The food processing industry is a continuously developing sector that uses innovative technologies to efficiently process food products. During processing, food industries generate substantial amounts of by-products in the form of waste materials. This food waste consists of organic matter rich in bioactive compounds, such as polyphenols, carotenoids, and flavonoids. Improper management of food waste can adversely affect both the environment and human health, leading to environmental pollution and the release of greenhouse gas emissions. Thus, proper food waste management has become an urgent global issue. The presence of bioactive compounds (mainly polyphenols, flavonoids, and anthocyanins, but also carotenoids, alkaloids, proteins, lipids, and carbohydrates) in food waste holds the potential to transform them into valuable resources. Several sectors, including food and energy, have recognized food waste as an innovative source.
- food waste
- valorization
- bioactive compounds
- extraction
- pulsed electric field
1. Introduction
2. Method Principles and Parameters Affecting Bioactive Compound Extraction
2.1. PEF Apparatus
A standard PEF processing unit consists of essential components, including a pulse generator, treatment chamber, and monitoring devices (Figure 1). The primary component generates high-voltage pulses that possess the requisite attributes in terms of duration and magnitude. The pulses that are produced are administered to a set of electrodes located within the treatment chamber, with the treated product being positioned between these electrodes [39]. Based on the type of product undergoing PEF processing (i.e., solid, liquid), treatment chambers can be categorized into two distinct classifications: batch treatment chambers and continuous treatment chambers [40]. The static treatment chamber is ideal for solid food processing.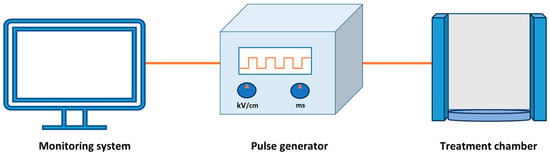
2.2. Solvent and Sample Characteristics
2.3. Electric Field Strength
Electric field strength is an important parameter in calculating extraction degree since it influences the physical characteristics of the targeted molecule, such as surface tension, diffusivity, solubility, and viscosity [44]. The PEF technique employs electric waves characterized by a high amplitude of voltage. Any product positioned within the chamber is subjected to brief electrical pulses, ranging from μs to ms in duration, that exhibit high voltage levels, typically ranging from 10 to 80 kV/cm [45]. Modifications to the technique conditions, including the electric field strength, pulse frequency, pulse width, shape of the pulse wave, and exposure time (which is influenced by the flow rate and volume of fluid in the electrode chamber), can be made depending on the desired effects and the specific properties of the processed food product [46]. Porosity is a flexible characteristic; thus, electroporation can result in reversible or irreversible effects. According to Vaessen et al. [47], an electric breakdown can be reversed if the induced holes are small compared to the membrane surface area and are caused by low-intensity PEF treatment (0.5–3 kV/cm). Prolonged and intensified treatment (15–40 kV/cm) causes the development of large pores, leading to irreversible membrane rupture [46][47]. Figure 2 illustrates the electroporation process by the efficacy of membrane electroporation is dependent upon several factors, involving electric field strength, duration, membrane constitution, pulse parameters (e.g., pulse count, duration, strength, and repetition rate), treatment technique, treatment chamber arrangement, ambient medium, and cellular morphology and dimensions [48][49].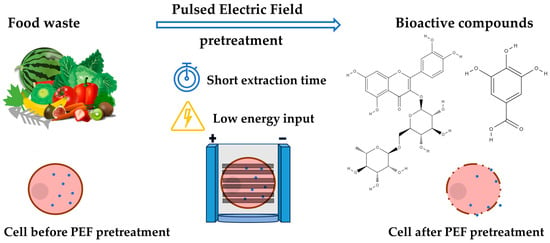
2.4. Temperature and Time of PEF Extraction
Temperature is a key factor that influences the technique of PEF extraction. Due to the nonthermal nature of PEF extraction technique, it is often performed at ambient temperatures. Elevated temperatures (>90 °C) typically lead to a reduction in the viscosity of liquid solvents, thereby detrimentally impacting the extraction technique [50]. The duration of treatment, including the number of pulses and the width of each pulse, is an additional parameter that can be used to assess the effectiveness of PEF. However, an extended duration of treatment may result in an elevation of the product’s temperature [43].3. Applications of PEF in Food Waste
3.1. Fruit Waste
3.1.1. Grapes
Grape by-products usually consist of peels, seeds, marc, stems, pulp, and pomace. Corrales et al. [51] investigated the valorization of grape by-products (skins, stems, and seeds) by extracting the bioactive compounds polyphenols and anthocyanins. They combined conventional extraction (stirring) at 70 °C with emerging novel pretreatment technologies like ultrasonication (US) (at 35 KHz), high hydrostatic pressure (HHP) (at 600 MPa), and PEF (at 3 kV/cm) in order to maximize the anthocyanin extraction. A pulse voltage of 9 kV was employed, leading to an electric field strength of 3 kV/cm. A series of 30 pulses was administered in order to achieve a targeted energy input of 10 kJ/kg. The overall temperature rise after the pretreatment was below 3 °C. The pulse repetition rate utilized in the experiment was 2 Hz, while the total duration of the treatment was 15 s. The results showed that total polyphenol content (TPC) was measured at ~200 μmol GAE/g dm. Both the pretreatments of US and PEF resulted in a ~75% increase in TPC, while HHP led to a 50% increase from the control extraction (~200 μmol GAE/g dm). In antiradical activity (AAR), the conventional extraction recorded ~200 μmol TE/g dm. The extractions performed using PEF showed a four-fold increase, HHP resulted in a three-fold increase, and the US led to a two-fold increase compared to the control extraction. Regarding anthocyanins extraction, PEF pretreatment was the most efficient technique to extract anthocyanins monoglucosides and acylated anthocyanins monoglucosides, recording a total ~77% increase from the conventional extraction, ~81% from US, and ~25% from HHP. However, the extraction of some acylated anthocyanins was more efficient with HHP pretreatment. HHP has the potential to lower the pH level due to the deprotonation of the extracted compounds. This decrease in pH level results in a better extraction of these compounds since, at pH < 4, they are in a more stable form. Thise study proved that the utilization of efficient extraction technologies and cost-effective raw materials can eliminate the need for large quantities of organic solvents and extended extraction periods, thereby circumventing conventional extraction procedures. In another study regarding the valorization of grape pomace, Carpentieri et al. [52] examined the effectiveness of pretreating white grape pomace with PEF in order to enhance the extraction of bioactive compounds, specifically total polyphenols and flavonoids, which possess strong antioxidant properties. Water and ethanol were the solvents used for extraction. The extracts obtained from both untreated and PEF-treated samples were analyzed using HPLC-PDA analysis. To optimize the PEF treatment along with the extraction technique, response surface methodology was used in each case. Under optimal conditions (electric field strength 3.8 kV/cm and energy input 10 kJ/kg) before solid–liquid extraction at a fixed temperature of 50 °C using 50% ethanol enhanced the extraction yield of bioactive compounds. The extracts obtained from samples treated with PEF exhibited significantly higher TPC by 8%, total flavonoid content by 31%, and ferric-reducing antioxidant power (FRAP) values by 36% compared to the control extraction. Epicatechin, p-coumaric acid, and quercetin were identified as the major phenolic components recovered by HPLC analysis. Furthermore, no degradation occurred as a result of the PEF application. Based on the information presented in the studies above, it can be concluded that the utilization of low voltage (ranging from 1 to 5 kV/cm) demonstrates efficacy in the recovery of polyphenols from grape processing by-products. The observed improvement leads to the enhancement of the antioxidant properties of the extracts, thereby transforming the by-products into value-added compounds. PEF has actually proved to be the best extraction technique in grapes waste. Meini et al. [53] implemented enzymatic extraction on grape pomace in acetate buffer using three different enzymes: pectinase, cellulase, and tannase. The TPC they obtained were 0.76 and 0.74 mg GAE/g grape pomace in the case of cellulase and tannase, respectively. In another study conducted by Pintać et al. [54], six solvents were evaluated in moderate shaking in order to evaluate their efficacy. The best one was proved to be 80% methanol, which resulted in a higher yield. Caldas et al. [55] evaluated UAE and MAE, along with conventional extraction. UAE was found to be the best extraction technique, 80 mg GAE/g. Up until now, PEF seems to be a more effective pretreatment technique, increasing the recoveries of the target compounds, but not to a great extent.3.1.2. Citrus Fruits
