Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Lindsay Dong and Version 1 by Cody Dalton.
Caveolin-1 (Cav1) is a protein that exists in many different forms and locations in cells and tissues throughout the body. We can understand more about cell growth, death, and cellular processes by further understanding the structure and function of Cav1. The increasing knowledge of Cav1 and its roles in different organs and disease processes helps delineate its potential use in the development of treatments and therapies.
- Caveolin-1
- caveolae
- tight junction
- barrier function
1. Introduction
Caveolin-1 is a highly ubiquitous protein found in many tissues throughout the human body. It is involved in processes including cell membrane maintenance, cellular signaling, differentiation, proliferation, and migration as well as regulation of programmed cell death and autophagy [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23][1][2][3][4][5][6][7][8][9][10][11][12][13][14][15][16][17][18][19][20][21][22][23]. This diverse set of roles varies depending on the tissue-specific location of caveolin-1 (Cav1), which includes but is not limited to endothelium, adipose, pneumocytes, and muscle and intestinal epithelium [24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46][24][25][26][27][28][29][30][31][32][33][34][35][36][37][38][39][40][41][42][43][44][45][46]. It is recognized that Cav1 is implicated in both the positive and negative regulation of many cellular processes [47,48,49,50,51,52][47][48][49][50][51][52]. For example, downregulation of Cav1 in the early stages of tumorigenesis of certain cancer types promotes proliferation, angiogenesis, and tumor progression while in the later stages re-expression of the protein seems to support cell invasion and metastasis [53]. This seemingly paradoxical description of function is one of many reasons that Cav1 is an important and interesting target protein for ongoing research and discovery. Cav1 has been studied as a critical participant in cytoskeletal interactions, intracellular signaling, cholesterol transport, and tight junction regulation (Figure 1) [38,39,40,46,47,48,49,50,51,52,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77][38][39][40][46][47][48][49][50][51][52][54][55][56][57][58][59][60][61][62][63][64][65][66][67][68][69][70][71][72][73][74][75][76][77].
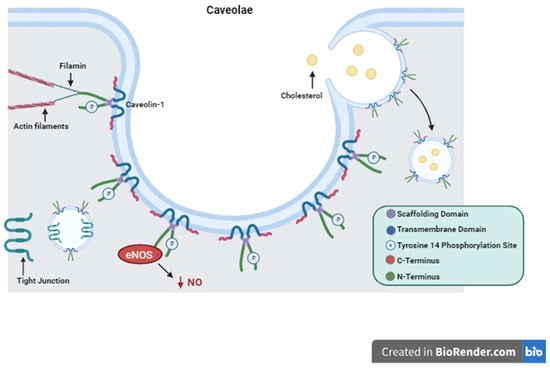
Figure 1. Caveolin-1 Structure and Function. Depiction of Caveolin-1 structure including its scaffolding domain, transmembrane domain, and Tyrosine 14 phosphorylation site. The structure of caveolae is represented with Caveolin-1 proteins forming the surface of the plasma membrane invagination. Important functions of Caveolin-1 are illustrated showing cytosolic interactions with actin filaments, endocytosis and cholesterol trafficking, tight junction shuttling, and intracellular signaling eNOS inactivation and decreased production of NO as an example.
