciferous vegetables including broccoli, cabbage, brussel sprouts, and radishes [200]. In breast cancer cells, SFN significantly reduced HDAC activity [201] and increased the expression of acetylated histones H3 and H4 [201]. Moreover, SFN enhanced the expression of the anti-oncogene proteins dual-specificity phosphatase 4 (DUSP4) and cyclin-dependent kinases (CDKs), which are associated with the downregulation of the HDAC5 and HDAC11 genes in the hepatocarcinoma HepG2 cell line [202]. A further benefit of SFN is that it increases let-7 expression, which may have anti-IAV effects [112].2.31. Tanshinone IIA
Tanshinone IIA is a natural bioactive compound found in Salvia miltiorrhiza Bunge’s rhizome [203]. Wang et al. investigated tanshinone IIA’s role in epigenetic modifications, demonstrating its effect on HDAC modification [204]. This bioactive molecule decreased the enzymatic activity of HDACs. Tanshinone IIA significantly reduced the protein levels of HDAC1, HDAC3, and HDAC8 by lowering mRNA expression [204]. Tanshinone IIA was reported to be an inhibitor of MAPK p38 [205]. MAPK p38 is explored by many viruses for their efficient replications [206]. Natural products that inhibit MAPK p38 activity might be a good candidate to exhibit broad-spectrum anti-viral activity [205], including DENV [60], coronavirus [61], Venezuelan equine encephalitis virus (VEEV) [62], EV71 [63], severe fever with thrombocytopenia syndrome virus (SFTSV), HSV-1, and SARS-CoV-2 [205][206].2.32. Ursolic Acid
Ursolic acid (3-beta-3-hydroxy-urs-12-ene-28-oic-acid) is a triterpenic acid found in ginseng (Panax Ginseng C. A. Meyer), rosemary (Rosmarinus officinalis L.), apple peel, pear, cranberry, and plum (Prunus domestica L.) [207]. It has been extensively studied for its chemopreventive and chemotherapeutic effects on a variety of malignancies. Ursolic acid significantly reduces the expression of various epigenetic regulatory factors, including HDAC1, HDAC2, HDAC3, and HDAC8 (Class I), as well as HDAC6 and HDAC7 (Class II) [208].2.33. Withaferin A
Withaferin A (WFA) is a steroidal lactone derived from the plant Withania somnifera (L.) Dunal [209], known for its anticancer properties and ability to target several cancer hallmarks such as cell proliferation, migration, invasion, and angiogenesis, as well as the epigenetic process [209]. WFA displayed chemopreventive benefits by reversing epigenetic alterations via the downregulation of HDAC1 protein levels [209].3. Conclusions
Not all bioactive substances that alter epigenetic modifications were reported to have anti-viral activity. On the other hand, other bioactive substances exhibited anti-viral activity without any evidence of epigenetic effects. This suggests that epigenetic pathways might contribute to the anti-viral effect of these bioactive compounds, but are not the exclusive mechanisms explaining their action. This missing information will need to be evaluated experimentally.
It has shown that a variety of bioactive compounds can modulate epigenetic modifications such as DNA methylation, histone modifications, and miRNA expression. Bioactive substances such as EGCG, apigenin, curcumin, quercetin, berberine, resveratrol, genistein, silibinin, and sulforaphane were particularly interesting for their complex effect on different epigenetic pathways. Some bioactive substances, such as ellagic acid, tanshinone IIA, selenium, cordyceptin, grifolin, andrographolide, ursolic acid, corosolic acid, and betulinic acid, can affect just one epigenetic mark, while others, such as EGCG, showed activities on all epigenetic features.
It should be noted that not all bioactive chemicals that influence the activity of epigenetic mediators have been evaluated for anti-viral activity. Others showed anti-viral efficacy but without a definite mechanism of action. Some bioactive substances showed anti-viral activity against a limited number of viruses, whilst others were efficient in inhibiting a wide range of viruses. Some bioactive substances have a broad-spectrum anti-viral activity, inhibiting both RNA and DNA viruses.
Several bioactive agents exhibited a broad spectrum of antiviral activities against both RNA and DNA viruses. For example, baicalin, sulforaphane, apigenin, ginkgolic acid, andrographolide, EGCG, resveratrol, berberine, quercetin, curcumin, and glycyrrhizic acid showed inhibitory effects on 5, 5, 9, 11, 12, 13, 13, 14, 17, and 18 distinct viruses, respectively.
References
- Kitazato, K.; Wang, Y.; Kobayashi, N. Viral infectious disease and natural products with antiviral activity. Drug Discov. Ther. 2007, 1, 14–22.
- Abookleesh, F.L.; Al-Anzi, B.S.; Ullah, A. Potential Antiviral Action of Alkaloids. Molecules 2022, 27, 903.
- Denaro, M.; Smeriglio, A.; Barreca, D.; De Francesco, C.; Occhiuto, C.; Milano, G.; Trombetta, D. Antiviral activity of plants and their isolated bioactive compounds: An update. Phytother. Res. 2020, 34, 742–768.
- Behl, T.; Rocchetti, G.; Chadha, S.; Zengin, G.; Bungau, S.; Kumar, A.; Mehta, V.; Uddin, M.S.; Khullar, G.; Setia, D.; et al. Phytochemicals from Plant Foods as Potential Source of Antiviral Agents: An Overview. Pharm. 2021, 14, 381.
- Jassim, S.A.; Naji, M.A. Novel antiviral agents: A medicinal plant perspective. J. Appl. Microbiol. 2003, 95, 412–427.
- Astani, A.; Reichling, J.; Schnitzler, P. Screening for antiviral activities of isolated compounds from essential oils. Evid. Based Complement. Altern. Med. 2011, 2011, 253643.
- Gavanji, S.; Sayedipour, S.S.; Larki, B.; Bakhtari, A. Antiviral activity of some plant oils against herpes simplex virus type 1 in Vero cell culture. J. Acute Med. 2015, 5, 62–68.
- Feriotto, G.; Marchetti, N.; Costa, V.; Beninati, S.; Tagliati, F.; Mischiati, C. Chemical Composition of Essential Oils from Thymus vulgaris, Cymbopogon citratus, and Rosmarinus officinalis, and Their Effects on the HIV-1 Tat Protein Function. Chem. Biodivers. 2018, 15, e1700436.
- Mori, K.; Obossou, E.K.; Suwa, S.; Miura, S.; Oh, S.-H.; Jinbo, N.; Ishibashi, Y.; Shikamoto, Y.; Hosono, T.; Toda, T. Human Immunodeficiency Virus Type 1 (HIV-1) Reverse Transcriptase Inhibitory Effect of Cymbopogon Nardus Essential Oil. Int. J. Adv. Res. 2016, 2, 7–13.
- Ma, L.; Yao, L. Antiviral Effects of Plant-Derived Essential Oils and Their Components: An Updated Review. Molecules 2020, 25, 2627.
- Naithani, R.; Huma, L.C.; Holland, L.E.; Shukla, D.; McCormick, D.L.; Mehta, R.G.; Moriarty, R.M. Antiviral activity of phytochemicals: A comprehensive review. Mini Rev. Med. Chem. 2008, 8, 1106–1133.
- Choi, H.J.; Lim, C.H.; Song, J.H.; Baek, S.H.; Kwon, D.H. Antiviral activity of raoulic acid from Raoulia australis against Picornaviruses. Phytomedicine 2009, 16, 35–39.
- Lin, L.T.; Chen, T.Y.; Lin, S.C.; Chung, C.Y.; Lin, T.C.; Wang, G.H.; Anderson, R.; Lin, C.C.; Richardson, C.D. Broad-spectrum antiviral activity of chebulagic acid and punicalagin against viruses that use glycosaminoglycans for entry. BMC Microbiol. 2013, 13, 187.
- Cotin, S.; Calliste, C.A.; Mazeron, M.C.; Hantz, S.; Duroux, J.L.; Rawlinson, W.D.; Ploy, M.C.; Alain, S. Eight flavonoids and their potential as inhibitors of human cytomegalovirus replication. Antivir. Res. 2012, 96, 181–186.
- Lyu, S.Y.; Rhim, J.Y.; Park, W.B. Antiherpetic activities of flavonoids against herpes simplex virus type 1 (HSV-1) and type 2 (HSV-2) in vitro. Arch. Pharm. Res. 2005, 28, 1293–1301.
