Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Camila Xu and Version 1 by Fuat Bicer.
Hepatocellular carcinoma (HCC) is the second most common cause of cancer-related deaths in the world. More than half of patients with HCC present with advanced stage, and highly active systemic therapies are crucial for improving outcomes. Immune checkpoint inhibitor (ICI)-based therapies have emerged as novel therapy options for advanced HCC.
- HCC
- immunotherapy
- PD1
- PDL1
- TIGIT
- LAG3
- TIM3
1. Introduction
Primary liver cancer is the sixth most diagnosed cancer worldwide. Hepatocellular carcinoma (HCC) is the predominant subtype, accounting for close to 90% of cases [1,2][1][2]. There were varying incidences ranging from 6.3 per 100,000 in the United States to >50 per 100,000 in some countries in East Asia in 2020 [1]. It is the second leading cause of cancer-related death in men and sixth in women [3]. The gap in incidence rates is due to disparities in the prevalence of risk factors [3,4,5][3][4][5].
Hepatitis C virus (HCV) is the leading cause of HCC in Western Europe, North America, and Japan, while Hepatitis B virus (HBV) is the leading cause of HCC in Asia (besides Japan), South America, and Africa [6]. The prevalence of some modifiable risk factors is on the rise globally, including alcohol consumption, metabolic syndrome, and non-alcoholic fatty liver disease (NAFLD). One study found that >3 drinks per day was associated with a 16% increased risk of HCC [7]. For another risk factor, metabolic syndrome, one study found it was associated with an 81% increased risk of developing HCC, and that risk can be reduced by treating one of the many conditions attributed to metabolic syndrome such as insulin resistance, obesity, hypertension, and dyslipidemia [7].
Immune checkpoint inhibitor (ICI)-based therapies have emerged as novel therapy options in advanced HCC. Early approved ICI’s included targets for programmed cell death-1 (PD-1)/programmed cell death ligand-1 (PD-L1) in the form of antibodies. ICI’s have shown progressive improvements in overall survival outcomes compared to sorafenib (TKI). Combination agents targeting cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and (PD-1) have been approved as well, surpassing the ICI monotherapy overall survival rates. Approximately two thirds of patients do not achieve the objective response with ICI-based therapies due to primary or acquired resistance created by the tumor’s overwhelming immunosuppressive state [8].
2. Current First-Line Therapies for Advanced/Metastatic HCC
2.1. TKI-Based Therapies
2.1.1. Sorafenib
Sorafenib, a multi-kinase inhibitor, (Figure 21) was the first systemic therapy to gain FDA approval for the treatment of HCC. The landmark SHARP trial was a multicenter, randomized control phase III trial that included 602 patients assigned in a 1:1 ratio to receive 400 mg sorafenib or placebo. Eligible patients were Child–Pugh Class A and had no previous systemic therapy. Most patients had HCC caused by chronic HCV (56%) and alcohol consumption (52%), and chronic HBV (37%) closely followed [25][9]. Median OS was 10.7 months in the sorafenib group and 7.9 months in the placebo group (Hazard Ratio (HR) = 0.69; 95% confidence interval (CI) = 0.55 to 0.87; p < 0.001). The incidence of drug related serious adverse events (AEs) was 9.4–14.6% in the sorafenib group and 5.0–25% in the placebo group [26][10]. The subsequent Asia–Pacific study confirmed the findings of the SHARP trial, showing that the mOS was 6.5 months in the sorafenib arm and 4.2 months in the placebo arm (HR = 0.68; 95% CI = 0.50–0.93; p = 0.014). Inclusion and exclusion criteria were similar as well [25,27,28,29][9][11][12][13].
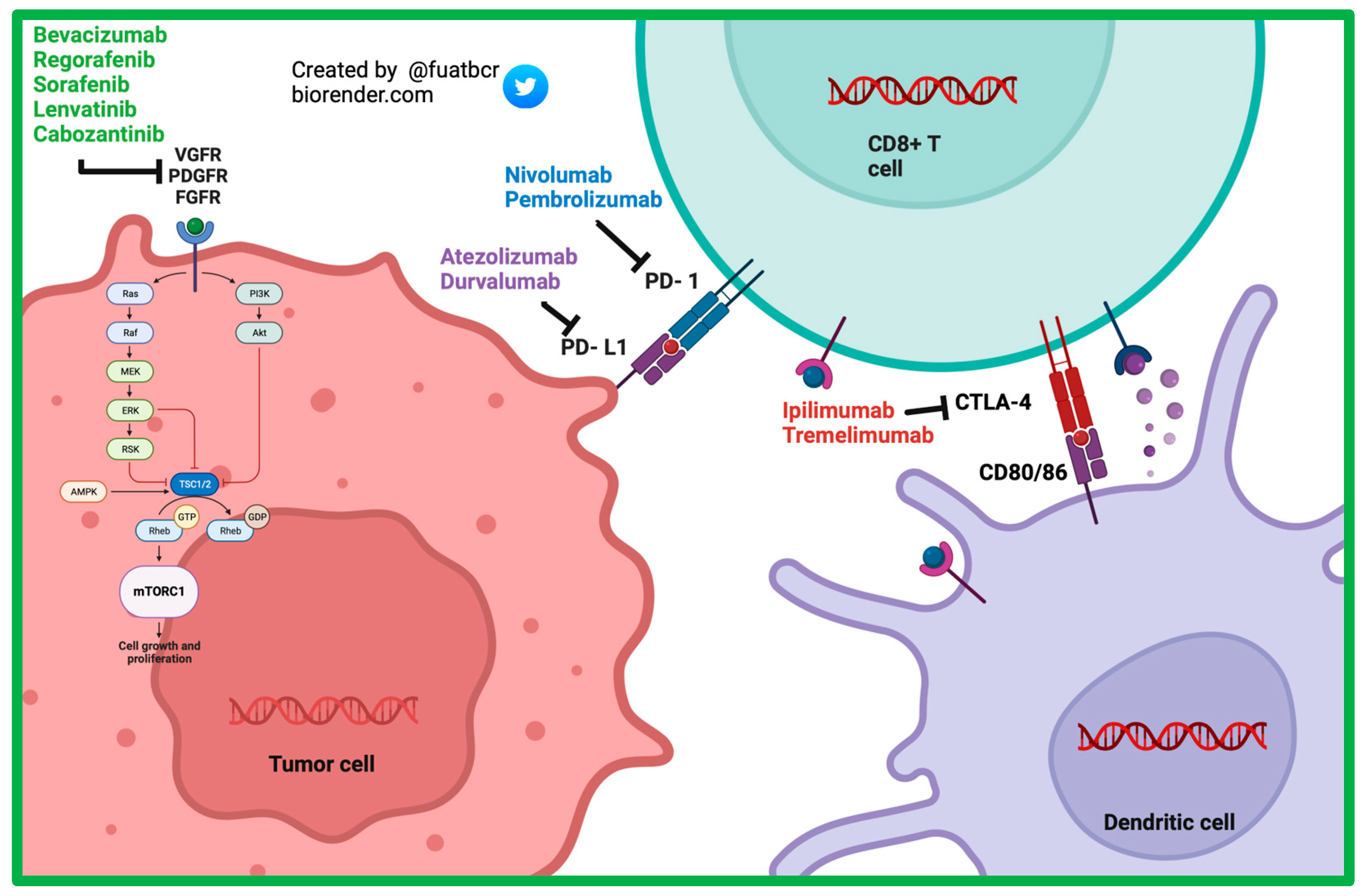
Figure 21.