2. Caveolin-1 Structure and Function
Cav1 is a 21 kDa protein that is associated with the plasma membrane having two hydrophilic termini residing on the cytosolic side separated by a hydrophobic segment lying within the membrane [78]. Here it performs its many functions with its two main functional domains: the tyrosine 14 phosphorylation site and its scaffolding domain [78]. The scaffolding domain is responsible for interactions with other proteins that regulate intracellular signaling cascades [78]. The Src family proteins, G proteins, protein kinase A and C, tyrosine kinase receptors (TKRs), and nitric oxide synthase (NOS) are just a few of these regulator proteins [79,80,81,82][79][80][81][82]. Phosphorylation of Cav1 on specific amino acid residues causes upregulation or downregulation of certain processes such as cholesterol trafficking [83,84][83][84]. Cav1 is also involved in the formation of caveolae. Caveolae, first described in 1955 while looking at gallbladder epithelium in mice, are invaginations of the cell plasma membrane that form vesicles which communicate extracellularly [85].Interestingly, Cav1 is a protein that is palmitoylated on three cysteine residues on the C-terminus [86,87][86][87]. This stabilizes the protein–membrane relationship and is required for certain protein–protein interactions as is the case with G proteins [86,87][86][87]. Cav1 has been demonstrated to induce cholesterol clustering and higher degrees of membrane curvature in cholesterol-rich membranes [86]. This is a process critical for cell signaling and the formation of caveolae [86]. Cav1 was first thought to perform only absorptive functions via caveolae formation without any mention of its secretory or intracellular functions [85].
3. Cellular Signaling
Cav1 serves as a key component of multiple cellular signaling pathways and both positive and negative regulatory functions have been identified [47,48,49,50,51,52][47][48][49][50][51][52]. For example, there is evidence of Cav1 involvement in the p38 mitogen-activated protein kinase (MAPK) signaling pathway, by which decreased Cav1-mediated activation of c-Jun N-terminal kinase (JNK), nuclear factor kappa B (NF-kB), and activator protein-1 (AP-1) leads to protection against inflammatory mechanisms in the setting of sepsis [47]. In contrast, Cav1 also interacts with toll-like receptor 4 (TLR4), a protein that activates the innate immune system, and increases endothelial cell permeability in the setting of hypoxia [48]. When Cav1 is knocked out, the inflammatory response mediated through the TLR4 pathway is suppressed [49]. C4. Cell Proliferation, Differentiation, Migration and Survival
4.1. Proliferation
Cav1 plays a part in multiple mechanisms that maintain cell viability and stabilization [1,2,3][1][2][3]. In general, Cav1 is noted to have inhibitory effects on cell proliferation as demonstrated in both mouse and human Cav1 knockout mesenchymal stem cells in which proliferation increased. There is further evidence of this phenomenon with multiple studies showing that upregulation of Cav1 has the opposite result and decreases proliferation [1,2][1][2]. In vivo, Cav1 overexpression arrested the cell cycle in mouse embryonic fibroblasts at the G0/G1 phase causing growth and mitotic activity to come to a halt [3].4.2. Differentiation
There is evidence to suggest that Cav1 has both inhibitory and promoter effects on cellular differentiation [50,51][50][51]. Knocking out Cav1 in mouse stem cells decreased mRNA expression of pluripotency markers while overexpression of Cav1 had the opposite effect [4]. It is hypothesized that Cav1 activity may allow progenitor cells to be more sensitive to certain stimuli that cause ongoing differentiation [4]. As cells reach their final phenotype, Cav1 can then function as part of a negative feedback loop to prevent ongoing growth and differentiation [5]. This was also demonstrated with mesenchymal stem cells undergoing osteogenic differentiation as Cav1 activity enhanced the process before stabilizing the final phenotype [6,7][6][7].4.3. Migration
One illustration of how Cav1 influences cell polarity and migration is how the protein assists with stromal-derived factor-1 (SDF-1) interactions with its receptor, CXCR4. This plays a crucial role cell adhesion during leukocyte recruitment [8]. Cav1 is required for mobilization of progenitor cells from mouse bone marrow [9]. Through interactions with the cytoskeleton, Cav1 promotes cellular polarity and morphological changes that allow for cellular migration [9]. This was further found to be regulated via Cav1 activation of Src and Rho GTPase [10]. Grande-Garcia et al. found that Cav1-deficient mouse embryonic fibroblasts did not exhibit polarized morphology via actin rearrangements [10]. Cells can also relocalize Cav1 depending on the type of movement they are performing [10,11][10][11]. During three-dimensional movement, endothelial cells release Cav1 from caveolar structures in the cell rear and relocalize it to the front [11].4.4. Survival