- Kim, D.E.; Min, J.S.; Jang, M.S.; Lee, J.Y.; Shin, Y.S.; Song, J.H.; Kim, H.R.; Kim, S.; Jin, Y.H.; Kwon, S. Natural Bis-Benzylisoquinoline Alkaloids-Tetrandrine, Fangchinoline, and Cepharanthine, Inhibit Human Coronavirus OC43 Infection of MRC-5 Human Lung Cells. Biomolecules 2019, 9, 696.
- Luganini, A.; Mercorelli, B.; Messa, L.; Palù, G.; Gribaudo, G.; Loregian, A. The isoquinoline alkaloid berberine inhibits human cytomegalovirus replication by interfering with the viral Immediate Early-2 (IE2) protein transactivating activity. Antivir. Res. 2019, 164, 52–60.
- Song, S.; Qiu, M.; Chu, Y.; Chen, D.; Wang, X.; Su, A.; Wu, Z. Downregulation of cellular c-Jun N-terminal protein kinase and NF-κB activation by berberine may result in inhibition of herpes simplex virus replication. Antimicrob. Agents Chemother. 2014, 58, 5068–5078.
- Latif, R.; Wang, C.Y. Andrographolide as a potent and promising antiviral agent. Chin. J. Nat. Med. 2020, 18, 760–769.
- Gupta, S.; Mishra, K.P.; Ganju, L. Broad-spectrum antiviral properties of andrographolide. Arch. Virol. 2017, 162, 611–623.
- Mishra, A.; Shaik, H.A.; Sinha, R.K.; Shah, B.R. Andrographolide: A Herbal-Chemosynthetic Approach for Enhancing Immunity, Combating Viral Infections, and Its Implication on Human Health. Molecules 2021, 26, 7036.
- Li, F.; Khanom, W.; Sun, X.; Paemanee, A.; Roytrakul, S.; Wang, D.; Smith, D.R.; Zhou, G.C. Andrographolide and Its 14-Aryloxy Analogues Inhibit Zika and Dengue Virus Infection. Molecules 2020, 25, 5037.
- Theerawatanasirikul, S.; Lueangaramkul, V.; Thangthamniyom, N.; Chankeeree, P.; Semkum, P.; Lekcharoensuk, P. Andrographolide and Deoxyandrographolide Inhibit Protease and IFN-Antagonist Activities of Foot-and-Mouth Disease Virus 3C(pro). Animals 2022, 12, 1995.
- Malat, P.; Ekalaksananan, T.; Heawchaiyaphum, C.; Suebsasana, S.; Roytrakul, S.; Yingchutrakul, Y.; Pientong, C. Andrographolide Inhibits Epstein-Barr Virus Lytic Reactivation in EBV-Positive Cancer Cell Lines through the Modulation of Epigenetic-Related Proteins. Molecules 2022, 27, 4666.
- Li, F.; Lee, E.M.; Sun, X.; Wang, D.; Tang, H.; Zhou, G.C. Design, synthesis and discovery of andrographolide derivatives against Zika virus infection. Eur. J. Med. Chem. 2020, 187, 111925.
- Chen, J.X.; Xue, H.J.; Ye, W.C.; Fang, B.H.; Liu, Y.H.; Yuan, S.H.; Yu, P.; Wang, Y.Q. Activity of andrographolide and its derivatives against influenza virus in vivo and in vitro. Biol. Pharm. Bull. 2009, 32, 1385–1391.
- Khole, S.; Mittal, S.; Jagadish, N.; Ghosh, D.; Gadgil, V.; Sinkar, V.; Ghaskadbi, S. Andrographolide enhances redox status of liver cells by regulating microRNA expression. Free Radic. Biol. Med. 2019, 130, 397–407.
- Shukla, S.; Gupta, S. Apigenin: A promising molecule for cancer prevention. Pharm. Res. 2010, 27, 962–978.
- Pandey, M.; Kaur, P.; Shukla, S.; Abbas, A.; Fu, P.; Gupta, S. Plant flavone apigenin inhibits HDAC and remodels chromatin to induce growth arrest and apoptosis in human prostate cancer cells: In vitro and in vivo study. Mol. Carcinog. 2012, 51, 952–962.
- Tseng, T.H.; Chien, M.H.; Lin, W.L.; Wen, Y.C.; Chow, J.M.; Chen, C.K.; Kuo, T.C.; Lee, W.J. Inhibition of MDA-MB-231 breast cancer cell proliferation and tumor growth by apigenin through induction of G2/M arrest and histone H3 acetylation-mediated p21(WAF1/CIP1) expression. Environ. Toxicol. 2017, 32, 434–444.
- Zakaryan, H.; Arabyan, E.; Oo, A.; Zandi, K. Flavonoids: Promising natural compounds against viral infections. Arch. Virol. 2017, 162, 2539–2551.
- Khan, T.; Khan, M.A.; Mashwani, Z.U.; Ullah, N.; Nadhman, A. Therapeutic potential of medicinal plants against COVID-19: The role of antiviral medicinal metabolites. Biocatal. Agric. Biotechnol. 2021, 31, 101890.
- Sharma, R.; Bhattu, M.; Tripathi, A.; Verma, M.; Acevedo, R.; Kumar, P.; Rajput, V.D.; Singh, J. Potential medicinal plants to combat viral infections: A way forward to environmental biotechnology. Environ. Res. 2023, 227, 115725.
- Xu, X.; Miao, J.; Shao, Q.; Gao, Y.; Hong, L. Apigenin suppresses influenza A virus-induced RIG-I activation and viral replication. J. Med. Virol. 2020, 92, 3057–3066.
- Ożarowski, M.; Karpiński, T.M. The Effects of Propolis on Viral Respiratory Diseases. Molecules 2023, 28, 359.
- Chiang, L.C.; Ng, L.T.; Cheng, P.W.; Chiang, W.; Lin, C.C. Antiviral activities of extracts and selected pure constituents of Ocimum basilicum. Clin. Exp. Pharmacol. Physiol. 2005, 32, 811–816.
- Tao, Y.; Zhan, S.; Wang, Y.; Zhou, G.; Liang, H.; Chen, X.; Shen, H. Baicalin, the major component of traditional Chinese medicine Scutellaria baicalensis induces colon cancer cell apoptosis through inhibition of oncomiRNAs. Sci. Rep. 2018, 8, 14477.
- Huang, H.; Zhou, W.; Zhu, H.; Zhou, P.; Shi, X. Baicalin benefits the anti-HBV therapy via inhibiting HBV viral RNAs. Toxicol. Appl. Pharmacol. 2017, 323, 36–43.
- Li, B.Q.; Fu, T.; Yan, Y.D.; Baylor, N.W.; Ruscetti, F.W.; Kung, H.F. Inhibition of HIV infection by baicalin--a flavonoid compound purified from Chinese herbal medicine. Cell Mol. Biol. Res. 1993, 39, 119–124.
- Zandi, K.; Teoh, B.-T.; Sam, S.-S.; Wong, P.-F.; Mustafa, M.R.; AbuBakar, S. Novel antiviral activity of baicalein against dengue virus. BMC Complement. Altern. Med. 2012, 12, 214.
- Luo, Z.; Kuang, X.-P.; Zhou, Q.-Q.; Yan, C.-Y.; Li, W.; Gong, H.-B.; Kurihara, H.; Li, W.-X.; Li, Y.-F.; He, R.-R. Inhibitory effects of baicalein against herpes simplex virus type 1. Acta Pharm. Sin. B 2020, 10, 2323–2338.
- Yu, X.; Li, H.; Hu, P.; Qing, Y.; Wang, X.; Zhu, M.; Wang, H.; Wang, Z.; Xu, J.; Guo, Q.; et al. Natural HDAC-1/8 inhibitor baicalein exerts therapeutic effect in CBF-AML. Clin. Transl. Med. 2020, 10, e154.
- Jiang, H.; Yao, Q.; An, Y.; Fan, L.; Wang, J.; Li, H. Baicalin suppresses the progression of Type 2 diabetes-induced liver tumor through regulating METTL3/m(6)A/HKDC1 axis and downstream p-JAK2/STAT1/clevaged Capase3 pathway. Phytomedicine 2022, 94, 153823.