Mechanism of action of currently approved systemic therapy options in advanced HCC.
After sorafenib received FDA approval, multiple clinical trials with other drugs failed to improve survival when compared to sorafenib. These drugs include sunitinib, brivanib, linifanib, everolimus, and tivantinib [30,31,32,33,34,35][14][15][16][17][18][19].
2.1.2. Lenvatinib
Lenvatinib is a multi-kinase inhibitor targeting VEGFR, fibroblast growth factor receptor (FGFR), platelet-derived growth factor receptor (PDGFR alpha), RET protooncogene (RET), and kit-protooncogene (KIT). In the REFLECT trial, a randomized phase III noninferiority trial, lenvatinib was found to be noninferior in overall survival compared to sorafenib (mOS 13.6 vs. 12.3 months, HR = 0.92; 95% CI = 0.79–1.06). The secondary endpoint of median progression-free survival (PFS) was 7.4 months in the lenvatinib group versus 3.7 months in the sorafenib group (HR = 0.66, 95% CI 0.79–1.06). Hypothyroidism, decreased appetite, and hypertension were more common in the lenvatinib group, and diarrhea and a hand–foot–skin reaction was less common the lenvatinib group. In 2018, the FDA approved lenvatinib for first-line treatment of patients with advanced HCC [36][20].
2.2. ICI-Based Therapies
2.2.1. Atezolizumab and Bevacizumab
The combination of atezolizumab, an anti-PD-L1 antibody, and bevacizumab, an anti-VEGF antibody, has shown synergistic anticancer activity [37,38][21][22]. Bevacizumab blocks VEGF, enabling maturation of dendritic cells that would have otherwise been downregulated with VEGF activity. Blocking VEGF prevents upregulation of MDSCs, which in turn allows for proliferation of CD8+ T cells and suppression of Tregs. With this environment, there is adequate antigen presentation, but tumor cells still can inhibit cytotoxic activity of the T cells with PD-L1/PD-1 upregulation. With the addition of an anti PD-L1 antibody, the T cells are able to destroy cancer cells without inhibition [37][21].
The phase Ib clinical trial GO30140 was an open-label, multi-arm trial where one of the HCC cohorts (A) studied the safety and efficacy of atezolizumab plus bevacizumab, while the other cohort (F) studied atezolizumab plus bevacizumab versus atezolizumab. Both cohorts met their primary endpoints with statistical significance [39][23].
IMbrave150 is a phase III randomized trial in patients who had unresectable HCC, no prior history of systemic therapy, and well-compensated liver disease (Child–Pugh A (CPA)). Patients were randomized to atezolizumab + bevacizumab versus sorafenib in a 2:1 ratio. The mOS was 19.2 months in the combination arm and 13.4 in the control arm (HR = 0.66; 95% CI = 0.52–0.85; p < 0.001). Grade 3 or 4 treatment-related adverse events occurred in 43% of the atezolizumab + bevacizumab group and 46% of the sorafenib group [40][24].
A recent study analyzed the correlative baseline tumor samples from a group of patients enrolled in the GO30140 or IMbrave150 phase III trial and provided insight to potentially significant biomarkers (key correlative findings are summarized in Table 1) [41][25]. It was demonstrated that genes or immune markers associated with pre-existing immunity, including expression of PD-L1 mRNA and effector T cell (Teff), were correlated with higher response to atezolizumab + bevacizumab in both GO30140 cohort A and in the IMbrave150 trial. Validation with immunohistochemical analysis was not able to demonstrate a clinically significant relationship between PD-L1 levels and ORR but revealed higher rate of infiltration of CD8+ T cells in the responders in arm A of GO30140 cohort A and in the IMbrave150 trial. Additionally, the study revealed that patients in the IMbrave150 trial with a low ratio of Treg/Teff signatures had a statistically significant higher PFS and OS in the atezolizumab + bevacizumab combination therapy group compared to sorafenib.