Cav1 involvement in the regulation of cell survival and programmed death in response to stress or insult has important implications for tissue repair and wound healing. For example, downregulation of Cav1 expression in myogenic precursor cells is mandatory for regeneration and healing in skeletal muscle tissue [12]. Dysregulation of these mechanisms is pertinent to certain disease states. Cav1 is an integral player in both cell survival and apoptosis. Apoptosis is cell death that can occur as a controlled part of an organism’s natural growth cycle or in response to extra or intracellular stressors. Just as essential to the cell cycle is the process of autophagy, in which Cav1 has also been shown to play a major role. Autophagy is the degradation of damaged or unnecessary cellular components through a lysosome-dependent mechanism. This process can be part of an adaptive response that promotes cell survival in the setting of starvation. There are multiple examples of Cav1 being a positive regular of autophagy and a negative regulator of apoptosis [13,14,15][13][14][15].4.5. Caveolin-1 Role in Cancer Cell Death and Survival
The role of Cav1 in tumor growth, metastasis, and response to treatment appears to be most closely linked with its role in regulating apoptosis and cell death mechanisms. Cav1 can act in accordance with tumor suppressor or promotor modalities depending on the cell type, tumor stage, and nature of the tumor stroma [17,18,53][17][18][53]. In specific cancers including breast, lung, colon, and ovarian, Cav1 is expressed in low levels, which is maintained throughout the process of tumor cell proliferation and metastasis [19,20,21,22,23][19][20][21][22][23].5. Caveolin-1 Tissue-Specific Roles
To further understand the roles that Cav1 plays in individual tissue types, it must first be explained that Cav1 takes on multiple different forms within the cell itself in order to perform these specific functions. As a membrane protein, Cav1 forms caveolae to interact with cytoskeletal elements and signaling cascade proteins [47,48,49,50,51,52,54][47][48][49][50][51][52][54]. In the cytoplasm, it is a soluble protein that participates in transporting and delivering lipids to the cell surface, mitochondria, endoplasmic reticulum, and other cellular compartments [24]. Examples of cell types in which Cav1 is localized to the membrane in the form of caveolae include fibroblasts, endothelial, and polarized epithelial cells of the intestine [24].5.1. Vascular
Cav1 expression in vascular endothelial cells regulates the progression of atherosclerosis in large vessels [27]. Increased autophagy in Cav1 knockout cells has shown to protect against the progression of atherosclerosis via a decrease in vascular inflammation, macrophage infiltration, and LDL transcytosis [27]. Cav1 also inhibits endothelial nitric oxide synthase activity via direct binding of endothelial nitric oxide synthase (eNOS) and regulation of its expression [28].5.2. Adipose
Adipose tissue has been described as one of the most abundant sources of caveolin proteins [31]. Caveolins are established cholesterol binding proteins [31,78][31][78]. Exogenous, extracellular lipids induce plasma membrane caveolins to associate with lipid droplets in adipocytes [32,33][32][33]. This indicates a trafficking pathway by which Cav1 shuttles cholesterol and fatty acids from plasma membrane to the organelle and vice versa [32,33][32][33]. In Cav1 null mice there is evidence of aberrant lipid metabolism, namely a lipoatrophic phenotype and hyperlipidemia, which results from reduced lipid storage within adipocytes and therefore increased levels of lipids remaining in circulation [34]. Cav1 null mice also have resistance to diet-induced obesity [35]. Cav1 performs key interactions with insulin receptors by affecting downstream signaling and stabilizing the receptors to the plasma membrane [32].5.3. Brain
There are a host of factors that influence the integrity and permeability of the blood-brain barrier (BBB). Caveolins are thought to perform vital functions in the regulation of the junctional proteins that create the barrier between vascular endothelial cells and the brain parenchyma [38,39,40][38][39][40]. Choi et al. delineated this relationship by showing that Cav1 null mice had significant degradation of tight junction proteins and an increase in matrix me5.4. Pneumocytes