- Cicero, A.F.; Baggioni, A. Berberine and Its Role in Chronic Disease. Adv. Exp. Med. Biol. 2016, 928, 27–45.
- Jin, Y.; Liu, S.; Ma, Q.; Xiao, D.; Chen, L. Berberine enhances the AMPK activation and autophagy and mitigates high glucose-induced apoptosis of mouse podocytes. Eur. J. Pharmacol. 2017, 794, 106–114.
- Kedhari Sundaram, M.; Hussain, A.; Haque, S.; Raina, R.; Afroze, N. Quercetin modifies 5′CpG promoter methylation and reactivates various tumor suppressor genes by modulating epigenetic marks in human cervical cancer cells. J. Cell Biochem. 2019, 120, 18357–18369.
- Lou, G.; Liu, Y.; Wu, S.; Xue, J.; Yang, F.; Fu, H.; Zheng, M.; Chen, Z. The p53/miR-34a/SIRT1 Positive Feedback Loop in Quercetin-Induced Apoptosis. Cell Physiol. Biochem. 2015, 35, 2192–2202.
- Lü, Y.; Han, B.; Yu, H.; Cui, Z.; Li, Z.; Wang, J. Berberine regulates the microRNA-21-ITGΒ4-PDCD4 axis and inhibits colon cancer viability. Oncol. Lett. 2018, 15, 5971–5976.
- Warowicka, A.; Nawrot, R.; Goździcka-Józefiak, A. Antiviral activity of berberine. Arch. Virol. 2020, 165, 1935–1945.
- Cui, Y.; Zhang, L.; Hu, D.; Yang, Y. Berberine Inhibits Herpes Simplex Virus 1 Replication in HEK293T Cells. Comput. Math. Methods Med. 2022, 2022, 7137401.
- Hayashi, K.; Minoda, K.; Nagaoka, Y.; Hayashi, T.; Uesato, S. Antiviral activity of berberine and related compounds against human cytomegalovirus. Bioorg Med. Chem. Lett. 2007, 17, 1562–1564.
- Mahata, S.; Bharti, A.C.; Shukla, S.; Tyagi, A.; Husain, S.A.; Das, B.C. Berberine modulates AP-1 activity to suppress HPV transcription and downstream signaling to induce growth arrest and apoptosis in cervical cancer cells. Mol. Cancer 2011, 10, 39.
- Ratanakomol, T.; Roytrakul, S.; Wikan, N.; Smith, D.R. Berberine Inhibits Dengue Virus through Dual Mechanisms. Molecules 2021, 26, 5501.
- Zha, W.; Liang, G.; Xiao, J.; Studer, E.J.; Hylemon, P.B.; Pandak, W.M., Jr.; Wang, G.; Li, X.; Zhou, H. Berberine inhibits HIV protease inhibitor-induced inflammatory response by modulating ER stress signaling pathways in murine macrophages. PLoS ONE 2010, 5, e9069.
- Hung, T.C.; Jassey, A.; Liu, C.H.; Lin, C.J.; Lin, C.C.; Wong, S.H.; Wang, J.Y.; Yen, M.H.; Lin, L.T. Berberine inhibits hepatitis C virus entry by targeting the viral E2 glycoprotein. Phytomedicine 2019, 53, 62–69.
- Pizzorno, A.; Padey, B.; Dubois, J.; Julien, T.; Traversier, A.; Dulière, V.; Brun, P.; Lina, B.; Rosa-Calatrava, M.; Terrier, O. In vitro evaluation of antiviral activity of single and combined repurposable drugs against SARS-CoV-2. Antivir. Res. 2020, 181, 104878.
- Wu, Y.; Li, J.Q.; Kim, Y.J.; Wu, J.; Wang, Q.; Hao, Y. In vivo and in vitro antiviral effects of berberine on influenza virus. Chin. J. Integr. Med. 2011, 17, 444–452.
- Keck, F.; Ataey, P.; Amaya, M.; Bailey, C.; Narayanan, A. Phosphorylation of Single Stranded RNA Virus Proteins and Potential for Novel Therapeutic Strategies. Viruses 2015, 7, 5257–5273.
- Adamson, A.L.; Darr, D.; Holley-Guthrie, E.; Johnson, R.A.; Mauser, A.; Swenson, J.; Kenney, S. Epstein-Barr virus immediate-early proteins BZLF1 and BRLF1 activate the ATF2 transcription factor by increasing the levels of phosphorylated p38 and c-Jun N-terminal kinases. J. Virol. 2000, 74, 1224–1233.
- Sreekanth, G.P.; Yenchitsomanus, P.T.; Limjindaporn, T. Role of mitogen-activated protein kinase signaling in the pathogenesis of dengue virus infection. Cell Signal 2018, 48, 64–68.
- Fung, T.S.; Liu, D.X. Activation of the c-Jun NH(2)-terminal kinase pathway by coronavirus infectious bronchitis virus promotes apoptosis independently of c-Jun. Cell Death Dis. 2017, 8, 3215.
- Voss, K.; Amaya, M.; Mueller, C.; Roberts, B.; Kehn-Hall, K.; Bailey, C.; Petricoin, E., 3rd; Narayanan, A. Inhibition of host extracellular signal-regulated kinase (ERK) activation decreases new world alphavirus multiplication in infected cells. Virology 2014, 468–470, 490–503.
- Shi, W.; Hou, X.; Peng, H.; Zhang, L.; Li, Y.; Gu, Z.; Jiang, Q.; Shi, M.; Ji, Y.; Jiang, J. MEK/ERK signaling pathway is required for enterovirus 71 replication in immature dendritic cells. Virol. J. 2014, 11, 227.
- Varghese, F.S.; Thaa, B.; Amrun, S.N.; Simarmata, D.; Rausalu, K.; Nyman, T.A.; Merits, A.; McInerney, G.M.; Ng, L.F.P.; Ahola, T. The Antiviral Alkaloid Berberine Reduces Chikungunya Virus-Induced Mitogen-Activated Protein Kinase Signaling. J. Virol. 2016, 90, 9743–9757.
- Kim, S.Y.; Kim, H.; Kim, S.W.; Lee, N.R.; Yi, C.M.; Heo, J.; Kim, B.J.; Kim, N.J.; Inn, K.S. An Effective Antiviral Approach Targeting Hepatitis B Virus with NJK14047, a Novel and Selective Biphenyl Amide p38 Mitogen-Activated Protein Kinase Inhibitor. Antimicrob. Agents Chemother. 2017, 61, e00214-17.
- Zuco, V.; Supino, R.; Righetti, S.C.; Cleris, L.; Marchesi, E.; Gambacorti-Passerini, C.; Formelli, F. Selective cytotoxicity of betulinic acid on tumor cell lines, but not on normal cells. Cancer Lett. 2002, 175, 17–25.
- Fulda, S. Betulinic Acid for cancer treatment and prevention. Int. J. Mol. Sci. 2008, 9, 1096–1107.
- Adewole, K.E.; Ishola, A.A. A Computational Approach to Investigate the HDAC6 and HDAC10 Binding Propensity of Psidium guajava-derived Compounds as Potential Anticancer Agents. Curr. Drug Discov. Technol. 2021, 18, 423–436.
- Liu, X.; Jutooru, I.; Lei, P.; Kim, K.; Lee, S.O.; Brents, L.K.; Prather, P.L.; Safe, S. Betulinic acid targets YY1 and ErbB2 through cannabinoid receptor-dependent disruption of microRNA-27a:ZBTB10 in breast cancer. Mol. Cancer Ther. 2012, 11, 1421–1431.
- Prinsloo, G.; Marokane, C.K.; Street, R.A. Anti-HIV activity of southern African plants: Current developments, phytochemistry and future research. J. Ethnopharmacol. 2018, 210, 133–155.
- Peyrat, L.A.; Eparvier, V.; Eydoux, C.; Guillemot, J.C.; Litaudon, M.; Stien, D. Betulinic Acid, The First Lupane-Type Triterpenoid Isolated from Both a Phomopsis sp. and Its Host Plant Diospyros carbonaria Benoist. Chem. Biodivers. 2017, 14, e1600171.
- Yao, D.; Li, H.; Gou, Y.; Zhang, H.; Vlessidis, A.G.; Zhou, H.; Evmiridis, N.P.; Liu, Z. Betulinic acid-mediated inhibitory effect on hepatitis B virus by suppression of manganese superoxide dismutase expression. Febs J. 2009, 276, 2599–2614.