| GO30140 Cohort A: Atezolizumab + Bevacizumab | ||
|---|---|---|
| Gene Alterations or Immune Signatures | Immune Cell Types | TMB |
| Gene alterations or immune signatures associated with greater response: CD274 (PD-L1 mRNA): high expression associated with longer PFS compared to those with low expression (p = 0.0011). Teff: high expression associated with longer PFS in combination compared to those with low expression (0.0035). |
Higher density of CD8+ T associated with better response (p = 0.007). | Greater ORR in TMB-high group (56%) compared to TMB-low group (35%). |
| Phase III IMbrave 150 Trial: Atezolizumab + Bevacizumab vs. Sorafenib | ||
| Gene Alterations or Immune Signatures | Immune Cell Types | TMB |
| Gene alterations or signatures associated with greater response: CD274 (PD-L1 mRNA): high expression associated with longer PFS in combination group versus sorafenib (p = 0.015), as well as greater OS (0.002) Teff: high expression associated with longer PFS in combination group versus sorafenib (p = 0.047), as well as greater OS (0.0002) |
Higher density of intra-tumoral CD8+ T cells showed longer PFS (0.053) and OS (0.001) Low ratio of Treg/Teff signatures had higher PFS and OS compared to sorafenib Higher density of CD8+ T cells associated with longer OS and PFS compared with sorafenib |
No associations of TMB with outcome |
| GO30140 Cohort F: Atezolizumab + Bevacizumab vs. Atezolizumab | ||
| Gene Alterations or Immune Signatures | Blood Vessel Density | |
| Genes or signatures associated with greater response: Myeloid inflammation: high expression associated with greater PFS (p = 0.036 versus monotherapy Gene signatures of Teff: high expression associated with greater PFS (p = 0.034 versus monotherapy KDR (VEGF receptor 2): high expression associated with greater PFS in combination group compared to monotherapy (p = 0.011) |
High vessel density in baseline tumors associated with longer PFS in combination group compared to monotherapy (p = 0.0018) | |
The study was able to use the GO30140 cohort F correlative samples to study the benefits of the added bevacizumab. Immune subsets of CD8+ T cells, Treg cells, and macrophages were associated with outcomes with the addition of bevacizumab [41,42][25][26]. Effector T cell and myeloid gene signatures at baseline were associated with improved outcomes in the combination group. It was also shown that the expression of the VEGFR-2 gene (KDR) was decreased in the combination group compared to monotherapy in pre- and post-treatment tumor biopsies. In addition, 80% of the responders had a decrease in the Treg signature in the combination group compared to 33% of the responders in the atezolizumab group. Lastly, higher blood vessel density was associated with longer PFS in the combination group compared to monotherapy.
The study also investigated the impact of TMB on therapy outcomes using whole-genome sequencing (WES) on tumor-blood samples of patients in both trials. TMB was not associated with outcomes in the IMbrave150 group [41][25].
2.2.2. Durvalumab + Tremelimumab
In a phase II trial involving patients with unresectable HCC, the combination of tremelimumab (anti-CTLA-4 antibody) plus durvalumab (a PD-L1 inhibitor) demonstrated promising clinical activity and safety [43][27]. Patients were randomly assigned to receive either 300 mg of tremelimumab for one dose plus 1500 mg of durvalumab every 4 weeks, 1500 mg of durvalumab every 4 weeks, 75 mg of tremelimumab every 4 weeks for a total of four doses plus 1500 mg of durvalumab every 4 weeks (a combination given the term T75 +D), or 400 mg of sorafenib twice a day.
The phase III HIMALAYA trial randomized previously untreated advanced HCC patients using the STRIDE (Single Tremelimumab Regular Interval Durvalumab) regimen, sorafenib, durvalumab, or 75 mg of tremelimumab every 4 weeks for a total of four doses plus 1500 mg of durvalumab every 4 weeks (T75 +D). Later, the T75 + D arm was closed based on the data from the phase II trial. The HIMALAYA trial demonstrated that the STRIDE regimen improved overall survival with mOS of 16.43 months versus 13.77 months for sorafenib (HR = 0.78; 96 CI = 0.65–0.92; p = 0.0035). The trial also demonstrated that durvalumab was noninferior to sorafenib. Based on the results of the HIMALAYA trial, the STRIDE regimen was approved for first-line therapy in advanced HCC [43,44][27][28].
An international, randomized phase III trial NCT03764293 studying camrelizumab plus rivoceranib, also known as apatinib (an anti-angiogenic), versus sorafenib recently revealed pivotal results in a front-line setting, with OS 22.1 months versus 15.2 months; HR = 0.62; 95% CI = 0.49–0.80; p < 0.0001. This study is now known as CARES-310, and a new drug application has just been submitted for the combination as a first-line treatment option [45][29].
3. Current Second-Line Therapies for Advanced/Metastatic HCC
3.1. ICI-Based Therapies
3.1.1. Nivolumab
Checkpoint 040 trial was a multi-cohort, open-label clinical trial studying nivolumab as both monotherapy and in combination with ipilimumab in advanced HCC patients with prior sorafenib use and those who were sorafenib naive. Patients with Child–Pugh A were included in cohorts 1–3, which included a dose-escalation phase for safety and a dose-expansion phase to assess safety and clinical data for different doses of nivolumab monotherapy. The trial revealed ORRs of 15 and 20% in dose escalation and expansion phases, respectively. The FDA approved this drug for second-line treatment in HCC; however, it was later withdrawn [46][30]. In cohort 5, only patients with Child–Pugh B were included, and most patients were Child–Pugh B7 (76%). Patients were treated with nivolumab alone in this non-comparative study. The mOS of the Child–Pugh B group was 7.6 months (95% CI = 4.4–10.5). Patients who responded to nivolumab monotherapy in cohort 5 showed stable or improved liver function, evidenced by five of the six responders improving from Child–Pugh B to Child–Pugh A. All responders showed stable ALBI grades [47][31]. These findings signify the potential role that immunotherapy may have in reversing tumor-mediated decline in liver function.
The confirmatory Check-Mate-459 trial randomized previously untreated advanced HCC patients to nivolumab versus sorafenib but failed to improve OS. Therefore, the nivolumab approval based on the Checkpoint 040 trial was withdrawn [48][32].
3.1.2. Pembrolizumab
Pembrolizumab originally received accelerated second-line approval for advanced HCC based on the findings of the Keynote-224 trial, which revealed an ORR of 17% per the RECIST v1.1 (95% CI = 11–26) and mOS of 12.9 months (95% CI 9.7–15.5) [49][33]. In the subsequent Keynote-240 trial assessing the safety and efficacy of pembrolizumab, mOS was 13.8 months in the pembrolizumab group and 10.6 months in the placebo group (HR = 0.78; 95% CI = 0.611–0.998; p = 0023), which did not meet the prespecified boundary of p = 0.0174 for OS [50][34].