Orange, lemon, and Satsuma mandarin belong to the Citrus genus. Commonly, their main by-products are their peels, which can be divided into albedo and flavedo. Luengo et al. [56] investigated the effect of PEF treatment on the extraction of polyphenols and flavonoids from orange peel by pressing. The highest Zp was achieved with a treatment time of 60 μs (20 pulses of 3 μs) at the various electric field strengths tested. The results showed that a PEF treatment of 5 kV/cm increased the amounts of naringin from 1 to 3.1/100 g fw and of hesperidin from 1.3 to 4.6 mg/100 g fw, respectively. Furthermore, the overall extraction yield of polyphenols was quantified to be 34.80 mg/100 g fw. Notably, the application of PEF at an electric field strength of 1, 3, 5, and 7 kV/cm resulted in a respective increase of 20, 129, 153, and 159% in the extraction yield of polyphenols from orange peel. PEF treatments of 1, 3, 5, and 7 kV/cm improved the antioxidant activity of the extract by 51, 94, 148, and 192%, respectively, as compared to the untreated sample. The measured total polyphenol yield in orange peel waste was substantially lower than found in other studies using stirred-tank extraction with organic solvents [56] or UAE [57]. However, the primary advantages of the PEF technique compared to other techniques for enhancing polyphenol extraction from orange peels through pressing are its ability to avoid sample dehydration and its utilization of water as a solvent. Furthermore, it is a cost-effective and environmentally feasible alternative to conventional extraction techniques, which require the product to be dried, use large amounts of organic solvents, and need long extraction times. Afifi et al. [58] conducted a comprehensive comparison of four extraction techniques: conventional, US, HHP, and PEF. The aim was to determine the most effective technique for extracting bioactive compounds from the orange peels. The investigation demonstrated that the extracts derived from this waste product contain a diverse range of bioactive compounds, with a particular focus on polymethoxy flavones. The antioxidant activity of the flavedo samples was found to be significantly higher when subjected to extraction techniques involving PEF and HHP, thereby demonstrating superior effectiveness compared to alternative extraction techniques. The ethyl acetate extract had the most notable antioxidant properties among the albedo samples. The aforementioned phenomenon can be ascribed to the distinctive qualitative composition of the compounds, as opposed to merely possessing larger quantities of identical metabolites. The study determined that the most effective processing conditions for extracting albedo using HHP and PEF were observed at a pressure of 200 MPa and an energy input of 15 kJ/kg, with a voltage of 10 kV/cm. The results showed that the most efficient conditions for extracting flavedo were achieved by subjecting the sample to HHP at a moderate pressure level of 400 MPa in conjunction with PEF treatment at an energy input of 15 kJ/kg and an electric field strength of 3 kV/cm. The analysis yielded a collective count of 57 metabolites, wherein 15 metabolites were found to be significantly present in both the flavedo and albedo. This finding suggests a noteworthy qualitative convergence of dispersed flavonoids. The chemometric analysis of the dataset indicates that orange flavedo can be considered a dependable source of soluble polyphenols, specifically polymethoxy flavones. Peiró et al. [59] examined the impact of varying intensities of PEF (3–9 kV/cm and 0–300 μs treatment time pulses) on the extraction of total polyphenols from lemon (Citrus limon) peel residues through pressing. Zp determined that the optimal treatment duration for increasing permeability is 30 pulses of 3 μs (total 90 μs) and electric field strength of 7 kV/cm, as statistically non-significant (p > 0.05) differences were found between 7 and 9 kV/cm. In contrast to the control samples, the impact of PEF was found to be unrelated to the size of lemon residue (1, 2, and 3 cm) in polyphenol extraction yield. However, PEF treatment significantly increased TPC, as an average of ~160 mg GAE/100 g of dw was an up to ~60% increase from the control sample, whereas 3 cm lemon peels were selected as optimal. The concentrations of hesperidin and eriocitrin, which are the predominant polyphenols present in lemon residues, were observed to exhibit a notable increase in response to the application of pressure and electric field. This resulted in an approximate 300% increase, yielding maximum observed values of hesperidin (84 mg/100 g fw) and eriocitrin (176 mg/100 g fw).3.1.3. Olives
The main by-products from olive trees are leaves, kernels, and olive mill wastewater. Roselló-Soto et al. [60] explored the possibility of enhancing the recovery of intracellular valuable bioactive compounds from olive (Olea europaea) kernels. Pretreatments such as high-voltage electrical discharges (HVED), PEF, and US were employed prior to extraction. The study examined the impact of energy in HVED with varying input levels (0–109 kJ/kg), pH values (2.5–12), and ethanol concentrations (0–50%) on extraction efficiency. PEF conditions required an electric field strength of 13.3 kV/cm, pulse length of 10 μs, and pulse rate of 10 Hz. Total polyphenols, proteins, antioxidant activity, and pigments were all determined in the extracts produced following corresponding pretreatments. The superiority of HVED treatment over US and PEF in terms of energy input and effective treatment time for the extraction of polyphenols and proteins was demonstrated. In the same energy input of 109 kJ/kg, the TPC was found to be 255 mg GAE/L for high-voltage electrical discharges, while for US and PEF, it was 140 mg GAE/L and 146 mg GAE/L, respectively. One potential hypothesis is that the fragmentation of treated particles of olive kernels occurs when electrical discharges are applied to various biological materials. This fragmentation might be caused by the propagation of shock waves and the subsequent explosion of cavitation bubbles. This technique is thought to enhance the extraction of polyphenols, as suggested by Ohsima et al. [61]. The same research team, Pappas et al. [62], evaluated the olive leaves valorization by maximizing polyphenol extract concentration by using PEF. They conducted a thorough investigation into the optimization of key parameters in PEF technology for the extraction of olive leaves. They focused on the extraction chamber geometry, electric field strength, pulse duration, pulse period (and frequency), and extraction duration. Through their experiments, they were able to gain insights into the optimal PEF assistance span for static solid–liquid extraction of olive leaves. PEF-treated extracts were compared to extracts obtained without the use of PEF. The results showed that the contribution of PEF on the extractability of total polyphenols (25.35 mg GAE/g dw) showed an increase of ~38% from the control sample. Furthermore, specific metabolites exhibited a remarkable increase of ~117%. This optimal contribution was observed when using a rectangular extraction chamber, a solvent mixture of 25% v/v ethanol, an electric field strength of 0.85 kV/cm, a pulse period of 100 μs with a pulse duration of 2 μs, and an extraction duration of 15 min. Regarding oxidative stability of the samples tested with differential scanning calorimetry, samples treated with a pulse duration of 10 μs, pulse period of 1000 μs, electric field of 0.85 kV/cm, and a time of extraction of 30 min exhibited the highest oxidation peak at 488 °C, which was higher by ~16% compared to the control sample. These findings highlighted the significant impact of PEF assistance on extraction performance and physicochemical properties enhancement, which were influenced by the specific parameters chosen.3.1.4. Blueberry
Berry fruit by-products mainly consist of skins and seeds. They also contain their pomace and their press cake. Bobinaitė et al. [63] examined the application of PEF pretreatment in order to enhance the permeability of cell membranes in fresh blueberry (Vaccinium myrtillus L.) tissue prior to pressing. The ultimate goal was to improve both the quality and quantity of the obtained juice that came from mechanical pressing, as well as to increase the extraction yield of bioactive compounds from the by-products of blueberry processing (press cake). The impact of varying combinations of electric field strengths (1, 3, and 5 kV/cm) and total specific energy input (1, 5, and 10 kJ/kg) on the Zp of blueberry juice yield and tissue was investigated. PEF treatment intensity of 1 kV/cm and 5 kJ/kg yielded the lowest Zp (0.61). The strongest treatment (5 kV/cm and 10 kJ/kg) boosted Zp to 0.87, and this specific energy input value was chosen for further procedures. In blueberry juice, PEF treatment at 1 or 3 kV/cm increased the extraction yield by ~38% compared to untreated samples. Electric field strength variation showed statistically non-significant differences (p < 0.05) in TPC, as PEF-treated samples averaged ~110 mg GAE/100 mL of juice, but this value was considerably higher than the control sample (~46%). The same pattern was observed in both total anthocyanin content (TAC) and FRAP, with PEF-treated samples increasing this content by ~60% and ~33% compared to the control sample, respectively (~25 mg/100 mL of juice and ~4.5 μmol TE/mL of juice). In blueberry press cake, however, the results revealed the statistically significant (p < 0.05) impact in each electric field strength value of all the above values per gram