Caveolin-1 is essential for pulmonary function and development. As previously discussed, Cav1 is involved in the proliferation and differentiation of stem cells [1,2,3,4,5,6,7][1][2][3][4][5][6][7]. This process is also true for fetal lung development, specifically as it relates to the differentiation into type II alveolar epithelial cells, also known as pneumocyte [43]. Type II pneumocytes have a high expression of Cav1 and structurally contain an abundance of caveolae [44,45][44][45]. It is thought that these caveolae are partly responsible for maintaining the homeostasis of water and protein transcytosis across the epithelial membrane [43,44,45,46][43][44][45][46]. In bronchopulmonary dysplasia, exposure of type II pneumocytes to hyperoxia showed disruption of the pulmonary epithelial barrier [46].5.4. Pneumocytes
Caveolin-1 is essential for pulmonary function and development. As previously discussed, Cav1 is involved in the proliferation and differentiation of stem cells [1,2,3,4,5,6,7][1][2][3][4][5][6][7]. This process is also true for fetal lung development, specifically as it relates to the differentiation into type II alveolar epithelial cells, also known as pneumocyte [43]. Type II pneumocytes have a high expression of Cav1 and structurally contain an abundance of caveolae [44,45][44][45]. It is thought that these caveolae are partly responsible for maintaining the homeostasis of water and protein transcytosis across the epithelial membrane [43,44,45,46][43][44][45][46].6. Caveolin-1 and the Intestinal Barrier
Multiple components contribute to the maintenance of the intestinal barrier. The intestinal epithelial layer acts as a semipermeable membrane allowing the absorption of nutrients and performance of immune functions while preventing the transport of harmful substances [55]. The mucosal layer is the first line of defense for epithelial cells coming into direct contact with potentially toxic microorganisms. IgA and antimicrobial proteins are secreted into this layer in order to neutralize these microorganisms [56]. The layer of polarized epithelial cells that separates the intestinal lumen from the lamina propria underneath is highly selective in what is allowed to be transported across [55]. This is in part due to regulation via the presence of junctional complexes, which consist of tight junctions, adherens junctions, and desmosomes [57,58][57][58]. Tight junctions determine a healthy epithelial barrier. Disruption of the tight junctions by systemic stressors can lead to intestinal hyperpermeability and trigger systemic immune responses [59]. These components of the intestinal barrier are the major points of interest when researching and describing the pathogenesis of gastrointestinal diseases. Although Cav1 is seen in the cytosolic and intracellular compartment forms, it is most commonly thought to be associated with the cell surface [24]. One purpose this might serve is to integrate junctional complexes into the plasma membrane. This anchors epithelial cells at the basolateral surface both to each other and to the basement membrane (Figure 2). The location of junctional complexes within the cell and their level of expression is, in part, Cav1-dependent [60,61,62,63,64,65,66,67][60][61][62][63][64][65][66][67]. Cav1-mediated shuttling and endocytosis of tight junctions during their relocation has been described previously [60,61,62,63,64,65,66,67][60][61][62][63][64][65][66][67].7. Conclusions
Caveolin-1 is a vital protein for many cellular processes and its involvement in both the positive and negative regulation of these processes has been clearly demonstrated. Its pervasiveness throughout a variety of tissue and cell types has established the protein as a point of interest for many researchers to better understand the pathophysiologic mechanisms of disease. Cav1 as a plasma membrane protein carries out regulatory functions of many intracellular signaling cascades.References
- Kumar, V. T cells and their immunometabolism: A novel way to understanding sepsis immunopathogenesis and future therapeutics. Eur. J. Cell Biol. 2018, 97, 379–392.
- Michell, D.L.; Shihata, W.A.; Andrews, K.L.; Abidin, N.A.Z.; Jefferis, A.-M.; Sampson, A.K.; Lumsden, N.G.; Huet, O.; Parat, M.-O.; Jennings, G.L.; et al. High intraluminal pressure promotes vascular inflammation via caveolin-1. Sci. Rep. 2021, 11, 5894.