Keynote-394, another phase III trial conducted in Asian patients with previously treated advanced HCC, revealed improvements in mOS and mPFS in those receiving pembrolizumab over best supportive care; mOS was 14.6 months for the pembrolizumab arm and 13.0 months for the placebo (HR = 0.70; 95% CI = 0.63–0.99; p = 0.0180). This trial is promising for the role of second-line ICIs for HCC in Asian patients [51,52][35][36].
3.1.3. Nivolumab/Ipilimumab
In 2020, a nivolumab plus ipilimumab combination was approved for the treatment of patients with advanced HCC who were previously treated with sorafenib. This was based on results of Arm A of cohort 4 of the Checkmate 040 trial, where patients received nivolumab (1 mg/kg) with ipilimumab (3 mg/kg) every 3 weeks for a total of four doses followed by nivolumab (240 mg) every 2 weeks. The ORR was 32% (RECIST v1.1) while the median response duration was 17.5 months (4.6–30.5 months). A follow-up showed that the ORR continued to stay at 32% while the 24-month OS rate improved to 46% (95% CI = 32–59%) [46,53][30][37].
Currently approved TKI-based therapies in second-line and beyond are summarized in Table 2 with key findings of the landmark trials.
Table 2.
Results from clinical trials from approved systemic therapies in advanced HCC.
| Clinical Trials in HCC | Phase | Line of Therapy | Arms | Primary Outcome(s) | Median OS (Months) | ORR (%) | Year Approved |
|---|---|---|---|---|---|---|---|
| Multikinase inhibitors and monoclonal antibody against VEGFR2 | |||||||
| SHARP [26][10] | III | First | Sorafenib (S) Placebo (P) |
OS | S: 10.7 P: 7.9 (HR = 0.69; 95% confidence interval (CI) = 0.55–0.87; p < 0.001) |
S: 43 P: 32 p = 0.002 |
2007 |
| RESORCE [54][38] | III | Second (post-SOR) | Regorafenib (R) Placebo |
OS | R: 10.6 P: 7.8 (HR = 0.63; 95% CI = 0.50–0.79; p < 0.0001) |
R:11 P: 4 p = 0.0047 |
2017 |
| REFLECT [36][20] | III | First | Lenvatinib (L), Sorafenib |
OS | L: 13.6 S: 12.3 (HR = 0.92; 95% CI = 0.79–1.06) |
L: 18.8 S: 6.5 p < 0.0001 |
2018 |
| CELESTIAL [55][39] | III | Second (post-SOR or other) | Cabozantinib (C) Placebo |
OS | C: 10.2 P: 8.0 (HR = 0.76; p < 0.005) |
C:4 P < 1 p = 0.009 |
2019 |
| REACH-2 [56][40] | III | Second | Ramucirumab (Ra), Placebo (AFP ≥ 400 ng/mL) |
OS | Ra: 8.5 P: 7.3 (HR = 0.71; p < 0.019) |
R:5 P:1 p = 0·1697 |
2019 |
| Immunotherapy (monotherapy) | |||||||
| Keynote-224 [49][33] | II | Second | Pembrolizumab (Pem) (post-SOR) | ORR | Pem: 12.9 months (95% CI = 9.7–15.5) |
17 (95% CI = 11–26) |
2018 |
| Checkmate 040 (cohorts 1–3 in dose expansion phase) [46][30] | I/II | Second | Nivolumab (N) (post-SOR) | ORR | 6 months:83% 9 months:74% |
20 (CI = 15–26) |
2017 |
| MKI with ICI | |||||||
| IMbrave150 (2020) [57][41] | III | First | Atezolizumab + Bevacizumab (AB), Sorafenib | AB: 19.2 S: 13.4 |
A + B:30 S:11 |
2020 | |
| Dual checkpoint inhibitors | |||||||
| Checkmate 040 (cohort 4) | I/II | Second | Nivolumab + ipilimumab | ORR | Arm A: 22.8 months (95% CI, 9.4-not reached) Arm B: 12.5 months (95% CI, 7.6–16.4) Arm C: 12.7 months (95% CI, 7.4–33.0) |
ARM A: 32 (95 = CI 20–47) ARM B: 27 (95% CI = 15–41) ARM C: 29 (95% CI = 29 (17–43) |
2020 |
| HIMALAYA [44][28] | III | First | Durvalumab + Tremelimumab (STRIDE), Durvalumab (D), Sorafenib |
OS | STRIDE: 16.4 S: 13.8 (HR = 0.78; 96% CI = 0.65–0.92; p = 0.0035) Durvalumab did not demonstrate superiority to sorafenib (p = 0.0674) |
STRIDE:20.1 D: 17 S: 5.1 |
2022 |
4. Combination Therapy of ICI with Anti-Angiogenic Therapy and TKI
The combination of immune checkpoint inhibitors with anti-angiogenic agents has changed HCC frontline therapy. Several other studies have taken advantage of the synergistic effect of an ICI with another novel agent [33][17].
The Keynote 524 trial was an open-label, phase Ib, multicenter, single-arm study where patients with unresectable HCC received lenvatinib and pembrolizumab. The primary objective was ORR via modified RECIST (mRECIST), RECIST version 1.1 (v1.1) per independent imaging review (IIR). One hundred out of the 104 patients did not receive any prior systemic therapy, and patients had BCLC stage B or C disease. The combination proved to have confirmed ORR of 46% (95% CI = 36–56) per mRECIST and 36% (95% CI = 26.6.0–46.2) per RECIST v1.1 [58][42].
Leap-002 was a phase III study that compared lenvatinib plus pembrolizumab versus lenvatinib plus placebo in previously untreated advanced HCC in a 1:1 ratio Child–Pugh Class A. The trial had co-primary endpoints of OS and PFS. The primary endpoints of OS and PFS in the combination of lenvatinib and pembrolizumab arm did not meet pre-specified statistical significance [59][43]. Although this trial failed to meet the pre-specified outcomes, it revealed significant survival data in both arms, with 21.2 months (95% CI = 19.0–23.6) in the lenvatinib plus pembrolizumab arm and 19.0 months (95% CI = 17.2–21.7) in the lenvatinib plus placebo arm.