or 100 g of press cake. Comparing the PEF-treated sample at 10 kV/cm with the control sample, TPC, TAC, and FRAP recorded an increase up to ~79% (~1800 mg GAE/100 g of press cake), ~106% (~1600 mg/100 g of press cake), and ~62% (~60 μmol TE/g of press cake), respectively. Thise study showed that PEF treatment could also be utilized for solid waste, which had better results compared to liquid samples. Pataro et al. [64] also assessed the utilization of PEF treatment in blueberry by-products (press cake). The electric field strength was increased to 3 kV/cm, and the energy input (1–10 kJ/kg) was modified prior to pressing in order to enhance both the quantity and quality of the expressed juice from blueberry fruits. Additionally, anthocyanins were extracted from the by-products of blueberry pressing, specifically from the press cake, using solid–liquid extraction techniques. The juice obtained from the pretreated sample, subjected to PEF treatment at an energy level of 1 kJ/kg, exhibited a significant increase of ~32% when compared to the untreated sample, which had a juice output of 42.7 g/100 g of fw. The application of higher energy input, specifically 10 kJ/kg, resulted in the most favorable outcome in terms of the largest increase in anthocyanin content by ~55%. Additionally, the antioxidant capacity, as determined by FRAP and DPPH assay, exhibited an increase of approximately ~36% and ~41%, respectively, in the juice. The extracts derived from the press cake of blueberries treated with PEF at an input energy of 10 kJ/kg exhibited higher anthocyanin content (~75%) and antioxidant capacity (determined by FRAP and DPPH tests at ~71% and ~109%, respectively) compared to extracts obtained from untreated blueberries. There was no significant degradation of individual anthocyanins following the application of PEF. HPLC analysis revealed that the predominant types of anthocyanins found were glycosides of delphinidin, cyanidin, petunidin, peonidin, and malvidin. The findings of the study indicate that the application of PEF as a pretreatment technique for blueberries and their by-products (press cake) shows promising results in facilitating the extraction of juice and antioxidants.3.1.5. Prunus Fruits
Cherry, peach, and apricot belong to the Prunus genus. Their main by-products are their pomace, peels (or skin), kernels (seeds), and leaves. In the case of almond, which also belongs to the Prunus genus, its main waste is its hull. The research conducted by Pataro et al. [65] focused on investigating the impact of PEF pretreatment on the extraction yield and antioxidant properties of juice obtained from the “Duroni Nero” variety of sweet cherry (Prunus avium L.) fruits. Additionally, the study examined the extraction of bioactive compounds from cherry by-products (specifically, press cake). PEF pretreatment was carried out with a specific energy input of 10 kJ/kg and electric field strength (0.5–3 kV/cm) before a pressure of 1.64 bar was applied for 5 min. The application of PEF with an electric field strength of 1 kV/cm resulted in a significant enhancement in juice extraction, with a notable increase of 40% in juice yield. Furthermore, the PEF-assisted pressing technique exhibited a substantial improvement of 80% in anthocyanin content and a 27% increase in antioxidative activity when compared to untreated samples. The application of PEF to press cake extracts resulted in a significant increase of 38% in anthocyanin content and 21% in antioxidant activity compared to untreated samples. This enhancement was observed at an electric field strength of 0.5 kV/cm. PEF treatment of juice and press cake extracts did not result in the degradation of certain anthocyanins. Overall, the findings of thise study revealed the potential of PEF as a moderate technique to increase the efficiency of industrial cherry fruit processing. Makrygiannis et al. [66] investigated the valorization of defatted biomass of apricot (Prunus armeniaca) kernels to generate extracts with high polyphenol content. PEF was evaluated as an independent extraction technique and as an addition to the prior extraction technique, utilizing water as solvent. Deep eutectic solvent (DES) and PEF integration were examined to increase extraction yield. The samples underwent PEF treatment for a total duration of 15 min, utilizing an electric field strength of 1 kV/cm. The pulses lasted for 10 μs and occurred at a frequency of 1000 μs. The defatted apricot kernel biomass was stirred for 15 min with water or deep eutectic solvent (glycerol:choline chloride 2:1 w/w), and the samples underwent PEF treatment for 15 min, either with extraction for 3 h at 60 °C or PEF + extraction. The results indicated that applying PEF prior to the extraction procedure resulted in an 88% increase in TPC. Similarly, the utilization of DES resulted in an approximately 70% increase in TPC. The combination of the two approaches resulted in a 173% increase (~12 mg GAE/g dw). DES with PEF prior to extraction was found to be the most effective way to extract bioactive compounds from defatted apricot kernels. Thus, a similar pattern was observed in total flavonoid content (TFC), FRAP, and AAR. Consequently, by implementing the above parameters, TFC, PR, and AAR were measured at ~10 mg RtE/g dw, ~18 μmol AAE/g dw, and ~12 μmol AAE/g dw. The corresponding increases were ~150%, ~80%, and ~71% compared to the control extraction with water, respectively. Thise study highlighted that low-voltage PEF treatment could be employed with several green solvents, such as deep eutectic solvents, in order to increase bioactive compound extraction.3.1.6. Quince
Quince by-products include its peels and leaves. Athanasiadis et al. [67] studied the recovery of bioactive compounds from quince peels. Firstly, they investigated the effects of various extraction parameters such as solvent composition, temperature, and time, as well as techniques such as PEF and US, either separately or in conjunction. Then, they optimized these parameters using response surface methodology, aiming to enhance the extraction of bioactive compounds. The PEF conditions utilized were an electric field strength of 1 kV/cm, a pulse duration of 10 μs, a pulse period of 1 ms, and a frequency of 1000 Hz. The US treatment was carried out in a bath, operating at 3 kHz, at 30 °C for 20 min. The most effective extraction technique proved to be simple, economical stirring at a relatively high temperature of 65 °C for 120 min. Following the application of principal component analysis and partial least squares analysis, it has been determined that quince peels exhibit notable quantities of various compounds. These include total polyphenols (43.99 mg gallic acid equivalents/g dw), total flavonoids (3.86 mg rutin equivalents/g dw), chlorogenic acid (2.12 mg/g dw), and ascorbic acid (543.93 mg/100 g dw). Additionally, the antioxidant activity of the quince peels has been measured to be 627.73 μmol AAE/g dw and 699.61 μmol DPPH/g dw through the employment of FRAP and DPPH assays, respectively. Nevertheless, PEF pretreatment of the samples appeared to enhance the DPPH radical scavenging value, thus increasing the antioxidant properties of the extracts.3.1.7. Papaya
An investigation conducted by Parniakov et al. [68] focused on comparing the efficiency of PEF and HVED in extracting papaya (Carica papaya) peels for their antioxidant and nutraceutical components. In thise study, the influence of varying pH levels (2.5–11) and temperatures (20–60 °C) on the extraction efficiency of nutritionally advantageous compounds was explored. Furthermore, a two-step extraction technique was utilized, wherein the sample was initially subjected to PEF treatment, followed by a subsequent aqueous extraction technique known as supplementary aqueous extraction (SAE), conducted at a relatively low temperature of 50 °C. The variation in extraction temperature or the use of a basic medium with a pH of 11 did not consistently result in higher yields or TEAC values. The protein content and TEAC values at pH levels of 7 and 11 exhibited a significant decrease when the temperature rose from 50 to 60 °C. The extraction of high-value chemicals using HVED demonstrated superior performance compared to PEF-assisted extraction. The protein concentrations utilized in the HVED- and PEF-assisted extractions were 60 and 20 mg/L, respectively. The experiments were performed under specific conditions, including a pH of 7, a temperature of 20 °C, and an extraction time of 45 min. The utilization of electrical discharges, turbulence, and pill fragmentation has been found to significantly enhance the efficiency of HVED-assisted extraction. Chemical by-products can be produced through the process of electrolysis and the generation of reactive free radicals resulting from electrical discharges. The aforementioned by-products possess the ability to decompose compounds that possess a significant amount of nutritional value. The combination of PEF treatment and subsequent aqueous extraction at 50 °C resulted in enhanced yields of papaya peel components and increased antioxidant capacities, even when the pH conditions were neutral.3.1.8. Mango