- Baker, N.; Zhang, G.; You, Y.; Tuan, R.S. Caveolin-1 regulates proliferation and osteogenic differentiation of human mesenchymal stem cells. J. Cell. Biochem. 2012, 113, 3773–3787.
- Park, J.-S.; Kim, H.-Y.; Kim, H.-W.; Chae, G.-N.; Oh, H.-T.; Park, J.-Y.; Shim, H.; Seo, M.; Shin, E.Y.; Kim, E.G.; et al. Increased caveolin-1, a cause for the declined adipogenic potential of senescent human mesenchymal stem cells. Mech. Ageing Dev. 2005, 126, 551–559.
- Volonte, D.; Zhang, K.; Lisanti, M.P.; Galbiati, F. Expression of Caveolin-1 Induces Premature Cellular Senescence in Primary Cultures of Murine Fibroblasts. Mol. Biol. Cell 2002, 13, 2502–2517.
- Lee, M.Y.; Ryu, J.M.; Lee, S.H.; Park, J.H.; Han, H.J. Lipid rafts play an important role for maintenance of embryonic stem cell self-renewal. J. Lipid Res. 2010, 51, 2082–2089.
- Simons, K.; Toomre, D. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 2000, 1, 31–39.
- Discher, D.E.; Mooney, D.J.; Zandstra, P.W. Growth Factors, Matrices, and Forces Combine and Control Stem Cells. Science 2009, 324, 1673–1677.
- Park, D.S.; Lee, H.; Frank, P.G.; Razani, B.; Nguyen, A.V.; Parlow, A.F.; Russell, R.G.; Hulit, J.; Pestell, R.G.; Lisanti, M.P.; et al. Caveolin-1-deficient Mice Show Accelerated Mammary Gland Development During Pregnancy, Premature Lactation, and Hyperactivation of the Jak-2/STAT5a Signaling Cascade. Mol. Biol. Cell 2002, 13, 3416–3430.
- Ratliff, B.B.; Singh, N.; Yasuda, K.; Park, H.-C.; Addabbo, F.; Ghaly, T.; Rajdev, M.; Jasmin, J.F.; Plotkin, M.; Lisanti, M.P.; et al. Mesenchymal Stem Cells, Used as Bait, Disclose Tissue Binding Sites: A Tool in the Search for the Niche? Am. J. Pathol. 2010, 177, 873–883.
- Sbaa, E.; DeWever, J.; Martinive, P.; Bouzin, C.; Frérart, F.; Balligand, J.-L.; Dessy, C.; Feron, O. Caveolin Plays a Central Role in Endothelial Progenitor Cell Mobilization and Homing in SDF-1–Driven Postischemic Vasculogenesis. Circ. Res. 2006, 98, 1219–1227.
- Grande-García, A.; Echarri, A.; de Rooij, J.; Alderson, N.B.; Waterman-Storer, C.M.; Valdivielso, J.M.; del Pozo, M.A. Caveolin-1 regulates cell polarization and directional migration through Src kinase and Rho GTPases. J. Cell Biol. 2007, 177, 683–694.
- Parat, M.-O.; Anand-Apte, B.; Fox, P.L.; Meng, F.; Saxena, S.; Liu, Y.; Joshi, B.; Wong, T.H.; Shankar, J.; Foster, L.J.; et al. Differential Caveolin-1 Polarization in Endothelial Cells during Migration in Two and Three Dimensions. Mol. Biol. Cell 2003, 14, 3156–3168.
- Baker, N.; Tuan, R.S. The less-often-traveled surface of stem cells: Caveolin-1 and caveolae in stem cells, tissue repair and regeneration. Stem Cell Res. Ther. 2013, 4, 90.
- Nah, J.; Yoo, S.-M.; Jung, S.; Jeong, E.I.; Park, M.; Kaang, B.-K.; Jung, Y.-K. Phosphorylated CAV1 activates autophagy through an interaction with BECN1 under oxidative stress. Cell Death Dis. 2017, 8, e2822.