The COSMIC-312 study is a phase III, multicenter, and open-label trial that studied the combination of cabozantinib and atezolizumab versus sorafenib. It randomized 837 patients with advanced HCC and no prior history of receiving systemic therapy to atezolizumab and cabozantinib versus sorafenib versus cabozantinib in a 2:1:1 ratio. The study had dual primary endpoints of PFS in the first 372 patients for the atezolizumab and cabozantinib versus sorafenib arm and OS for the atezolizumab and cabozantinib versus sorafenib in all patients. The primary endpoint of PFS was longer in the combination group (6.8 vs. 4.2 months; HR 0.63, 99% CI5.6–8.3, p = 0.0012), but there was no significant difference in the overall survival (15.4 vs. 15.5 months; HR 0.90, 96% CI 0.69–1.18, p = 0.44) between the groups [60][44]. Given the lack of improvement in survival, this combination therapy is unlikely to be adopted for first-line therapy in advanced HCC.
The ICI/anti-angiogenic combination trials so far have rendered impressive results as shown in the IMbrave 150 trial underscoring the unique synergistic activity of this combination approach. Although early phase trials with ICI/TKI combinations were promising, the survival benefit over single-agent TKI was not shown in larger trials. Several other studies involving anti-angiogenics or TKIs and ICIs are underway, as shown in Table 3, including tivozanib plus durvalumab (NCT03970616) and regorafenib plus tiselizumab (NCT04183088) [61,62,63][45][46][47].
Table 3.
Ongoing clinical trials of ICI-based approaches in HCC.
| Trial Name and ID | Cancer Type | Estimated Enrollment | Targeting Mechanism | Control Arm | Phase | Start and Completion Dates | Primary Measures |
|---|---|---|---|---|---|---|---|
| RATIONALE—301 [64][48] NCT03412773 |
HCC | December 2017 | Tislelizumab (anti-PD-1 antibody) | Sorafenib | III | December 2017 July 2023 |
OS |
| Checkmate 9DW [65][49] NCT04039607 |
HCC | September 2019 | Nivolumab + Ipilimumab | Sorafenib or lenvatinib | III | September 2019 June 2025 |
OS |
| NCT03764293 [45][29] (CARES-310) | Locally advanced or metastatic and unresectable HCC | June 2019 | Camrelizumab (anti-PD-1 antibody) + Apatinib (VEGF inhibitor) | Sorafenib | III | June 2019 April 2023 |
OS PFS |
| DEDUCTIVE [62][46] NCT03970616 |
Advanced HCC | September 2019 | Tivozanib (selective VEGFR 1,2,3 TKI) + Durvalumab (PD-L1 inhibitor) | N/A | 1/IIb | September 2019 March 2023 |
TEAEs |
| NCT04183088 [63][47] | Advanced HCC | December 2020 | Tislelizumab (anti-PD-1 antibody) + regorafenib (TKI) | N/A | II | December 2020 March 2025 |
TRAE ORR PFS |
| NCT04401813 [66][50] | Advanced HCC | June 2020 | IBI308 (anti-CTLA4 antibody) + Sintilizumab (anti-PD-1 antibody) | N/A | I | June 2020 April 2023 |
AE ORR |
| NCT04212221 [67][51] | Advanced HCC | April 2020 | MGD013 (anti PD 1 antiobdy and anti-LAG-3 antibody) + Brivanib | N/A | I/II | Completed Pending results | DLTs ORR |
| NCT03680508 [68][52] | Advanced HCC | December 2019 | Cobolimab (TIM-3 binding antibody) + Dostarlimab (anti PD-1 antibody) | N/A | II | December 2019 October 2025 |
ORR |
| NCT03841201 [69][53] | Advanced HCC | June 2019 | Lenvatinib (TKI) + Nivolumab (anti-PD-1 antibody) | N/A | II | June 2019 March 2023 |
ORR AE SAE |
| RENOBATE [70][54] NCT04310709 |
Advanced HCC | June 2020 | Regorafenib + Nivolumab | Completed Pending results |
Response Rate | ||
| ARYA-1 [71][55] NCT04502082 |
Advanced HCC | April 2021 | ET140203 autologous T cell product | N/A | I/II | April 2021 June 2024 |
Incidence of AE and severity rates of AE Incidence rates of DLT RP2D |
| TRIPLET [72][56] NCT05665348 |
HCC—Hepatocellular Carcinoma | September 2021 | Atezolizumab (anti-PD-L1 antibody) + Bevacizumab (VEGF inhibitor) + Ipilimumab (anti-CTLA-4 antibody) | Atezolizumab + Bevacizumab | II/III | September 2021 April 2026 |
Objective response Overall survival |
| NCT05022927 [73][57] | Advanced HCC | June 2021 | ERY974 + Tocilicumab + Atezolizumab + Bevacizumab | N/A | I | June 2021 September 2024 |
Incidence of treatment-emergent adverse events (TEAE) |
| The PNeoVCA Study [74][58] NCT05269381 |
Various advanced solid tumors including HCC | March 2022 | Cyclophosphamide (alkylating agent) + Neoantigen vaccine (containing sargramostim (GM-CSF)) + Pembrolizumab (anti-PD-1 antibody) | N/A | I | March 2022 February 2025 |
Incidence of AE |
| RELATIVITY—106 [75][59] NCT05337137 |
Advanced HCC | April 2022 | Relatinib + Nivolumab + Bevacizumab | Nivolumab + Bevacizumab | 1/2 | April 2022 March 2023 |
Incidence of DLT PFS |
References
- Rumgay, H.; Arnold, M.; Ferlay, J.; Lesi, O.; Cabasag, C.J.; Vignat, J.; Laversanne, M.; McGlynn, K.A.; Soerjomataram, I. Global burden of primary liver cancer in 2020 and predictions to 2040. J. Hepatol. 2022, 77, 1598–1606.