Parniakov et al. [69] implemented PEF pretreatment in order to recover bioactive compounds from mango peels. In their work, they examined the efficacy of traditional extraction at various pH values, ranging from 2.5 to 11, and various temperatures, varying between 20 and 60 °C. Then, they investigated the extraction aided by PEF or HVED of compounds identified in mango peels that are nutritionally valuable. For PEF and HVED treatments, exponential decay pulses with initial electric field strengths of 13.3 kV/cm and 40 kV/cm, respectively, were utilized. The aqueous extraction of proteins and carbohydrates was not influenced by temperature. Aqueous extraction at 60 °C and pH 6 yielded the highest concentrations of antioxidant and nutritionally valuable compounds, but the extracts were not stable. The use of the two-stage PEF and supplementary aqueous extraction approach proved to be the most effective technique. The first step included PEF pretreated extraction. The second step was a supplementary extraction at pH 6, at 50 °C, for 3 h. The combination of the two provided an exceptional increase in the yields of TPC (~400%) even at normal pH levels.3.1.9. Pomegranate
In their study, Rajha et al. [70] employed various extraction techniques, including infrared (IR), US, PEF, and HVED, to facilitate the recovery of polyphenols, flavonoids, and tannins from pomegranate peels. The temperature remained constant at 50 °C for each extraction, whereas electric field strength was set at 10 kV/cm. The temperature increase observed during the PEF treatment did not surpass 5 °C and was effectively regulated through the implementation of a cold-water bath. The specific energy input for the PEF treatment ranged from 90 to 100 kJ/kg, while the energy delivered per pulse was measured to be 0.29 kJ. While the utilization of HVED-assisted extraction resulted in a 1.3-fold increase in the recovery of polyphenols compared to PEF-assisted extraction, the latter technique exhibited a higher recovery of ellagic acid. After a duration of 7 min, the phenolic extractable fraction by PEF exhibited a mean value of 39 ± 2 mg GAE/g DM. This measurement was found to be 15.22% lower than the HVED treatment. However, it was observed to be 168.97% higher than the ultrasonic US treatment, 387.5% higher than the IR treatment, and 680% superior to the water bath treatment. Thise study demonstrates the considerable effectiveness of both HVED and PEF treatments in extracting polyphenols from pomegranate peels. The effectiveness of PEF in recovering polyphenols is lower compared to HVED. However, PEF is characterized by less damage and a higher level of selectivity in its treatment technique when compared to HVED.3.2. Vegetables
3.2.1. Tomato
Commonly, tomato waste includes peel, pulp, and seeds. Luengo et al. [71] investigated the impact of applying PEF with varying intensities (3–7 kV/cm) and durations from 0 to 300 μs on the extraction of carotenoids from both tomato peel and pulp. The extraction technique was conducted using 50:25:25 v:v:v hexane:acetone:ethanol. Based on the Zp, it has been determined that the most favorable duration for the permeabilization process of tomato peel and pulp is 5 kV/cm and 90 μs (30 pulses of 3 μs). Moreover, Zp in PEF-treated pulp increased from 0 to 0.7 with increasing treatment time (0–300 μs) and was almost twice that of the Zp in PEF-treated peels. However, a switch from 5 to 7 kV/cm led to a statistically non-significant (p > 0.05) increase in carotenoid extraction of tomato peels. Compared to the untreated sample, PEF treatment at 5 kV/cm led to a 39% increase in the carotenoid extraction efficiency of tomato peels using the abovementioned solvent mixture. The inclusion of acetone in the solvent mixture did not yield a significant improvement in carotenoid extraction when tomato peels were subjected to PEF treatment. The employment of response surface methodology to assess the efficacy of PEF treatment in reducing the proportion of hexane within a hexane:ethanol mixture facilitated a decrease in the hexane concentration from 45 to 30% while maintaining the carotenoid extraction yield and keeping it unaffected. The correlation between the carotenoid concentration and the antioxidant capacity of the extracts derived from tomato peel was observed, while the application of PEF treatment did not have any significant impact on the antioxidant capacity.3.2.2. Potato
Hossain et al. [72] the influence of PEF pretreatment and pulsed light pretreatment on the recovery of steroidal alkaloids (glycoalkaloids and aglycone alkaloids) from potato peels via solid–liquid extraction. An electric field strength of 0.75 kV/cm and a total duration of 600 μs resulted in a maximum recovery of total steroidal alkaloids that was increased by ~99.9% compared to the untreated (control) peels. However, electric field strength higher than 0.75 kV/cm diminished steroidal alkaloid recoveries during treatment periods ranging from 150 to 1500 s. The quantities of glycoalkaloids and aglycone alkaloids in potato peels were likewise enhanced by pulsed-light treatments, reaching a plateau at fluences of 7.86 and 9.38 J/cm2, respectively. However, PEF treatment increased extraction yield more than pulsed-light-treated peels. For instance, α-solanine and solanidine were measured at ~80 and ~1300 μg/g dw, respectively. The corresponding values from pulsed light treatment for the above compounds were ~70 and ~600 μg/g dw, respectively.3.2.3. Carrot
Roohinejad et al. [73] assessed the recovery of β-carotene from carrot pomace via oil-in-water, utilizing a PEF pretreatment. The optimum conditions for PEF treatment were determined as an electric field strength of 0.6 kV/cm with a constant frequency of 5 Hz, a treatment duration of 3 ms, and a pulse width of 20 μs. The results showed that the β-carotene content of PEF-treated carrot pomace extracted using microemulsions was greater than that of untreated carrot pomace. To forecast the ideal extraction conditions employing transparent microemulsions with high β-carotene loading, low polydispersity index, and small microemulsion particle size, a mathematical model was developed. The model indicated that the optimum conditions were a temperature of 52.2 °C, an extraction time of 49.4 min, and a ratio of 1:70 w/w carrot/microemulsion. The extract would yield microemulsions with a polydispersity index of 0.27, a particle size of 74 nm, and α-carotene loading of 19.6 μg/g at these conditions. The results of thise study support the use of oil-in-water microemulsions for the extraction of β-carotene.3.2.4. Corn
Corn silk is known as the main agricultural corn by-product. Zhao et al. [74] implemented a response surface methodology in order to enhance the recovery of polysaccharides from corn silk via PEF pretreatment. There were three tested parameters: the electric field intensity, the pulse duration, and the liquid-to-raw solid material ratio. The experimental values of the electric field intensity were 25, 30, and 35 kV/cm. The pulse duration was between 4, 6, and 8 μs. The liquid-to-raw-solid material ratio varied between 45, 50, and 55. These independent variables were statistically analyzed, and the optimum conditions were proved to be electric field intensity of 30 kV/cm, 6 μs pulse duration, and a ratio of liquid-to-solid raw material of 50:1. Under these conditions, the yield of the polysaccharides was 2.36%, and of the crude protein was 0.14%. It has become clear that increasing the polysaccharide recovery depends on the PEF intensity. The yield of extracted polysaccharides increases as the electric field intensity under 30 kV/cm elevates.3.2.5. Onion
Skin is the main onion by-product. In a study valorizing onion, Kim et al. [75] studied the most effective extraction conditions for obtaining quercetin from dehydrated onion skin and investigated the potential enhancement of yield by combining pretreatment with PEF treatment and SWE. Onion skin samples were treated with PEF with varying electric field strengths (0.5–2.5 kV/cm) and durations (5–120 s). SWE was subsequently conducted using a 15 min extraction duration and several temperatures (105–185 °C). The highest yield of total quercetin was observed when pretreated with PEF at 2.5 kV/cm for 15 s, followed by SWE at 145 °C for 15 min (19.25 mg/g onion skin dw). The inclusion of PEF resulted in a decrease in the optimal extraction temperature (at 145 from 165 °C) and a ~31% increase in the yield of quercetin (from 14.60 mg/g dried onion skin). The results indicate that pretreating onion skin with PEF has the potential to enhance flavonoid extraction.3.3. Other
3.3.1. Coffee and Cocoa
Cocoa bean shell is the most common cocoa by-product. As for coffee seeds, their most well-known by-products are their silverskin and their parchment, which consists of their skin and pulp. Barbosa-Pereira et al. [76] implemented PEF as a new pretreatment technique in order to enhance polyphenol recovery from two food by-products, coffee silver skin, and cocoa bean shell. The optimal PEF conditions were established by a response surface methodology as 1.74 kV/cm electric field strength and 991 pulses in 11.99 μs. Then, a solid–liquid extraction followed utilizing ethanol 39.15% as a solvent for 118.54 min. The findings revealed the potential of PEF pretreatment to increase the extraction of bioactive compounds from cocoa bean shell and coffee silver skin as a greener alternative extraction option compared to traditional extraction techniques with industrial-scale use. Appropriately chosen PEF pretreatment extraction parameters customized to each matrix can increase the yield of bioactive compounds in cocoa bean shell and coffee silver skin samples by 20 and 21.3%, respectively, when compared to untreated samples and may be adopted to produce extracts with high nutritional specific phytochemical profiles.3.3.2. Rapeseed