- Tiwari, P.; Gupta, K.P. Modulation of miR-203 and its regulators as a function of time during the development of 7, 12 dimethylbenz anthracene induced mouse skin tumors in presence or absence of the antitumor agents. Toxicol. Appl. Pharmacol. 2014, 278, 148–158.
- Pant, K.; Mishra, A.K.; Pradhan, S.M.; Nayak, B.; Das, P.; Shalimar, D.; Saraya, A.; Venugopal, S.K. Butyrate inhibits HBV replication and HBV-induced hepatoma cell proliferation via modulating SIRT-1/Ac-p53 regulatory axis. Mol. Carcinog. 2019, 58, 524–532.
- Voon, F.L.; Sulaiman, M.R.; Akhtar, M.N.; Idris, M.F.; Akira, A.; Perimal, E.K.; Israf, D.A.; Ming-Tatt, L. Cardamonin (2′,4′-dihydroxy-6′-methoxychalcone) isolated from Boesenbergia rotunda (L.) Mansf. inhibits CFA-induced rheumatoid arthritis in rats. Eur. J. Pharmacol. 2017, 794, 127–134.
- Jin, Y.H.; Min, J.S.; Kwon, S. Cardamonin as a p38 MAPK Signaling Pathway Activator Inhibits Human Coronavirus OC43 Infection in Human Lung Cells. Nutrients 2023, 15, 1335.
- Das, S.K.; Masuda, M.; Sakurai, A.; Sakakibara, M. Medicinal uses of the mushroom Cordyceps militaris: Current state and prospects. Fitoterapia 2010, 81, 961–968.
- Ryu, E.; Son, M.; Lee, M.; Lee, K.; Cho, J.Y.; Cho, S.; Lee, S.K.; Lee, Y.M.; Cho, H.; Sung, G.H.; et al. Cordycepin is a novel chemical suppressor of Epstein-Barr virus replication. Oncoscience 2014, 1, 866–881.
- Rabie, A.M. Potent Inhibitory Activities of the Adenosine Analogue Cordycepin on SARS-CoV-2 Replication. ACS Omega 2022, 7, 2960–2969.
- Ling, J.Y.; Sun, Y.J.; Zhang, H.; Lv, P.; Zhang, C.K. Measurement of cordycepin and adenosine in stroma of Cordyceps sp. by capillary zone electrophoresis (CZE). J. Biosci. Bioeng. 2002, 94, 371–374.
- Hou, W.; Li, Y.; Zhang, Q.; Wei, X.; Peng, A.; Chen, L.; Wei, Y. Triterpene acids isolated from Lagerstroemia speciosa leaves as alpha-glucosidase inhibitors. Phytother. Res. 2009, 23, 614–618.
- Zhao, J.; Zhou, H.; An, Y.; Shen, K.; Yu, L. Biological effects of corosolic acid as an anti-inflammatory, anti-metabolic syndrome and anti-neoplasic natural compound. Oncol. Lett. 2021, 21, 84.
- Hudlikar, R.R.; Sargsyan, D.; Wu, R.; Su, S.; Zheng, M.; Kong, A.N. Triterpenoid corosolic acid modulates global CpG methylation and transcriptome of tumor promotor TPA induced mouse epidermal JB6 P+ cells. Chem. Biol. Interact. 2020, 321, 109025.
- Ratovitski, E.A. Anticancer Natural Compounds as Epigenetic Modulators of Gene Expression. Curr. Genom. 2017, 18, 175–205.
- Hewlings, S.J.; Kalman, D.S. Curcumin: A Review of Its Effects on Human Health. Foods 2017, 6, 92.
- Liu, H.L.; Chen, Y.; Cui, G.H.; Zhou, J.F. Curcumin, a potent anti-tumor reagent, is a novel histone deacetylase inhibitor regulating B-NHL cell line Raji proliferation. Acta Pharmacol. Sin. 2005, 26, 603–609.
- Shu, L.; Khor, T.O.; Lee, J.H.; Boyanapalli, S.S.; Huang, Y.; Wu, T.Y.; Saw, C.L.; Cheung, K.L.; Kong, A.N. Epigenetic CpG demethylation of the promoter and reactivation of the expression of Neurog1 by curcumin in prostate LNCaP cells. Aaps J. 2011, 13, 606–614.
- Wei, Z.Q.; Zhang, Y.H.; Ke, C.Z.; Chen, H.X.; Ren, P.; He, Y.L.; Hu, P.; Ma, D.Q.; Luo, J.; Meng, Z.J. Curcumin inhibits hepatitis B virus infection by down-regulating cccDNA-bound histone acetylation. World J. Gastroenterol. 2017, 23, 6252–6260.
- Sohn, E.J.; Bak, K.M.; Nam, Y.K.; Park, H.T. Upregulation of microRNA 344a-3p is involved in curcumin induced apoptosis in RT4 schwannoma cells. Cancer Cell Int. 2018, 18, 199.
- Hassan, F.U.; Rehman, M.S.; Khan, M.S.; Ali, M.A.; Javed, A.; Nawaz, A.; Yang, C. Curcumin as an Alternative Epigenetic Modulator: Mechanism of Action and Potential Effects. Front. Genet. 2019, 10, 514.
- Sharifi-Rad, J.; Quispe, C.; Castillo, C.M.S.; Caroca, R.; Lazo-Vélez, M.A.; Antonyak, H.; Polishchuk, A.; Lysiuk, R.; Oliinyk, P.; De Masi, L.; et al. Ellagic Acid: A Review on Its Natural Sources, Chemical Stability, and Therapeutic Potential. Oxid. Med. Cell Longev. 2022, 2022, 3848084.
- Kang, I.; Okla, M.; Chung, S. Ellagic acid inhibits adipocyte differentiation through coactivator-associated arginine methyltransferase 1-mediated chromatin modification. J. Nutr. Biochem. 2014, 25, 946–953.
- Dinata, R.; Nisa, N.; Arati, C.; Rasmita, B.; Uditraj, C.; Siddhartha, R.; Bhanushree, B.; Saeed-Ahmed, L.; Manikandan, B.; Bidanchi, R.M.; et al. Repurposing immune boosting and anti-viral efficacy of Parkia bioactive entities as multi-target directed therapeutic approach for SARS-CoV-2: Exploration of lead drugs by drug likeness, molecular docking and molecular dynamics simulation methods. J. Biomol. Struct. Dyn. 2023, 1–39.
- Prasanth, M.I.; Sivamaruthi, B.S.; Chaiyasut, C.; Tencomnao, T. A Review of the Role of Green Tea (Camellia sinensis) in Antiphotoaging, Stress Resistance, Neuroprotection, and Autophagy. Nutrients 2019, 11, 474.
- Ngwe Tun, M.M.; Luvai, E.; Nwe, K.M.; Toume, K.; Mizukami, S.; Hirayama, K.; Komatsu, K.; Morita, K. Anti-SARS-CoV-2 activity of various PET-bottled Japanese green teas and tea compounds in vitro. Arch. Virol. 2022, 167, 1547–1557.
- Cheng, Z.; Zhang, Z.; Han, Y.; Wang, J.; Wang, Y.; Chen, X.; Shao, Y.; Cheng, Y.; Zhou, W.; Lu, X.; et al. A review on anti-cancer effect of green tea catechins. J. Funct. Foods 2020, 74, 104172.
- Li, F.; Qasim, S.; Li, D.; Dou, Q.P. Updated review on green tea polyphenol epigallocatechin-3-gallate as a cancer epigenetic regulator. Semin. Cancer Biol. 2022, 83, 335–352.
- Bag, A.; Bag, N. Tea Polyphenols and Prevention of Epigenetic Aberrations in Cancer. J. Nat. Sci. Biol. Med. 2018, 9, 2–5.
- Henning, S.M.; Wang, P.; Carpenter, C.L.; Heber, D. Epigenetic effects of green tea polyphenols in cancer. Epigenomics 2013, 5, 729–741.
- Moseley, V.R.; Morris, J.; Knackstedt, R.W.; Wargovich, M.J. Green tea polyphenol epigallocatechin 3-gallate, contributes to the degradation of DNMT3A and HDAC3 in HCT 116 human colon cancer cells. Anticancer. Res. 2013, 33, 5325–5333.