- Asafo-Agyei, K.O.; Samant, H. Hepatocellular Carcinoma. In StatPearls; StatPearls Publishing: St. Petersburg, FL, USA, 2023. Available online: http://www.ncbi.nlm.nih.gov/books/NBK559177/ (accessed on 3 October 2023).
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2021, 71, 209–249.
- Zhu, R.X.; Seto, W.-K.; Lai, C.-L.; Yuen, M.-F. Epidemiology of Hepatocellular Carcinoma in the Asia-Pacific Region. Gut Liver 2016, 10, 332–339.
- McGlynn, K.A.; Petrick, J.L.; El-Serag, H.B. Epidemiology of Hepatocellular Carcinoma. Hepatology 2021, 73, 4–13.
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Primer 2021, 7, 6.
- Testino, G.; Leone, S.; Borro, P. Alcohol and hepatocellular carcinoma: A review and a point of view. World J. Gastroenterol. 2014, 20, 15943–15954.
- Pinter, M.; Jain, R.K.; Duda, D.G. The Current Landscape of Immune Checkpoint Blockade in Hepatocellular Carcinoma: A Review. JAMA Oncol. 2021, 7, 113–123.
- Ben Mousa, A. Sorafenib in the treatment of advanced hepatocellular carcinoma. Saudi J. Gastroenterol. 2008, 14, 40–42.
- Bruix, J.; Raoul, J.-L.; Sherman, M.; Mazzaferro, V.; Bolondi, L.; Craxi, A.; Galle, P.R.; Santoro, A.; Beaugrand, M.; Sangiovanni, A.; et al. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma: Subanalyses of a phase III trial. J. Hepatol. 2012, 57, 821–829.
- Su, G.L.; Altayar, O.; O’Shea, R.; Shah, R.; Estfan, B.; Wenzell, C.; Sultan, S.; Falck-Ytter, Y. AGA Clinical Practice Guideline on Systemic Therapy for Hepatocellular Carcinoma. Gastroenterology 2022, 162, 920–934.
- Llovet, J.M.; Castet, F.; Heikenwalder, M.; Maini, M.K.; Mazzaferro, V.; Pinato, D.J.; Pikarsky, E.; Zhu, A.X.; Finn, R.S. Immunotherapies for hepatocellular carcinoma. Nat. Rev. Clin. Oncol. 2022, 19, 151–172.
- Cheng, A.-L.; Kang, Y.-K.; Chen, Z.; Tsao, C.-J.; Qin, S.; Kim, J.S.; Luo, R.; Feng, J.; Ye, S.; Yang, T.-S.; et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009, 10, 25–34.
- Zhu, A.X.; Kudo, M.; Assenat, E.; Cattan, S.; Kang, Y.-K.; Lim, H.Y.; Poon, R.T.P.; Blanc, J.-F.; Vogel, A.; Chen, C.-L.; et al. Effect of everolimus on survival in advanced hepatocellular carcinoma after failure of sorafenib: The EVOLVE-1 randomized clinical trial. JAMA 2014, 312, 57–67.
- Cheng, A.-L.; Kang, Y.-K.; Lin, D.-Y.; Park, J.-W.; Kudo, M.; Qin, S.; Chung, H.-C.; Song, X.; Xu, J.; Poggi, G.; et al. Sunitinib versus sorafenib in advanced hepatocellular cancer: Results of a randomized phase III trial. J. Clin. Oncol. 2013, 31, 4067–4075.
- Johnson, P.J.; Qin, S.; Park, J.-W.; Poon, R.T.P.; Raoul, J.-L.; Philip, P.A.; Hsu, C.-H.; Hu, T.-H.; Heo, J.; Xu, J.; et al. Brivanib versus sorafenib as first-line therapy in patients with unresectable, advanced hepatocellular carcinoma: Results from the randomized phase III BRISK-FL study. J. Clin. Oncol. 2013, 31, 3517–3524.
- Cainap, C.; Qin, S.; Huang, W.-T.; Chung, I.J.; Pan, H.; Cheng, Y.; Kudo, M.; Kang, Y.-K.; Chen, P.-J.; Toh, H.-C.; et al. Linifanib versus Sorafenib in patients with advanced hepatocellular carcinoma: Results of a randomized phase III trial. J. Clin. Oncol. 2015, 33, 172–179.
- Rimassa, L.; Assenat, E.; Peck-Radosavljevic, M.; Pracht, M.; Zagonel, V.; Mathurin, P.; Rota Caremoli, E.; Porta, C.; Daniele, B.; Bolondi, L.; et al. Tivantinib for second-line treatment of MET-high, advanced hepatocellular carcinoma (METIV-HCC): A final analysis of a phase 3, randomised, placebo-controlled study. Lancet Oncol. 2018, 19, 682–693.
- Zhu, A.X.; Rosmorduc, O.; Evans, T.R.J.; Ross, P.J.; Santoro, A.; Carrilho, F.J.; Bruix, J.; Qin, S.; Thuluvath, P.J.; Llovet, J.M.; et al. SEARCH: A phase III, randomized, double-blind, placebo-controlled trial of sorafenib plus erlotinib in patients with advanced hepatocellular carcinoma. J. Clin. Oncol. 2015, 33, 559–566.
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.-H.; Ikeda, K.; Piscaglia, F.; Baron, A.; Park, J.-W.; Han, G.; Jassem, J.; et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet 2018, 391, 1163–1173.
- Brackenier, C.; Kinget, L.; Cappuyns, S.; Verslype, C.; Beuselinck, B.; Dekervel, J. Unraveling the Synergy between Atezolizumab and Bevacizumab for the Treatment of Hepatocellular Carcinoma. Cancers 2023, 15, 348.
- Liu, X.; Lu, Y.; Qin, S. Atezolizumab and bevacizumab for hepatocellular carcinoma: Mechanism, pharmacokinetics and future treatment strategies. Future Oncol. 2021, 17, 2243–2256.