The main residue of rapeseed is its stems. Rapeseed (Brassica napus L.), a seasonal oilseed plant cultivated during winter or spring, ranks as the third most extensively cultivated variety of vegetable oil globally, following palm and soybean oil. Yu et al. [77] investigated the recovery of bioactive compounds, such as polyphenols and proteins, from rapeseed stems and leaves. The thermocouple-controlled temperature elevation during PEF treatment did not rise above 5 °C. The maximum polyphenol recovery was obtained after treatment at 5 kV/cm. More specifically, the polyphenols extraction yield reached 52% after 1 h of extraction with the mild PEF treatment (electric field strength 0.80 kV/cm, energy input 6.4 kJ/kg, and Zp 0.7), but under the same conditions, protein yield was not significantly improved. The findings also demonstrate that the polyphenol and protein content of rapeseed stems and leaves varied depending on the maturity of the plant, which may help to pinpoint the ideal harvest period for by-product valorization.3.3.3. Drumstick Tree
Bozinou et al. [78] investigated the employment of PEF treatment in freeze-dried drumstick tree (Moringa oleifera) leaves. The comparative analysis involved evaluating the efficacy of PEF extraction in relation to other established techniques, including MAE and UAE, simple boiling water extraction, and plain maceration (serving as the control). The control sample was prepared by employing equal amounts of freeze-dried leaves, which remained in double-distilled water at ambient temperature and immersed in water for a period of 40 min, which corresponded to the duration of the PEF procedure. Electric field strength was constant at 7 kV/cm. The variables examined in thise study encompassed the pulse duration (PD), which denotes the duration of time in which the electric field is administered, and the pulse interval (PI), which represents the duration between two consecutive pulse applications. Pulse duration ranged from 10–100 ms, whereas pulse interval ranged from 25–100 μs. PEF-treated samples had eight different combinations of PD and PI. Regarding TPC, the PEF-5 sample (20 ms PD and 100 μs PI) was the most effective way by extracting 40.24 mg GAE/g dw, achieving ~45% more than the control sample. The same pattern was also observed in other antioxidant capacity assays, such as % scavenging activity and FRAP, as expected. Other PEF-treated samples with high PI (100 μs) and increasing PD (from 50 to 100 ms) had decreasing TPC. Hence, it should be highlighted that a combination of low PD and high PI is the optimal condition for the extraction of total polyphenols from freeze-dried M. oleifera leaves.3.3.4. Flaxseed
Flaxseed sole by-product is the seed hulls. An interesting study regarding hull valorization was conducted by Boussetta et al. [79]. The authors assessed the feasibility of extracting polyphenols from flaxseed hulls through the application of PEF treatment. They examined the impact of various operating parameters on the extraction of polyphenols, specifically focusing on the duration of the treatment (1–10 ms), the electric field strength (10–20 kV/cm), the composition of the solvent (including ethanol: water mixtures, 0.05–0.3 M citric or sodium hydroxide), and the duration of product rehydration (0–60 min). Flaxseed hulls underwent acidic or alkaline extraction with the solvents above while being agitated at 20 °C prior to PEF. The utilization of 50% ethanol resulted in a more efficient extraction of polyphenols (~300 mg GAE/100 g dm), achieving an increase of ~42% compared to extraction of 20%. However, the authors declared that industrial PEF applications should not exceed this ethanol level (20%), so this level was used as optimal. The findings of the study revealed that the optimum conditions of a PEF treatment were at 20 kV/cm for 10 ms, with an energy output of 300 kJ/kg, after 40 min of rehydration at 150 rpm. The use of 0.3 M citric acid obtained ~270 mg GAE/100 g of dm, whereas the use of 0.3 M sodium hydroxide obtained ~1000 mg GAE/100 g of dm. The control sample with no sodium hydroxide obtained ~200 mg GAE/100 g of dm instead. The use of PEF produced satisfactory results in the recovery of antioxidant compounds; however, it would be interesting to recover proteins from this by-product.3.3.5. Sage
Leaves and stems are the two by-products of sage. The study conducted by Athanasiadis et al. [80] had as its primary objective to assess the efficacy of PEF-assisted extraction in obtaining phytochemicals from the leaves of sage (Salvia officinalis L.). The experimental conditions involved a range of parameters, specifically the pulse duration of the PEF, which was set at either 10 or 100 μs for a duration of 30 min. They also investigated the use of various “green” extraction solvents, namely pure water, ethanol, and their mixtures at concentrations of 25–75% v/v. The extracts that were obtained as a result were assessed in comparison to reference extracts that were obtained without the use of PEF. The extraction efficiency was evaluated by determining the levels of total polyphenols, individual polyphenols, volatile compounds, and oxidation resistance. The optimal conditions involved a 25% v/v aqueous ethanol solvent with a pulse duration of 100 μs and electric field strength of 1 kV/cm, which led to the highest PEF contribution to both total and individual polyphenols, as well as rosmarinic acid extractability. This led to a significant increase of up to 73.2 and 403.1%, compared to reference extract, respectively. The findings were also validated by the differential scanning calorimetry technique. The PEF-treated extracts exhibited an average increase in oxidation temperature of 61.5% compared to the reference extracts (182 °C).3.4. Seafood
The main by-products of fishery and seafood consist of viscera, skin, bones, fins, and heads, and in the case of shrimps, their main by-product is their side streams. The utilization of fishery by-products is significant due to their abundance of biologically active compounds, such as proteins and astaxanthins. Therefore, it is crucial to employ environmentally friendly and efficient extraction techniques to retrieve these valuable compounds. To that end, a study conducted Wang et al. [81] utilized the head, skin, and viscera of rainbow trout and sole as the focal matrices. Two extraction techniques, namely accelerated solvent extraction (ASE) and PEF, were employed. ASE was conducted at temperatures ranging from 45 to 55 °C for a duration of 15 min, with pH levels between 5.2 and 6.8 and pressure set at 103.4 bars. PEF, on the other hand, involved applying electric field strength ranging from 1 to 3 kV/cm, with energy levels between 123 and 300 kJ/kg, and a duration of 15 to 24 h. The parameters of PEF were dependent on the part of the fish studied. The results of the study indicated that the utilization of both ASE and PEF treatments had ~80% protein extraction efficiency of fish by-products. The SDS-PAG electrophoresis revealed that the application of ASE and PEF treatments resulted in alterations to the molecular size distribution of the protein present in the extracts. The findings of the study indicated that the application of ASE and PEF treatments resulted in a significant enhancement of the antioxidant capacity (ORAC) in extracts obtained from the skin and head of rainbow trout and sole fish (p < 0.05). However, ASE treatment was found to be the most effective way to extract antioxidant compounds in both fish species. ASE treatments in the skin of rainbow trout and in the viscera of sole recorded ~43 and ~50% higher values than the corresponding PEF-treated samples. On the other hand, PEF was found to be more effective in ABTS assay. The utilization of PEF has proven advantageous for the extraction of soluble proteins from by-products. This technique not only serves as a substitute for the environmental pollution caused by organic reagents in conventional extraction techniques but also effectively preserves the antioxidant properties of bioactive compounds. This aligns with the demands of contemporary industry and environmental progress and is poised to significantly contribute to the future transformation and exploitation of aquatic resources.4. Conclusions
PEF technology recovers bioactive compounds, including polyphenols, pigments, and macronutrients, from food processing wastes utilizing low-energy and concentrated solvents. It may also be used to recycle waste products from various food processing industries. The primary problem with PEF applications is that the efficacy of the treatment can be affected by PEF device parameters and external factors such as conductivity, pH, and the concentration of the treated solution. Until definitive pathways are established, further investigation into the thermal, chemical, and biophysical effects of PEF on protein structures is necessary. It is also critical that authors supply all necessary experimental details so that similar studies can be carried out and outcomes can be compared.