- Wang, R.; He, W.; Li, Z.; Chang, W.; Xin, Y.; Huang, T. Caveolin-1 functions as a key regulator of 17β-estradiol-mediated autophagy and apoptosis in BT474 breast cancer cells. Int. J. Mol. Med. 2014, 34, 822–827.
- Xu, Q.; Shi, W.; Lv, P.; Meng, W.; Mao, G.; Gong, C.; Chen, Y.; Wei, Y.; He, X.; Zhao, J.; et al. Critical role of caveolin-1 in aflatoxin B1-induced hepatotoxicity via the regulation of oxidation and autophagy. Cell Death Dis. 2020, 11, 6.
- Gargalovic, P.; Dory, L. Cellular apoptosis is associated with increased caveolin-1 expression in macrophages. J. Lipid Res. 2003, 44, 1622–1632.
- Chidlow, J.H.; Sessa, W.C. Caveolae, caveolins, and cavins: Complex control of cellular signalling and inflammation. Cardiovasc. Res. 2010, 86, 219–225.
- Sotgia, F.; Martinez-Outschoorn, U.E.; Howell, A.; Pestell, R.G.; Pavlides, S.; Lisanti, M.P. Caveolin-1 and Cancer Metabolism in the Tumor Microenvironment: Markers, Models, and Mechanisms. Annu. Rev. Pathol. Mech. Dis. 2012, 7, 423–467.
- Ando, T.; Ishiguro, H.; Kimura, M.; Mitsui, A.; Mori, Y.; Sugito, N.; Tomoda, K.; Mori, R.; Harada, K.; Katada, T.; et al. The overexpression of caveolin-1 and caveolin-2 correlates with a poor prognosis and tumor progression in esophageal squamous cell carcinoma. Oncol. Rep. 2007, 18, 601–609.
- Ho, C.-C.; Kuo, S.-H.; Huang, P.-H.; Huang, H.-Y.; Yang, C.-H.; Yang, P.-C. Caveolin-1 expression is significantly associated with drug resistance and poor prognosis in advanced non-small cell lung cancer patients treated with gemcitabine-based chemotherapy. Lung Cancer 2008, 59, 105–110.
- Hu, J.; Shao, S.; Song, Y.; Zhao, J.; Dong, Y.; Gong, L.; Yang, P. Hepatocyte Growth Factor Induces Invasion and Migration of Ovarian Cancer Cells by Decreasing the Expression of E-cadherin, β-catenin, and Caveolin-1. Anat. Rec. 2010, 293, 1134–1139.
- Koleske, A.J.; Baltimore, D.; Lisanti, M.P. Reduction of caveolin and caveolae in oncogenically transformed cells. Proc. Natl. Acad. Sci. USA 1995, 92, 1381–1385.
- Williams, T.M.; Lisanti, M.P. Caveolin-1 in oncogenic transformation, cancer, and metastasis. Am. J. Physiol. Cell Physiol. 2005, 288, C494–C506.
- Li, W.-P.; Liu, P.; Pilcher, B.K.; Anderson, R.G.W. Cell-specific targeting of caveolin-1 to caveolae, secretory vesicles, cytoplasm or mitochondria. J. Cell Sci. 2001, 114, 1397–1408.
- Kurzchalia, T.V.; Dupree, P.; Parton, R.G.; Kellner, R.; Virta, H.; Lehnert, M.; Simons, K. VIP21, a 21-kD membrane protein is an integral component of trans-Golgi-network-derived transport vesicles. J. Cell Biol. 1992, 118, 1003–1014.
- Smart, E.J.; Ying, Y.S.; Conrad, P.A.; Anderson, R.G. Caveolin moves from caveolae to the Golgi apparatus in response to cholesterol oxidation. J. Cell Biol. 1994, 127, 1185–1197.