- Meeran, S.M.; Patel, S.N.; Chan, T.H.; Tollefsbol, T.O. A novel prodrug of epigallocatechin-3-gallate: Differential epigenetic hTERT repression in human breast cancer cells. Cancer Prev. Res. 2011, 4, 1243–1254.
- Nandakumar, V.; Vaid, M.; Katiyar, S.K. (−)-Epigallocatechin-3-gallate reactivates silenced tumor suppressor genes, Cip1/p21 and p16INK4a, by reducing DNA methylation and increasing histones acetylation in human skin cancer cells. Carcinogenesis 2011, 32, 537–544.
- Rasheed, Z.; Rasheed, N.; Al-Shaya, O. Epigallocatechin-3-O-gallate modulates global microRNA expression in interleukin-1β-stimulated human osteoarthritis chondrocytes: Potential role of EGCG on negative co-regulation of microRNA-140-3p and ADAMTS5. Eur. J. Nutr. 2018, 57, 917–928.
- Shin, S.; Kim, K.; Lee, M.J.; Lee, J.; Choi, S.; Kim, K.S.; Ko, J.M.; Han, H.; Kim, S.Y.; Youn, H.J.; et al. Epigallocatechin Gallate-Mediated Alteration of the MicroRNA Expression Profile in 5α-Dihydrotestosterone-Treated Human Dermal Papilla Cells. Ann. Dermatol. 2016, 28, 327–334.
- Chen, X.; Chang, L.; Qu, Y.; Liang, J.; Jin, W.; Xia, X. Tea polyphenols inhibit the proliferation, migration, and invasion of melanoma cells through the down-regulation of TLR4. Int. J. Immunopathol. Pharmacol. 2018, 32, 394632017739531.
- Arffa, M.L.; Zapf, M.A.; Kothari, A.N.; Chang, V.; Gupta, G.N.; Ding, X.; Al-Gayyar, M.M.; Syn, W.; Elsherbiny, N.M.; Kuo, P.C.; et al. Epigallocatechin-3-Gallate Upregulates miR-221 to Inhibit Osteopontin-Dependent Hepatic Fibrosis. PLoS ONE 2016, 11, e0167435.
- Mekky, R.Y.; El-Ekiaby, N.; El Sobky, S.A.; Elemam, N.M.; Youness, R.A.; El-Sayed, M.; Hamza, M.T.; Esmat, G.; Abdelaziz, A.I. Epigallocatechin gallate (EGCG) and miR-548m reduce HCV entry through repression of CD81 receptor in HCV cell models. Arch. Virol. 2019, 164, 1587–1595.
- Kunden, R.D.; Khan, J.Q.; Ghezelbash, S.; Wilson, J.A. The Role of the Liver-Specific microRNA, miRNA-122 in the HCV Replication Cycle. Int. J. Mol. Sci. 2020, 21, 5677.
- Jopling, C.L.; Yi, M.; Lancaster, A.M.; Lemon, S.M.; Sarnow, P. Modulation of hepatitis C virus RNA abundance by a liver-specific MicroRNA. Science 2005, 309, 1577–1581.
- Panigrahi, M.; Thibault, P.A.; Wilson, J.A. MicroRNA 122 Affects both the Initiation and the Maintenance of Hepatitis C Virus Infections. J. Virol. 2022, 96, e0190321.
- Yousefpouran, S.; Mostafaei, S.; Manesh, P.V.; Iranifar, E.; Bokharaei-Salim, F.; Nahand, J.S.; Mirzaei, H.; Taran, M.; Babaei, F.; Sayad, B.; et al. The assessment of selected MiRNAs profile in HIV, HBV, HCV, HIV/HCV, HIV/HBV Co-infection and elite controllers for determination of biomarker. Microb. Pathog. 2020, 147, 104355.
- Meerson, A.; Khatib, S.; Mahajna, J. Natural Products Targeting Cancer Stem Cells for Augmenting Cancer Therapeutics. Int. J. Mol. Sci. 2021, 22, 13044.
- Kong, Y.; Feng, Z.; Chen, A.; Qi, Q.; Han, M.; Wang, S.; Zhang, Y.; Zhang, X.; Yang, N.; Wang, J.; et al. The Natural Flavonoid Galangin Elicits Apoptosis, Pyroptosis, and Autophagy in Glioblastoma. Front. Oncol. 2019, 9, 942.
- Zeng, H.; Huang, P.; Wang, X.; Wu, J.; Wu, M.; Huang, J. Galangin-induced down-regulation of BACE1 by epigenetic mechanisms in SH-SY5Y cells. Neuroscience 2015, 294, 172–181.
- Deng, X.; Zuo, M.; Pei, Z.; Xie, Y.; Yang, Z.; Zhang, Z.; Jiang, M.; Kuang, D. MicroRNA-455-5p Contributes to Cholangiocarcinoma Growth and Mediates Galangin’s Anti-Tumor Effects. J. Cancer 2021, 12, 4710–4721.
- Meyer, J.J.; Afolayan, A.J.; Taylor, M.B.; Erasmus, D. Antiviral activity of galangin isolated from the aerial parts of Helichrysum aureonitens. J. Ethnopharmacol. 1997, 56, 165–169.
- Aggarwal, V.; Tuli, H.S.; Kaur, J.; Aggarwal, D.; Parashar, G.; Chaturvedi Parashar, N.; Kulkarni, S.; Kaur, G.; Sak, K.; Kumar, M.; et al. Garcinol Exhibits Anti-Neoplastic Effects by Targeting Diverse Oncogenic Factors in Tumor Cells. Biomedicines 2020, 8, 103.
- Laurson, J.; Khan, S.; Chung, R.; Cross, K.; Raj, K. Epigenetic repression of E-cadherin by human papillomavirus 16 E7 protein. Carcinogenesis 2010, 31, 918–926.
- Kopytko, P.; Piotrowska, K.; Janisiak, J.; Tarnowski, M. Garcinol-A Natural Histone Acetyltransferase Inhibitor and New Anti-Cancer Epigenetic Drug. Int. J. Mol. Sci. 2021, 22, 2828.
- Behera, A.K.; Swamy, M.M.; Natesh, N.; Kundu, T.K. Garcinol and Its Role in Chronic Diseases. Adv. Exp. Med. Biol. 2016, 928, 435–452.
- Kim, J.-Y.; Jo, J.; Leem, J.; Park, K.-K. Inhibition of p300 by Garcinol Protects against Cisplatin-Induced Acute Kidney Injury through Suppression of Oxidative Stress, Inflammation, and Tubular Cell Death in Mice. Antioxidants 2020, 9, 1271.
- Sharifi-Rad, J.; Quispe, C.; Imran, M.; Rauf, A.; Nadeem, M.; Gondal, T.A.; Ahmad, B.; Atif, M.; Mubarak, M.S.; Sytar, O.; et al. Genistein: An Integrative Overview of Its Mode of Action, Pharmacological Properties, and Health Benefits. Oxid. Med. Cell Longev. 2021, 2021, 3268136.
- Fang, M.Z.; Chen, D.; Sun, Y.; Jin, Z.; Christman, J.K.; Yang, C.S. Reversal of hypermethylation and reactivation of p16INK4a, RARbeta, and MGMT genes by genistein and other isoflavones from soy. Clin. Cancer Res. 2005, 11, 7033–7041.
- Zhang, Y.; Li, Q.; Chen, H. DNA methylation and histone modifications of Wnt genes by genistein during colon cancer development. Carcinogenesis 2013, 34, 1756–1763.
- Gerstmeier, J.; Seegers, J.; Witt, F.; Waltenberger, B.; Temml, V.; Rollinger, J.M.; Stuppner, H.; Koeberle, A.; Schuster, D.; Werz, O. Ginkgolic Acid is a Multi-Target Inhibitor of Key Enzymes in Pro-Inflammatory Lipid Mediator Biosynthesis. Front. Pharmacol. 2019, 10, 797.
- Fukuda, I.; Ito, A.; Hirai, G.; Nishimura, S.; Kawasaki, H.; Saitoh, H.; Kimura, K.; Sodeoka, M.; Yoshida, M. Ginkgolic acid inhibits protein SUMOylation by blocking formation of the E1-SUMO intermediate. Chem. Biol. 2009, 16, 133–140.