References
- Ebrahimi, P.; Lante, A. Environmentally Friendly Techniques for the Recovery of Polyphenols from Food By-Products and Their Impact on Polyphenol Oxidase: A Critical Review. Appl. Sci. 2022, 12, 1923.
- Poojary, M.M.; Roohinejad, S.; Barba, F.J.; Koubaa, M.; Puértolas, E.; Jambrak, A.R.; Greiner, R.; Oey, I. Application of Pulsed Electric Field Treatment for Food Waste Recovery Operations. In Handbook of Electroporation; Miklavčič, D., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 2573–2590. ISBN 978-3-319-32885-0.
- Velusamy, M.; Rajan, A.; Radhakrishnan, M. Valorisation of Food Processing Wastes Using PEF and Its Economic Advances—Recent Update. Int. J. Food Sci. Technol. 2023, 58, 2021–2041.
- Arshad, R.N.; Abdul-Malek, Z.; Roobab, U.; Qureshi, M.I.; Khan, N.; Ahmad, M.H.; Liu, Z.-W.; Aadil, R.M. Effective Valorization of Food Wastes and By-Products through Pulsed Electric Field: A Systematic Review. J. Food Process Eng. 2021, 44, e13629.
- Sarangi, P.K.; Mishra, S.; Mohanty, P.; Singh, P.K.; Srivastava, R.K.; Pattnaik, R.; Adhya, T.K.; Das, T.; Lenka, B.; Gupta, V.K.; et al. Food and Fruit Waste Valorisation for Pectin Recovery: Recent Process Technologies and Future Prospects. Int. J. Biol. Macromol. 2023, 235, 123929.
- Ben-Othman, S.; Jõudu, I.; Bhat, R. Bioactives from Agri-Food Wastes: Present Insights and Future Challenges. Molecules 2020, 25, 510.
- Hao, X.; Wang, X.; Liu, R.; Li, S.; van Loosdrecht, M.C.M.; Jiang, H. Environmental Impacts of Resource Recovery from Wastewater Treatment Plants. Water Res. 2019, 160, 268–277.
- Tongkham, N.; Juntasalay, B.; Lasunon, P.; Sengkhamparn, N. Dragon Fruit Peel Pectin: Microwave-Assisted Extraction and Fuzzy Assessment. Agric. Nat. Resour. 2017, 51, 262–267.
- Roy, P.; Mohanty, A.K.; Dick, P.; Misra, M. A Review on the Challenges and Choices for Food Waste Valorization: Environmental and Economic Impacts. ACS Environ. Au 2023, 3, 58–75.
- Martirosyan, D.; Miller, E. Bioactive Compounds: The Key to Functional Foods. Bioact. Compd. Health Dis. 2018, 1, 36.
- Shen, X.; Zhang, M.; Bhandari, B.; Gao, Z. Novel Technologies in Utilization of Byproducts of Animal Food Processing: A Review. Crit. Rev. Food Sci. Nutr. 2019, 59, 3420–3430.
- Gerasopoulos, K.; Petrotos, K. 15—Utilization of Olive Mill Waste Waters to Produce Bioactive Animal Feed. In Membrane Engineering in the Circular Economy; Iulianelli, A., Cassano, A., Conidi, C., Petrotos, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 393–412. ISBN 978-0-323-85253-1.
- Sharmila, V.G.; Kavitha, S.; Obulisamy, P.K.; Banu, J.R. Chapter 8—Production of Fine Chemicals from Food Wastes. In Food Waste to Valuable Resources; Banu, J.R., Kumar, G., Gunasekaran, M., Kavitha, S., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 163–188. ISBN 978-0-12-818353-3.
- Capanoglu, E.; Nemli, E.; Tomas-Barberan, F. Novel Approaches in the Valorization of Agricultural Wastes and Their Applications. J. Agric. Food Chem. 2022, 70, 6787–6804.
- Lomartire, S.; Gonçalves, A.M.M. An Overview of Potential Seaweed-Derived Bioactive Compounds for Pharmaceutical Applications. Mar. Drugs 2022, 20, 141.
- Carocho, M.; Morales, P.; Ferreira, I.C.F.R. Antioxidants: Reviewing the Chemistry, Food Applications, Legislation and Role as Preservatives. Trends Food Sci. Technol. 2018, 71, 107–120.
- Dias, C.; Fonseca, A.M.A.; Amaro, A.L.; Vilas-Boas, A.A.; Oliveira, A.; Santos, S.A.O.; Silvestre, A.J.D.; Rocha, S.M.; Isidoro, N.; Pintado, M. Natural-Based Antioxidant Extracts as Potential Mitigators of Fruit Browning. Antioxidants 2020, 9, 715.
- Vilas-Boas, A.A.; Pintado, M.; Oliveira, A.L.S. Natural Bioactive Compounds from Food Waste: Toxicity and Safety Concerns. Foods 2021, 10, 1564.
- Gowman, A.C.; Picard, M.C.; Lim, L.-T.; Misra, M.; Mohanty, A.K. Fruit Waste Valorization for Biodegradable Biocomposite Applications: A Review. BioResources 2019, 14, 10047–10092.
- Sogut, E.; Cakmak, H. Utilization of Carrot (Daucus carota L.) Fiber as a Filler for Chitosan Based Films. Food Hydrocoll. 2020, 106, 105861.
- Tsang, Y.F.; Kumar, V.; Samadar, P.; Yang, Y.; Lee, J.; Ok, Y.S.; Song, H.; Kim, K.-H.; Kwon, E.E.; Jeon, Y.J. Production of Bioplastic through Food Waste Valorization. Environ. Int. 2019, 127, 625–644.
- Kalak, T. Potential Use of Industrial Biomass Waste as a Sustainable Energy Source in the Future. Energies 2023, 16, 1783.
- Xu, Z.; Shen, J.; Qu, Y.; Chen, H.; Zhou, X.; Hong, H.; Sun, H.; Lin, H.; Deng, W.; Wu, F. Using Simple and Easy Water Quality Parameters to Predict Trihalomethane Occurrence in Tap Water. Chemosphere 2022, 286, 131586.
- Dhua, S.; Kumar, K.; Sharanagat, V.S.; Nema, P.K. Bioactive Compounds and Its Optimization from Food Waste: Review on Novel Extraction Techniques. Nutr. Food Sci. 2022, 52, 1270–1288.
- Wang, H.; Ding, J.; Ren, N. Recent Advances in Microwave-Assisted Extraction of Trace Organic Pollutants from Food and Environmental Samples. TrAC Trends Anal. Chem. 2016, 75, 197–208.
- Moradi, N.; Rahimi, M. Effect of Simultaneous Ultrasound/Pulsed Electric Field Pretreatments on the Oil Extraction from Sunflower Seeds. Sep. Sci. Technol. 2018, 53, 2088–2099.
- Damar, I.; Yilmaz, E. Ultrasound-Assisted Extraction of Phenolic Compounds in Blackthorn (Prunus spinosa L.): Characterization, Antioxidant Activity and Optimization by Response Surface Methodology. J. Food Meas. Charact. 2023, 17, 1467–1479.
- Wei, M.-C.; Xiao, J.; Yang, Y.-C. Extraction of α-Humulene-Enriched Oil from Clove Using Ultrasound-Assisted Supercritical Carbon Dioxide Extraction and Studies of Its Fictitious Solubility. Food Chem. 2016, 210, 172–181.