- Lee, M.S.; Ryoo, B.-Y.; Hsu, C.-H.; Numata, K.; Stein, S.; Verret, W.; Hack, S.P.; Spahn, J.; Liu, B.; Abdullah, H.; et al. Atezolizumab with or without bevacizumab in unresectable hepatocellular carcinoma (GO30140): An open-label, multicentre, phase 1b study. Lancet Oncol. 2020, 21, 808–820.
- Roy, A. Updated Efficacy and Safety Data from IMbrave150: Atezolizumab Plus Bevacizumab vs. Sorafenib for Unresectable Hepatocellular Carcinoma. J. Clin. Exp. Hepatol. 2022, 12, 1575–1576.
- Zhu, A.X.; Abbas, A.R.; De Galarreta, M.R.; Guan, Y.; Lu, S.; Koeppen, H.; Zhang, W.; Hsu, C.-H.; He, A.R.; Ryoo, B.-Y.; et al. Molecular correlates of clinical response and resistance to atezolizumab in combination with bevacizumab in advanced hepatocellular carcinoma. Nat. Med. 2022, 28, 1599–1611.
- Hack, S.P.; Zhu, A.X.; Wang, Y. Augmenting Anticancer Immunity Through Combined Targeting of Angiogenic and PD-1/PD-L1 Pathways: Challenges and Opportunities. Front. Immunol. 2020, 11, 598877.
- Abou-Alfa, G.K.; Lau, G.; Kudo, M.; Chan, S.L.; Kelley, R.K.; Furuse, J.; Sukeepaisarnjaroen, W.; Kang, Y.-K.; Van Dao, T.; De Toni, E.N.; et al. Tremelimumab plus Durvalumab in Unresectable Hepatocellular Carcinoma. NEJM Evid. 2022, 1, 8.
- Helwick, C. HIMALAYA Trial: First-Line Tremelimumab Plus Durvalumab Improves Overall Survival in Unresectable Hepatocellular Carcinoma. The ASCO Post, 10 February 2022.
- Elevar Therapeutics Submits New Drug Application to FDA for Combination of Rivoceranib and Camrelizumab as First-Line Treatment Option for Unresectable Hepatocellular Carcinoma. Available online: https://elevartherapeutics.com/2023/07/17/elevar-therapeutics-announces-fda-acceptance-for-filing-of-new-drug-application-for-rivoceranib-in-combination-with-camrelizumab-as-a-first-line-treatment-for-unresectable-hepatocellular-carcinoma/ (accessed on 17 July 2023).
- El-Khoueiry, A.B.; Sangro, B.; Yau, T.; Crocenzi, T.S.; Kudo, M.; Hsu, C.; Kim, T.-Y.; Choo, S.-P.; Trojan, J.; Welling, T.H.; et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017, 389, 2492–2502.
- Kudo, M.; Matilla, A.; Santoro, A.; Melero, I.; Gracián, A.C.; Acosta-Rivera, M.; Choo, S.-P.; El-Khoueiry, A.B.; Kuromatsu, R.; El-Rayes, B.; et al. CheckMate 040 cohort 5: A phase I/II study of nivolumab in patients with advanced hepatocellular carcinoma and Child-Pugh B cirrhosis. J. Hepatol. 2021, 75, 600–609.
- Yau, T.; Park, J.-W.; Finn, R.S.; Cheng, A.-L.; Mathurin, P.; Edeline, J.; Kudo, M.; Harding, J.J.; Merle, P.; Rosmorduc, O.; et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): A randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2022, 23, 77–90.
- Zhu, A.X.; Finn, R.S.; Edeline, J.; Cattan, S.; Ogasawara, S.; Palmer, D.; Verslype, C.; Zagonel, V.; Fartoux, L.; Vogel, A.; et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): A non-randomised, open-label phase 2 trial. Lancet Oncol. 2018, 19, 940–952.
- Finn, R.S.; Ryoo, B.-Y.; Merle, P.; Kudo, M.; Bouattour, M.; Lim, H.Y.; Breder, V.; Edeline, J.; Chao, Y.; Ogasawara, S.; et al. Pembrolizumab as Second-Line Therapy in Patients with Advanced Hepatocellular Carcinoma in KEYNOTE-240: A Randomized, Double-Blind, Phase III Trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020, 38, 193–202.
- Qin, S.; Chen, Z.; Fang, W.; Ren, Z.; Xu, R.; Ryoo, B.-Y.; Meng, Z.; Bai, Y.; Chen, X.; Liu, X.; et al. Pembrolizumab plus best supportive care versus placebo plus best supportive care as second-line therapy in patients in Asia with advanced hepatocellular carcinoma (HCC): Phase 3 KEYNOTE-394 study. J. Clin. Oncol. 2022, 40, 383.
- Foerster, F.; Gairing, S.J.; Ilyas, S.I.; Galle, P.R. Emerging immunotherapy for HCC: A guide for hepatologists. Hepatology 2022, 75, 1604–1626.
- Saung, M.T.; Pelosof, L.; Casak, S.; Donoghue, M.; Lemery, S.; Yuan, M.; Rodriguez, L.; Schotland, P.; Chuk, M.; Davis, G.; et al. FDA Approval Summary: Nivolumab Plus Ipilimumab for the Treatment of Patients with Hepatocellular Carcinoma Previously Treated with Sorafenib. Oncologist 2021, 26, 797–806.
- Bruix, J. Regorafenib and the RESORCE trial: A new second-line option for hepatocellular carcinoma patients. Hepatic Oncol. 2016, 3, 187–189.
- Abou-Alfa, G.K.; Meyer, T.; Cheng, A.-L.; El-Khoueiry, A.B.; Rimassa, L.; Ryoo, B.-Y.; Cicin, I.; Merle, P.; Chen, Y.; Park, J.-W.; et al. Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. N. Engl. J. Med. 2018, 379, 54–63.
- Zhu, A.X.; Kang, Y.-K.; Yen, C.-J.; Finn, R.S.; Galle, P.R.; Llovet, J.M.; Assenat, E.; Brandi, G.; Pracht, M.; Lim, H.Y.; et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019, 20, 282–296.