- Flotho, A.; Melchior, F. Sumoylation: A regulatory protein modification in health and disease. Annu. Rev. Biochem. 2013, 82, 357–385.
- Yang, W.S.; Campbell, M.; Chang, P.C. SUMO modification of a heterochromatin histone demethylase JMJD2A enables viral gene transactivation and viral replication. PLoS Pathog. 2017, 13, e1006216.
- Bhutta, M.S.; Shechter, O.; Gallo, E.S.; Martin, S.D.; Jones, E.; Doncel, G.F.; Borenstein, R. Ginkgolic Acid Inhibits Herpes Simplex Virus Type 1 Skin Infection and Prevents Zosteriform Spread in Mice. Viruses 2021, 13, 86.
- Pastorino, G.; Cornara, L.; Soares, S.; Rodrigues, F.; Oliveira, M. Liquorice (Glycyrrhiza glabra): A phytochemical and pharmacological review. Phytother. Res. 2018, 32, 2323–2339.
- Sun, Z.G.; Zhao, T.T.; Lu, N.; Yang, Y.A.; Zhu, H.L. Research Progress of Glycyrrhizic Acid on Antiviral Activity. Mini Rev. Med. Chem. 2019, 19, 826–832.
- Cao, L.; Ding, W.; Jia, R.; Du, J.; Wang, T.; Zhang, C.; Gu, Z.; Yin, G. Anti-inflammatory and hepatoprotective effects of glycyrrhetinic acid on CCl4-induced damage in precision-cut liver slices from Jian carp (Cyprinus carpio var. jian) through inhibition of the nf-kB pathway. Fish Shellfish Immunol. 2017, 64, 234–242.
- Zhong, L.L.D.; Lam, W.C.; Yang, W.; Chan, K.W.; Sze, S.C.W.; Miao, J.; Yung, K.K.L.; Bian, Z.; Wong, V.T. Potential Targets for Treatment of Coronavirus Disease 2019 (COVID-19): A Review of Qing-Fei-Pai-Du-Tang and Its Major Herbs. Am. J. Chin. Med. 2020, 48, 1051–1071.
- Omer, M.O.; Almalki, W.H.; Shahid, I.; Khuram, S.; Altaf, I.; Imran, S. Comparative study to evaluate the anti-viral efficacy of Glycyrrhiza glabra extract and ribavirin against the Newcastle disease virus. Pharmacogn. Res. 2014, 6, 6–11.
- Cinatl, J.; Morgenstern, B.; Bauer, G.; Chandra, P.; Rabenau, H.; Doerr, H.W. Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus. Lancet 2003, 361, 2045–2046.
- Liu, W.; Huang, S.; Li, Y.; Li, Y.; Li, D.; Wu, P.; Wang, Q.; Zheng, X.; Zhang, K. Glycyrrhizic acid from licorice down-regulates inflammatory responses via blocking MAPK and PI3K/Akt-dependent NF-κB signalling pathways in TPA-induced skin inflammation. Medchemcomm 2018, 9, 1502–1510.
- Kwon, H.S.; Oh, S.M.; Kim, J.K. Glabridin, a functional compound of liquorice, attenuates colonic inflammation in mice with dextran sulphate sodium-induced colitis. Clin. Exp. Immunol. 2008, 151, 165–173.
- Yu, X.Q.; Xue, C.C.; Zhou, Z.W.; Li, C.G.; Du, Y.M.; Liang, J.; Zhou, S.F. In vitro and in vivo neuroprotective effect and mechanisms of glabridin, a major active isoflavan from Glycyrrhiza glabra (licorice). Life Sci. 2008, 82, 68–78.
- Hsu, Y.L.; Wu, L.Y.; Hou, M.F.; Tsai, E.M.; Lee, J.N.; Liang, H.L.; Jong, Y.J.; Hung, C.H.; Kuo, P.L. Glabridin, an isoflavan from licorice root, inhibits migration, invasion and angiogenesis of MDA-MB-231 human breast adenocarcinoma cells by inhibiting focal adhesion kinase/Rho signaling pathway. Mol. Nutr. Food Res. 2011, 55, 318–327.
- Jiang, F.; Mu, J.; Wang, X.; Ye, X.; Si, L.; Ning, S.; Li, Z.; Li, Y. The repressive effect of miR-148a on TGF beta-SMADs signal pathway is involved in the glabridin-induced inhibition of the cancer stem cells-like properties in hepatocellular carcinoma cells. PLoS ONE 2014, 9, e96698.
- Jiang, F.; Li, Y.; Mu, J.; Hu, C.; Zhou, M.; Wang, X.; Si, L.; Ning, S.; Li, Z. Glabridin inhibits cancer stem cell-like properties of human breast cancer cells: An epigenetic regulation of miR-148a/SMAd2 signaling. Mol. Carcinog. 2016, 55, 929–940.
- Ye, M.; Liu, J.K.; Lu, Z.X.; Zhao, Y.; Liu, S.F.; Li, L.L.; Tan, M.; Weng, X.X.; Li, W.; Cao, Y. Grifolin, a potential antitumor natural product from the mushroom Albatrellus confluens, inhibits tumor cell growth by inducing apoptosis in vitro. FEBS Lett. 2005, 579, 3437–3443.
- Luo, X.; Yang, L.; Xiao, L.; Xia, X.; Dong, X.; Zhong, J.; Liu, Y.; Li, N.; Chen, L.; Li, H.; et al. Grifolin directly targets ERK1/2 to epigenetically suppress cancer cell metastasis. Oncotarget 2015, 6, 42704–42716.
- Nediani, C.; Ruzzolini, J.; Romani, A.; Calorini, L. Oleuropein, a Bioactive Compound from Olea europaea L., as a Potential Preventive and Therapeutic Agent in Non-Communicable Diseases. Antioxidants 2019, 8, 578.
- Fabiani, R.; Vella, N.; Rosignoli, P. Epigenetic Modifications Induced by Olive Oil and Its Phenolic Compounds: A Systematic Review. Molecules 2021, 26, 273.
- Juli, G.; Oliverio, M.; Bellizzi, D.; Gallo Cantafio, M.E.; Grillone, K.; Passarino, G.; Colica, C.; Nardi, M.; Rossi, M.; Procopio, A.; et al. Anti-tumor Activity and Epigenetic Impact of the Polyphenol Oleacein in Multiple Myeloma. Cancers 2019, 11, 990.
- Cuyàs, E.; Gumuzio, J.; Lozano-Sánchez, J.; Carreras, D.; Verdura, S.; Llorach-Parés, L.; Sanchez-Martinez, M.; Selga, E.; Pérez, G.J.; Scornik, F.S.; et al. Extra Virgin Olive Oil Contains a Phenolic Inhibitor of the Histone Demethylase LSD1/KDM1A. Nutrients 2019, 11, 1656.
- Lee-Huang, S.; Huang, P.L.; Zhang, D.; Lee, J.W.; Bao, J.; Sun, Y.; Chang, Y.T.; Zhang, J.; Huang, P.L. Discovery of small-molecule HIV-1 fusion and integrase inhibitors oleuropein and hydroxytyrosol: Part I. fusion inhibition. Biochem. Biophys. Res. Commun. 2007, 354, 872–878.
- Yamaguchi, Y.; Kumagai, H. Characteristics, biosynthesis, decomposition, metabolism and functions of the garlic odour precursor, S-allyl-L-cysteine sulfoxide. Exp. Ther. Med. 2020, 19, 1528–1535.
- Tolo, F.M.; Rukunga, G.M.; Muli, F.W.; Njagi, E.N.; Njue, W.; Kumon, K.; Mungai, G.M.; Muthaura, C.N.; Muli, J.M.; Keter, L.K.; et al. Anti-viral activity of the extracts of a Kenyan medicinal plant Carissa edulis against herpes simplex virus. J. Ethnopharmacol. 2006, 104, 92–99.
- Lin, L.T.; Hsu, W.C.; Lin, C.C. Antiviral natural products and herbal medicines. J. Tradit. Complement. Med. 2014, 4, 24–35.
- Tsai, Y.; Cole, L.L.; Davis, L.E.; Lockwood, S.J.; Simmons, V.; Wild, G.C. Antiviral properties of garlic: In vitro effects on influenza B, herpes simplex and coxsackie viruses. Planta Med. 1985, 51, 460–461.