- dos Santos, L.C.; Bitencourt, R.G.; dos Santos, P.; de Tarso Vieira e Rosa, P.; Martínez, J. Solubility of Passion Fruit (Passiflora edulis Sims) Seed Oil in Supercritical CO2. Fluid Phase Equilib. 2019, 493, 174–180.
- Kang, J.-H.; Kim, S.; Moon, B. Optimization by Response Surface Methodology of Lutein Recovery from Paprika Leaves Using Accelerated Solvent Extraction. Food Chem. 2016, 205, 140–145.
- Wu, K.; Ju, T.; Deng, Y.; Xi, J. Mechanochemical Assisted Extraction: A Novel, Efficient, Eco-Friendly Technology. Trends Food Sci. Technol. 2017, 66, 166–175.
- Xi, J.; Yan, L. Optimization of Pressure-Enhanced Solid-Liquid Extraction of Flavonoids from Flos Sophorae and Evaluation of Their Antioxidant Activity. Sep. Purif. Technol. 2017, 175, 170–176.
- Xi, J.; Li, Z.; Fan, Y. Recent Advances in Continuous Extraction of Bioactive Ingredients from Food-Processing Wastes by Pulsed Electric Fields. Crit. Rev. Food Sci. Nutr. 2021, 61, 1738–1750.
- Barba, F.J.; Zhu, Z.; Koubaa, M.; Sant’Ana, A.S.; Orlien, V. Green Alternative Methods for the Extraction of Antioxidant Bioactive Compounds from Winery Wastes and By-Products: A Review. Trends Food Sci. Technol. 2016, 49, 96–109.
- Cilla, A.; Bosch, L.; Barberá, R.; Alegría, A. Effect of Processing on the Bioaccessibility of Bioactive Compounds—A Review Focusing on Carotenoids, Minerals, Ascorbic Acid, Tocopherols and Polyphenols. J. Food Compos. Anal. 2018, 68, 3–15.
- Picart-Palmade, L.; Cunault, C.; Chevalier-Lucia, D.; Belleville, M.-P.; Marchesseau, S. Potentialities and Limits of Some Non-Thermal Technologies to Improve Sustainability of Food Processing. Front. Nutr. 2019, 5, 00130.
- Puértolas, E.; Barba, F.J. Electrotechnologies Applied to Valorization of By-Products from Food Industry: Main Findings, Energy and Economic Cost of Their Industrialization. Food Bioprod. Process. 2016, 100, 172–184.
- Geow, C.H.; Tan, M.C.; Yeap, S.P.; Chin, N.L. A Review on Extraction Techniques and Its Future Applications in Industry. Eur. J. Lipid Sci. Technol. 2021, 123, 2000302.
- Mohamed, M.E.A.; Amer Eiss, A.H. Pulsed Electric Fields for Food Processing Technology. In Structure and Function of Food Engineering; Amer Eissa, A., Ed.; InTech: London, UK, 2012; ISBN 978-953-51-0695-1.
- Raso, J.; Frey, W.; Ferrari, G.; Pataro, G.; Knorr, D.; Teissie, J.; Miklavčič, D. Recommendations Guidelines on the Key Information to Be Reported in Studies of Application of PEF Technology in Food and Biotechnological Processes. Innov. Food Sci. Emerg. Technol. 2016, 37, 312–321.
- Bocker, R.; Silva, E.K. Pulsed electric field assisted extraction of natural food pigments and colorings from plant matrices. Food Chem. X 2022, 15, 100398.
- Puértolas, E.; Koubaa, M.; Barba, F.J. An Overview of the Impact of Electrotechnologies for the Recovery of Oil and High-Value Compounds from Vegetable Oil Industry: Energy and Economic Cost Implications. Food Res. Int. 2016, 80, 19–26.
- Zia, S.; Khan, M.R.; Shabbir, M.A.; Aslam Maan, A.; Khan, M.K.I.; Nadeem, M.; Khalil, A.A.; Din, A.; Aadil, R.M. An Inclusive Overview of Advanced Thermal and Nonthermal Extraction Techniques for Bioactive Compounds in Food and Food-Related Matrices. Food Rev. Int. 2022, 38, 1166–1196.
- Ranjha, M.M.A.N.; Kanwal, R.; Shafique, B.; Arshad, R.N.; Irfan, S.; Kieliszek, M.; Kowalczewski, P.Ł.; Irfan, M.; Khalid, M.Z.; Roobab, U.; et al. A Critical Review on Pulsed Electric Field: A Novel Technology for the Extraction of Phytoconstituents. Molecules 2021, 26, 4893.
- Lasekan, O.; Ng, S.; Azeez, S.; Shittu, R.; Teoh, L.; Gholivand, S. Effect of Pulsed Electric Field Processing on Flavor and Color of Liquid Foods†. J. Food Process. Preserv. 2017, 41, e12940.
- Nowosad, K.; Sujka, M.; Pankiewicz, U.; Kowalski, R. The Application of PEF Technology in Food Processing and Human Nutrition. J. Food Sci. Technol. 2021, 58, 397–411.
- Vaessen, E.M.J.; Timmermans, R.A.H.; Tempelaars, M.H.; Schutyser, M.a.I.; den Besten, H.M.W. Reversibility of Membrane Permeabilization upon Pulsed Electric Field Treatment in Lactobacillus Plantarum WCFS1. Sci. Rep. 2019, 9, 19990.
- Morales-de la Peña, M.; Welti-Chanes, J.; Martín-Belloso, O. Novel Technologies to Improve Food Safety and Quality. Curr. Opin. Food Sci. 2019, 30, 1–7.
- Saletnik, B.; Zaguła, G.; Saletnik, A.; Bajcar, M.; Słysz, E.; Puchalski, C. Effect of Magnetic and Electrical Fields on Yield, Shelf Life and Quality of Fruits. Appl. Sci. 2022, 12, 3183.
- Zhao, Y.; Zheng, Y.; He, H.; Sun, Z.; Li, A. Effective Aluminum Extraction Using Pressure Leaching of Bauxite Reaction Residue from Coagulant Industry and Leaching Kinetics Study. J. Environ. Chem. Eng. 2021, 9, 104770.
- Corrales, M.; Toepfl, S.; Butz, P.; Knorr, D.; Tauscher, B. Extraction of Anthocyanins from Grape By-Products Assisted by Ultrasonics, High Hydrostatic Pressure or Pulsed Electric Fields: A Comparison. Innov. Food Sci. Emerg. Technol. 2008, 9, 85–91.
- Carpentieri, S.; Ferrari, G.; Pataro, G. Optimization of Pulsed Electric Fields-Assisted Extraction of Phenolic Compounds from White Grape Pomace Using Response Surface Methodology. Front. Sustain. Food Syst. 2022, 6, 854968.
- Meini, M.-R.; Cabezudo, I.; Boschetti, C.E.; Romanini, D. Recovery of Phenolic Antioxidants from Syrah Grape Pomace through the Optimization of an Enzymatic Extraction Process. Food Chem. 2019, 283, 257–264.
- Pintać, D.; Majkić, T.; Torović, L.; Orčić, D.; Beara, I.; Simin, N.; Mimica–Dukić, N.; Lesjak, M. Solvent Selection for Efficient Extraction of Bioactive Compounds from Grape Pomace. Ind. Crops Prod. 2018, 111, 379–390.
- Caldas, T.W.; Mazza, K.E.L.; Teles, A.S.C.; Mattos, G.N.; Brígida, A.I.S.; Conte-Junior, C.A.; Borguini, R.G.; Godoy, R.L.O.; Cabral, L.M.C.; Tonon, R.V. Phenolic Compounds Recovery from Grape Skin Using Conventional and Non-Conventional Extraction Methods. Ind. Crops Prod. 2018, 111, 86–91.
- Luengo, E.; Álvarez, I.; Raso, J. Improving the Pressing Extraction of Polyphenols of Orange Peel by Pulsed Electric Fields. Innov. Food Sci. Emerg. Technol. 2013, 17, 79–84.