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.-Y.; Lim, H.Y.; Kudo, M.; Breder, V.V.; Merle, P.; et al. IMbrave150: Updated overall survival (OS) data from a global, randomized, open-label phase III study of atezolizumab (atezo) + bevacizumab (bev) versus sorafenib (sor) in patients (pts) with unresectable hepatocellular carcinoma (HCC). J. Clin. Oncol. 2021, 39, 267.
- Finn, R.S.; Ikeda, M.; Zhu, A.X.; Sung, M.W.; Baron, A.D.; Kudo, M.; Okusaka, T.; Kobayashi, M.; Kumada, H.; Kaneko, S.; et al. Phase Ib Study of Lenvatinib Plus Pembrolizumab in Patients with Unresectable Hepatocellular Carcinoma. J. Clin. Oncol. 2020, 38, 2960–2970.
- Llovet, J.M.; Kudo, M.; Cheng, A.-L.; Finn, R.S.; Galle, P.R.; Kaneko, S.; Meyer, T.; Qin, S.; Dutcus, C.E.; Chen, E.; et al. Lenvatinib (len) plus pembrolizumab (pembro) for the first-line treatment of patients (pts) with advanced hepatocellular carcinoma (HCC): Phase 3 LEAP-002 study. J. Clin. Oncol. 2019, 37, TPS4152.
- Kelley, R.K.; Rimassa, L.; Cheng, A.-L.; Kaseb, A.; Qin, S.; Zhu, A.X.; Chan, S.L.; Melkadze, T.; Sukeepaisarnjaroen, W.; Breder, V.; et al. Cabozantinib plus atezolizumab versus sorafenib for advanced hepatocellular carcinoma (COSMIC-312): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2022, 23, 995–1008.
- Akce, M.; El-Rayes, B.F.; Bekaii-Saab, T.S. Frontline therapy for advanced hepatocellular carcinoma: An update. Ther. Adv. Gastroenterol. 2022, 15, 175628482210861.
- A Study of Tivozanib in Combination with Durvalumab in Subjects with Advanced Hepatocellular Carcinoma (DEDUCTIVE). Available online: https://clinicaltrials.gov/study/NCT03970616#more-information (accessed on 1 July 2023).
- Regorafenib Plus Tislelizumab as First-Line Systemic Therapy for Patients with Advanced Hepatocellular Carcinoma NCT04183088. Available online: https://www.clinicaltrials.gov/study/NCT04183088 (accessed on 1 July 2023).
- Phase 3 Study of Tislelizumab Versus Sorafenib in Participants with Unresectable HCC. ClinicalTrials.Gov ID NCT03412773. Available online: https://clinicaltrials.gov/study/NCT03412773 (accessed on 1 July 2023).
- A Study of Nivolumab in Combination with Ipilimumab in Participants with Advanced Hepatocellular Carcinoma (CheckMate 9DW) ClinicalTrials.Gov ID NCT04039607. Available online: https://clinicaltrials.gov/study/NCT04039607 (accessed on 27 June 2023).
- Efficacy and Safety of IBI310 Combined with Sintilimab in Patients with Advanced Hepatocellular Carcinoma. ClinicalTrials.Gov ID NCT04401813. Available online: https://clinicaltrials.gov/study/NCT04401813 (accessed on 27 June 2023).
- MGD013 Monotherapy and Combination With Brivanib Dose Escalation and Expansion Study in Advanced Liver Cancer Patients. Available online: https://clinicaltrials.gov/study/NCT04212221 (accessed on 27 June 2023).
- Acoba, J.D.; Rho, Y.; Fukaya, E. Phase II study of cobolimab in combination with dostarlimab for the treatment of advanced hepatocellular carcinoma. J. Clin. Oncol. 2023, 41, 580.
- Immunotherapy With Nivolumab in Combination with Lenvatinib for Advanced Stage Hepatocellular Carcinoma. ClinicalTrials.Gov ID NCT03841201. Available online: https://clinicaltrials.gov/study/NCT03841201 (accessed on 27 July 2023).
- Combination of Regorafenib and Nivolumab in Unresectable Hepatocellular Carcinoma (RENOBATE). ClinicalTrials.Gov ID NCT04310709. Available online: https://clinicaltrials.gov/study/NCT04310709 (accessed on 25 July 2023).
- Study of ET140203 T Cells in Adults With Advanced Hepatocellular Carcinoma (ARYA-1). ClinicalTrials.Gov ID NCT04502082. Available online: https://clinicaltrials.gov/study/NCT04502082 (accessed on 15 June 2023).
- Study Evaluating the Benefit of Adding Ipilimumab to the Combination of Atezolizumab and Bevacizumab in Patients with Hepatocellular Carcinoma Receiving First-Line Systemic Therapy (TRIPLET). ClinicalTrials.Gov ID NCT05665348. Available online: https://clinicaltrials.gov/study/NCT05665348 (accessed on 27 July 2023).
- A Phase I Study of ERY974 in Patients with Hepatocellular Carcinoma ClinicalTrials.Gov ID NCT05022927. Available online: https://clinicaltrials.gov/study/NCT05022927 (accessed on 16 July 2023).
- Personalized Neoantigen Peptide-Based Vaccine in Combination with Pembrolizumab for Treatment of Advanced Solid Tumors (PNeoVCA) ClinicalTrials.Gov ID NCT05269381. Available online: https://clinicaltrials.gov/study/NCT05269381 (accessed on 1 June 2023).
- Sangro, B.; Yau, T.; Harding, J.J.; Acosta Rivera, M.; Kazushi, N.; El-Khoueiry, A.B.; Cruz-Correa, M.; Perez-Callejo, D.; McLean, S.; Sparks, J.; et al. RELATIVITY-106: A phase 1/2 trial of nivolumab (NIVO) + relatlimab (RELA) in combination with bevacizumab (BEV) in first-line (1L) hepatocellular carcinoma (HCC). J. Clin. Oncol. 2023, 41, TPS636.
More