- Abiy, E.; Berhe, A. Anti-bacterial effect of garlic (Allium sativum) against clinical isolates of Staphylococcus aureus and Escherichia coli from patients attending Hawassa Referral Hospital, Ethiopia. J. Infect. Dis. Treat. 2016, 2, 1–5.
- Gebreyohannes, G.; Gebreyohannes, M. Medicinal values of garlic: A review. Int. J. Med. Med. Sci. 2013, 5, 401–408.
- Lawson, L.D.; Bauer, R. Phytomedicines of Europe: Chemistry and Biological Activity; ACS Publications: Washington, DC, USA, 1998.
- Borlinghaus, J.; Albrecht, F.; Gruhlke, M.C.; Nwachukwu, I.D.; Slusarenko, A.J. Allicin: Chemistry and biological properties. Molecules 2014, 19, 12591–12618.
- Wang, L.; Jiao, H.; Zhao, J.; Wang, X.; Sun, S.; Lin, H. Allicin Alleviates Reticuloendotheliosis Virus-Induced Immunosuppression via ERK/Mitogen-Activated Protein Kinase Pathway in Specific Pathogen-Free Chickens. Front. Immunol. 2017, 8, 1856.
- Weber, N.D.; Andersen, D.O.; North, J.A.; Murray, B.K.; Lawson, L.D.; Hughes, B.G. In vitro virucidal effects of Allium sativum (garlic) extract and compounds. Planta Med. 1992, 58, 417–423.
- Arreola, R.; Quintero-Fabián, S.; López-Roa, R.I.; Flores-Gutiérrez, E.O.; Reyes-Grajeda, J.P.; Carrera-Quintanar, L.; Ortuño-Sahagún, D. Immunomodulation and anti-inflammatory effects of garlic compounds. J. Immunol. Res. 2015, 2015, 401630.
- Metwally, D.M.; Al-Olayan, E.M.; Alanazi, M.; Alzahrany, S.B.; Semlali, A. Antischistosomal and anti-inflammatory activity of garlic and allicin compared with that of praziquantel in vivo. BMC Complement. Altern. Med. 2018, 18, 135.
- Prasad, K.; Laxdal, V.A.; Yu, M.; Raney, B.L. Antioxidant activity of allicin, an active principle in garlic. Mol. Cell Biochem. 1995, 148, 183–189.
- El-Saber Batiha, G.; Magdy Beshbishy, A.; Wasef, L.G.; Elewa, Y.H.; Al-Sagan, A.A.; Abd El-Hack, M.E.; Taha, A.E.; Abd-Elhakim, Y.M.; Prasad Devkota, H. Chemical Constituents and Pharmacological Activities of Garlic (Allium sativum L.): A Review. Nutrients 2020, 12, 872.
- Trio, P.Z.; You, S.; He, X.; He, J.; Sakao, K.; Hou, D.X. Chemopreventive functions and molecular mechanisms of garlic organosulfur compounds. Food Funct. 2014, 5, 833–844.
- Fang, F.; Li, H.; Cui, W.; Dong, Y. Treatment of hepatitis caused by cytomegalovirus with allitridin injection--an experimental study. J. Tongji Med. Univ. 1999, 19, 271–274.
- Keyaerts, E.; Vijgen, L.; Pannecouque, C.; Van Damme, E.; Peumans, W.; Egberink, H.; Balzarini, J.; Van Ranst, M. Plant lectins are potent inhibitors of coronaviruses by interfering with two targets in the viral replication cycle. Antivir. Res. 2007, 75, 179–187.
- Liu, Z.F.; Fang, F.; Dong, Y.S.; Li, G.; Zhen, H. Experimental study on the prevention and treatment of murine cytomegalovirus hepatitis by using allitridin. Antivir. Res. 2004, 61, 125–128.
- Terrasson, J.; Xu, B.; Li, M.; Allart, S.; Davignon, J.L.; Zhang, L.H.; Wang, K.; Davrinche, C. Activities of Z-ajoene against tumour and viral spreading in vitro. Fundam. Clin. Pharmacol. 2007, 21, 281–289.
- Walder, R.; Kalvatchev, Z.; Garzaro, D.; Barrios, M.; Apitz-Castro, R. In vitro suppression of HIV-1 replication by ajoene . Biomed. Pharmacother. 1997, 51, 397–403.
- Hall, A.; Troupin, A.; Londono-Renteria, B.; Colpitts, T.M. Garlic Organosulfur Compounds Reduce Inflammation and Oxidative Stress during Dengue Virus Infection. Viruses 2017, 9, 159.
- Li, Y.N.; Huang, F.; Liu, X.L.; Shu, S.N.; Huang, Y.J.; Cheng, H.J.; Fang, F. Allium sativum-derived allitridin inhibits Treg amplification in cytomegalovirus infection. J. Med. Virol. 2013, 85, 493–500.
- Yi, X.; Feng, F.; Xiang, Z.; Ge, L. The effects of allitridin on the expression of transcription factors T-bet and GATA-3 in mice infected by murine cytomegalovirus. J. Med. Food 2005, 8, 332–336.
- Kaschula, C.H.; Hunter, R.; Parker, M.I. Garlic-derived anticancer agents: Structure and biological activity of ajoene. Biofactors 2010, 36, 78–85.
- Yi, L.; Su, Q. Molecular mechanisms for the anti-cancer effects of diallyl disulfide. Food Chem. Toxicol. 2013, 57, 362–370.
- Choi, H.J.; Bae, E.Y.; Song, J.H.; Baek, S.H.; Kwon, D.H. Inhibitory effects of orobol 7-O-D-glucoside from banaba (Lagerstroemia speciosa L.) on human rhinoviruses replication. Lett. Appl. Microbiol. 2010, 51, 1–5.
- Pevear, D.C.; Tull, T.M.; Seipel, M.E.; Groarke, J.M. Activity of pleconaril against enteroviruses. Antimicrob. Agents Chemother. 1999, 43, 2109–2115.
- Burgett, A.W.; Poulsen, T.B.; Wangkanont, K.; Anderson, D.R.; Kikuchi, C.; Shimada, K.; Okubo, S.; Fortner, K.C.; Mimaki, Y.; Kuroda, M.; et al. Natural products reveal cancer cell dependence on oxysterol-binding proteins. Nat. Chem. Biol. 2011, 7, 639–647.
- Albulescu, L.; Bigay, J.; Biswas, B.; Weber-Boyvat, M.; Dorobantu, C.M.; Delang, L.; van der Schaar, H.M.; Jung, Y.S.; Neyts, J.; Olkkonen, V.M.; et al. Uncovering oxysterol-binding protein (OSBP) as a target of the anti-enteroviral compound TTP-8307. Antivir. Res. 2017, 140, 37–44.
- Roberts, B.L.; Severance, Z.C.; Bensen, R.C.; Le-McClain, A.T.; Malinky, C.A.; Mettenbrink, E.M.; Nuñez, J.I.; Reddig, W.J.; Blewett, E.L.; Burgett, A.W.G. Differing activities of oxysterol-binding protein (OSBP) targeting anti-viral compounds. Antivir. Res. 2019, 170, 104548.
- Albulescu, L.; Strating, J.R.; Thibaut, H.J.; van der Linden, L.; Shair, M.D.; Neyts, J.; van Kuppeveld, F.J. Broad-range inhibition of enterovirus replication by OSW-1, a natural compound targeting OSBP. Antivir. Res. 2015, 117, 110–114.
- Alonso-Álvarez, S.; Pardal, E.; Sánchez-Nieto, D.; Navarro, M.; Caballero, M.D.; Mateos, M.V.; Martín, A. Plitidepsin: Design, development, and potential place in therapy. Drug Des. Devel Ther. 2017, 11, 253–264.
- Gomes, N.G.M.; Valentão, P.; Andrade, P.B.; Pereira, R.B. Plitidepsin to treat multiple myeloma. Drugs Today 2020, 56, 337–347.
- Wei, T.; Li, D.; Marcial, D.; Khan, M.; Lin, M.H.; Snape, N.; Ghildyal, R.; Harrich, D.; Spann, K. The eukaryotic elongation factor 1A is critical for genome replication of the paramyxovirus respiratory syncytial virus. PLoS ONE 2014, 9, e114447.