- Khan, M.K.; Abert-Vian, M.; Fabiano-Tixier, A.-S.; Dangles, O.; Chemat, F. Ultrasound-Assisted Extraction of Polyphenols (Flavanone Glycosides) from Orange (Citrus sinensis L.) Peel. Food Chem. 2010, 119, 851–858.
- Afifi, S.M.; Gök, R.; Eikenberg, I.; Krygier, D.; Rottmann, E.; Stübler, A.-S.; Aganovic, K.; Hillebrand, S.; Esatbeyoglu, T. Comparative Flavonoid Profile of Orange (Citrus sinensis) Flavedo and Albedo Extracted by Conventional and Emerging Techniques Using UPLC-IMS-MS, Chemometrics and Antioxidant Effects. Front. Nutr. 2023, 10, 1158473.
- Peiró, S.; Luengo, E.; Segovia, F.; Raso, J.; Almajano, M.P. Improving Polyphenol Extraction from Lemon Residues by Pulsed Electric Fields. Waste Biomass Valorization 2019, 10, 889–897.
- Roselló-Soto, E.; Barba, F.J.; Parniakov, O.; Galanakis, C.M.; Lebovka, N.; Grimi, N.; Vorobiev, E. High Voltage Electrical Discharges, Pulsed Electric Field, and Ultrasound Assisted Extraction of Protein and Phenolic Compounds from Olive Kernel. Food Bioprocess Technol. 2015, 8, 885–894.
- Ohshima, T.; Tanino, T.; Guionet, A.; Takahashi, K.; Takaki, K. Mechanism of Pulsed Electric Field Enzyme Activity Change and Pulsed Discharge Permeabilization of Agricultural Products. Jpn. J. Appl. Phys. 2021, 60, 060501.
- Pappas, V.M.; Lakka, A.; Palaiogiannis, D.; Athanasiadis, V.; Bozinou, E.; Ntourtoglou, G.; Makris, D.P.; Dourtoglou, V.G.; Lalas, S.I. Optimization of Pulsed Electric Field as Standalone “Green” Extraction Procedure for the Recovery of High Value-Added Compounds from Fresh Olive Leaves. Antioxidants 2021, 10, 1554.
- Bobinaitė, R.; Pataro, G.; Lamanauskas, N.; Šatkauskas, S.; Viškelis, P.; Ferrari, G. Application of Pulsed Electric Field in the Production of Juice and Extraction of Bioactive Compounds from Blueberry Fruits and Their By-Products. J. Food Sci. Technol. 2015, 52, 5898–5905.
- Pataro, G.; Bobinaitė, R.; Bobinas, Č.; Šatkauskas, S.; Raudonis, R.; Visockis, M.; Ferrari, G.; Viškelis, P. Improving the Extraction of Juice and Anthocyanins from Blueberry Fruits and Their By-Products by Application of Pulsed Electric Fields. Food Bioprocess Technol. 2017, 10, 1595–1605.
- Pataro, G.; Carullo, D.; Bobinaite, R.; Donsì, G.; Ferrari, G. Improving the Extraction Yield of Juice and Bioactive Compounds from Sweet Cherries and Their By-Products by Pulsed Electric Fields. Chem. Eng. Trans. 2017, 57, 1717–1722.
- Makrygiannis, I.; Athanasiadis, V.; Bozinou, E.; Chatzimitakos, T.; Makris, D.P.; Lalas, S.I. Combined Effects of Deep Eutectic Solvents and Pulsed Electric Field Improve Polyphenol-Rich Extracts from Apricot Kernel Biomass. Biomass 2023, 3, 66–77.
- Athanasiadis, V.; Chatzimitakos, T.; Bozinou, E.; Kotsou, K.; Palaiogiannis, D.; Lalas, S.I. Optimization of Extraction Parameters for Enhanced Recovery of Bioactive Compounds from Quince Peels Using Response Surface Methodology. Foods 2023, 12, 2099.
- Parniakov, O.; Barba, F.J.; Grimi, N.; Lebovka, N.; Vorobiev, E. Impact of Pulsed Electric Fields and High Voltage Electrical Discharges on Extraction of High-Added Value Compounds from Papaya Peels. Food Res. Int. 2014, 65, 337–343.
- Parniakov, O.; Barba, F.J.; Grimi, N.; Lebovka, N.; Vorobiev, E. Extraction Assisted by Pulsed Electric Energy as a Potential Tool for Green and Sustainable Recovery of Nutritionally Valuable Compounds from Mango Peels. Food Chem. 2016, 192, 842–848.
- Rajha, H.N.; Abi-Khattar, A.-M.; El Kantar, S.; Boussetta, N.; Lebovka, N.; Maroun, R.G.; Louka, N.; Vorobiev, E. Comparison of Aqueous Extraction Efficiency and Biological Activities of Polyphenols from Pomegranate Peels Assisted by Infrared, Ultrasound, Pulsed Electric Fields and High-Voltage Electrical Discharges. Innov. Food Sci. Emerg. Technol. 2019, 58, 102212.
- Luengo, E.; Álvarez, I.; Raso, J. Improving Carotenoid Extraction from Tomato Waste by Pulsed Electric Fields. Front. Nutr. 2014, 1, 00012.
- Hossain, M.B.; Aguiló-Aguayo, I.; Lyng, J.G.; Brunton, N.P.; Rai, D.K. Effect of Pulsed Electric Field and Pulsed Light Pre-Treatment on the Extraction of Steroidal Alkaloids from Potato Peels. Innov. Food Sci. Emerg. Technol. 2015, 29, 9–14.
- Roohinejad, S.; Oey, I.; Everett, D.W.; Niven, B.E. Evaluating the Effectiveness of β-Carotene Extraction from Pulsed Electric Field-Treated Carrot Pomace Using Oil-in-Water Microemulsion. Food Bioprocess Technol. 2014, 7, 3336–3348.
- Zhao, W.; Yu, Z.; Liu, J.; Yu, Y.; Yin, Y.; Lin, S.; Chen, F. Optimized Extraction of Polysaccharides from Corn Silk by Pulsed Electric Field and Response Surface Quadratic Design. J. Sci. Food Agric. 2011, 91, 2201–2209.
- Kim, H.-S.; Ko, M.-J.; Park, C.-H.; Chung, M.-S. Application of Pulsed Electric Field as a Pre-Treatment for Subcritical Water Extraction of Quercetin from Onion Skin. Foods 2022, 11, 1069.
- Barbosa-Pereira, L.; Guglielmetti, A.; Zeppa, G. Pulsed Electric Field Assisted Extraction of Bioactive Compounds from Cocoa Bean Shell and Coffee Silverskin. Food Bioprocess Technol. 2018, 11, 818–835.
- Yu, X.; Gouyo, T.; Grimi, N.; Bals, O.; Vorobiev, E. Pulsed Electric Field Pretreatment of Rapeseed Green Biomass (Stems) to Enhance Pressing and Extractives Recovery. Bioresour. Technol. 2016, 199, 194–201.
- Bozinou, E.; Karageorgou, I.; Batra, G.; Dourtoglou, V.G.; Lalas, S.I. Pulsed Electric Field Extraction and Antioxidant Activity Determination of Moringa oleifera Dry Leaves: A Comparative Study with Other Extraction Techniques. Beverages 2019, 5, 8.
- Boussetta, N.; Soichi, E.; Lanoisellé, J.-L.; Vorobiev, E. Valorization of Oilseed Residues: Extraction of Polyphenols from Flaxseed Hulls by Pulsed Electric Fields. Ind. Crops Prod. 2014, 52, 347–353.
- Athanasiadis, V.; Lakka, A.; Palaiogiannis, D.; Pappas, V.M.; Bozinou, E.; Ntourtoglou, G.; Makris, D.P.; Dourtoglou, V.G.; Lalas, S.I. Pulsed Electric Field and Salvia officinalis L. Leaves: A Successful Combination for the Extraction of High Value Added Compounds. Foods 2021, 10, 2014.
- Wang, M.; Zhou, J.; Collado, M.C.; Barba, F.J. Accelerated Solvent Extraction and Pulsed Electric Fields for Valorization of Rainbow Trout (Oncorhynchus mykiss) and Sole (Dover sole) By-Products: Protein Content, Molecular Weight Distribution and Antioxidant Potential of the Extracts. Mar. Drugs 2021, 19, 207.