- Duke, S.O. Benefits of Resveratrol and Pterostilbene to Crops and Their Potential Nutraceutical Value to Mammals. Agriculture 2022, 12, 368.
- Kala, R.; Shah, H.N.; Martin, S.L.; Tollefsbol, T.O. Epigenetic-based combinatorial resveratrol and pterostilbene alters DNA damage response by affecting SIRT1 and DNMT enzyme expression, including SIRT1-dependent γ-H2AX and telomerase regulation in triple-negative breast cancer. BMC Cancer 2015, 15, 672.
- Liu, T.; Liu, P.Y.; Marshall, G.M. The critical role of the class III histone deacetylase SIRT1 in cancer. Cancer Res. 2009, 69, 1702–1705.
- Anand David, A.V.; Arulmoli, R.; Parasuraman, S. Overviews of Biological Importance of Quercetin: A Bioactive Flavonoid. Pharmacogn. Rev. 2016, 10, 84–89.
- Lee, W.J.; Chen, Y.R.; Tseng, T.H. Quercetin induces FasL-related apoptosis, in part, through promotion of histone H3 acetylation in human leukemia HL-60 cells. Oncol. Rep. 2011, 25, 583–591.
- Saiko, P.; Pemberger, M.; Horvath, Z.; Savinc, I.; Grusch, M.; Handler, N.; Erker, T.; Jaeger, W.; Fritzer-Szekeres, M.; Szekeres, T. Novel resveratrol analogs induce apoptosis and cause cell cycle arrest in HT29 human colon cancer cells: Inhibition of ribonucleotide reductase activity. Oncol. Rep. 2008, 19, 1621–1626.
- Koushki, M.; Amiri-Dashatan, N.; Ahmadi, N.; Abbaszadeh, H.A.; Rezaei-Tavirani, M. Resveratrol: A miraculous natural compound for diseases treatment. Food Sci. Nutr. 2018, 6, 2473–2490.
- Parashar, G.; Capalash, N. Promoter methylation-independent reactivation of PAX1 by curcumin and resveratrol is mediated by UHRF1. Clin. Exp. Med. 2016, 16, 471–478.
- Izquierdo-Torres, E.; Hernández-Oliveras, A.; Meneses-Morales, I.; Rodríguez, G.; Fuentes-García, G.; Zarain-Herzberg, Á. Resveratrol up-regulates ATP2A3 gene expression in breast cancer cell lines through epigenetic mechanisms. Int. J. Biochem. Cell Biol. 2019, 113, 37–47.
- Kala, R.; Tollefsbol, T.; Li, Y. Potential of resveratrol in inhibiting cancer and slowing aging. J. Nutr. Food Sci. S 2012, S5, 001.
- Bijak, M. Silybin, a Major Bioactive Component of Milk Thistle (Silybum marianum L. Gaernt.)-Chemistry, Bioavailability, and Metabolism. Molecules 2017, 22, 1942.
- Karim, M.; Saul, S.; Ghita, L.; Sahoo, M.K.; Ye, C.; Bhalla, N.; Lo, C.-W.; Jin, J.; Park, J.-G.; Martinez-Gualda, B.; et al. Numb-associated kinases are required for SARS-CoV-2 infection and are cellular targets for antiviral strategies. Antivir. Res. 2022, 204, 105367.
- Edwards, R.B.; Lucas, D.M.; Lozanski, G.; Johnson, A.J.; Su, B.-N.; Lin, T.S.; Byrd, J.C.; Kinghorn, A.D.; Grever, M.R. Silvestrol, a Rocaglate Derivative from the Indonesian Plant Aglaia foveolata, Has Significant Bcl-2- and p53-Independent Anti-Tumor Activity against Chronic Lymphocytic Leukemia Cells. Blood 2006, 108, 2600.
- Chu, J.; Galicia-Vázquez, G.; Cencic, R.; Mills, J.R.; Katigbak, A.; Porco, J.A., Jr.; Pelletier, J. CRISPR-Mediated Drug-Target Validation Reveals Selective Pharmacological Inhibition of the RNA Helicase, eIF4A. Cell Rep. 2016, 15, 2340–2347.
- Biedenkopf, N.; Lange-Grünweller, K.; Schulte, F.W.; Weißer, A.; Müller, C.; Becker, D.; Becker, S.; Hartmann, R.K.; Grünweller, A. The natural compound silvestrol is a potent inhibitor of Ebola virus replication. Antivir. Res. 2017, 137, 76–81.
- Elgner, F.; Sabino, C.; Basic, M.; Ploen, D.; Grünweller, A.; Hildt, E. Inhibition of Zika Virus Replication by Silvestrol. Viruses 2018, 10, 149.
- Henss, L.; Scholz, T.; Grünweller, A.; Schnierle, B.S. Silvestrol Inhibits Chikungunya Virus Replication. Viruses 2018, 10, 592.
- M de Figueiredo, S.; S Binda, N.; A Nogueira-Machado, J.; A Vieira-Filho, S.; B Caligiorne, R. The antioxidant properties of organosulfur compounds (sulforaphane). Recent. Pat. Endocr. Metab. Immune Drug Discov. 2015, 9, 24–39.
- Jiang, L.-L.; Zhou, S.-J.; Zhang, X.-M.; Chen, H.-Q.; Liu, W. Sulforaphane suppresses in vitro and in vivo lung tumorigenesis through downregulation of HDAC activity. Biomed. Pharmacother. 2016, 78, 74–80.
- Dos Santos, P.W.d.S.; Machado, A.R.T.; De Grandis, R.A.; Ribeiro, D.L.; Tuttis, K.; Morselli, M.; Aissa, A.F.; Pellegrini, M.; Antunes, L.M.G. Transcriptome and DNA methylation changes modulated by sulforaphane induce cell cycle arrest, apoptosis, DNA damage, and suppression of proliferation in human liver cancer cells. Food Chem. Toxicol. 2020, 136, 111047.
- Zeng, A.; Wang, D.; Wu, N.; Yang, R.; Nan, G.; Bian, X.; Yang, G. Extraction of Tanshinone IIA and Cryptotanshinone from the Rhizome of Salvia miltiorrhiza Bunge: Kinetics and Modeling. Sep. Sci. Technol. 2014, 49, 2330–2337.
- Wang, L.; Zhang, C.; Guo, Y.; Su, Z.-Y.; Yang, Y.; Shu, L.; Kong, A.-N.T. Blocking of JB6 cell transformation by tanshinone IIA: Epigenetic reactivation of Nrf2 antioxidative stress pathway. AAPS J. 2014, 16, 1214–1225.
- Valipour, M. Therapeutic prospects of naturally occurring p38 MAPK inhibitors tanshinone IIA and pinocembrin for the treatment of SARS-CoV-2-induced CNS complications. Phytother. Res. 2023, 37, 3724–3743.
- Cheng, Y.; Sun, F.; Wang, L.; Gao, M.; Xie, Y.; Sun, Y.; Liu, H.; Yuan, Y.; Yi, W.; Huang, Z.; et al. Virus-induced p38 MAPK activation facilitates viral infection. Theranostics 2020, 10, 12223–12240.
- Machado, D.G.; Neis, V.B.; Balen, G.O.; Colla, A.; Cunha, M.P.; Dalmarco, J.B.; Pizzolatti, M.G.; Prediger, R.D.; Rodrigues, A.L. Antidepressant-like effect of ursolic acid isolated from Rosmarinus officinalis L. in mice: Evidence for the involvement of the dopaminergic system. Pharmacol. Biochem. Behav. 2012, 103, 204–211.
- Zhang, C.; Wang, C.; Li, W.; Wu, R.; Guo, Y.; Cheng, D.; Yang, Y.; Androulakis, I.P.; Kong, A.N. Pharmacokinetics and Pharmacodynamics of the Triterpenoid Ursolic Acid in Regulating the Antioxidant, Anti-inflammatory, and Epigenetic Gene Responses in Rat Leukocytes. Mol. Pharm. 2017, 14, 3709–3717.
- Bungau, S.; Vesa, C.M.; Abid, A.; Behl, T.; Tit, D.M.; Purza, A.L.; Pasca, B.; Todan, L.M.; Endres, L. Withaferin A-A Promising Phytochemical Compound with Multiple Results in Dermatological Diseases. Molecules 2021, 26, 2407.