- Zhang, X.; Ramírez, C.M.; Aryal, B.; Madrigal-Matute, J.; Liu, X.; Diaz, A.; Torrecilla-Parra, M.; Suárez, Y.; Cuervo, A.M.; Sessa, W.C.; et al. Cav-1 (Caveolin-1) Deficiency Increases Autophagy in the Endothelium and Attenuates Vascular Inflammation and Atherosclerosis. Arter. Thromb. Vasc. Biol. 2020, 40, 1510–1522.
- Chen, Z.; Oliveira, S.D.S.; Zimnicka, A.M.; Jiang, Y.; Sharma, T.; Chen, S.; Lazarov, O.; Bonini, M.G.; Haus, J.M.; Minshall, R.D.; et al. Reciprocal regulation of eNOS and caveolin-1 functions in endothelial cells. Mol. Biol. Cell 2018, 29, 1190–1202.
- Pan, Y.-M.; Yao, Y.-Z.; Zhu, Z.-H.; Sun, X.-T.; Qiu, Y.-D.; Ding, Y.-T. Caveolin-1 is important for nitric oxide-mediated angiogenesis in fibrin gels with human umbilical vein endothelial cells. Acta Pharmacol. Sin. 2006, 27, 1567–1574.
- Murata, T.; Lin, M.I.; Huang, Y.; Yu, J.; Bauer, P.M.; Giordano, F.J.; Sessa, W.C. Reexpression of caveolin-1 in endothelium rescues the vascular, cardiac, and pulmonary defects in global caveolin-1 knockout mice. J. Exp. Med. 2007, 204, 2373–2382.
- Scherer, P.E.; Lisanti, M.P.; Baldini, G.; Sargiacomo, M.; Mastick, C.C.; Lodish, H.F. Induction of caveolin during adipogenesis and association of GLUT4 with caveolin-rich vesicles. J. Cell Biol. 1994, 127, 1233–1243.
- Le Lay, S.; Hajduch, E.; Lindsay, M.R.; Le Lièpvre, X.; Thiele, C.; Ferré, P.; Parton, R.G.; Kurzchalia, T.; Simons, K.; Dugail, I. Cholesterol-Induced Caveolin Targeting to Lipid Droplets in Adipocytes: A Role for Caveolar Endocytosis. Traffic 2006, 7, 549–561.
- Pol, A.; Martin, S.; Ingelmo-Torres, M.; Ferguson, C.; Enrich, C.; Parton, R.G.; Nishimura, T.; Uchida, Y.; Yachi, R.; Kudlyk, T.; et al. Cholesterol and Fatty Acids Regulate Dynamic Caveolin Trafficking through the Golgi Complex and between the Cell Surface and Lipid Bodies. Mol. Biol. Cell 2005, 16, 2091–2105.
- Razani, B.; Combs, T.P.; Wang, X.B.; Frank, P.G.; Park, D.S.; Russell, R.G.; Li, M.; Tang, B.; Jelicks, L.A.; Scherer, P.E.; et al. Caveolin-1-deficient Mice Are Lean, Resistant to Diet-induced Obesity, and Show Hypertriglyceridemia with Adipocyte Abnormalities*. J. Biol. Chem. 2002, 277, 8635–8647.
- Cohen, A.W.; Combs, T.P.; Scherer, P.E.; Lisanti, M.P. Role of caveolin and caveolae in insulin signaling and diabetes. Am. J. Physiol. Metab. 2003, 285, E1151–E1160.
- Brännmark, C.; Palmér, R.; Glad, S.T.; Cedersund, G.; Strålfors, P. Mass and Information Feedbacks through Receptor Endocytosis Govern Insulin Signaling as Revealed Using a Parameter-free Modeling Framework. J. Biol. Chem. 2010, 285, 20171–20179.
- Haddad, D.; Al Madhoun, A.; Nizam, R.; Al-Mulla, F. Role of Caveolin-1 in Diabetes and Its Complications. Oxidative Med. Cell. Longev. 2020, 2020, 9761539.
- Choi, K.-H.; Kim, H.-S.; Park, M.-S.; Lee, E.-B.; Lee, J.-K.; Kim, J.-T.; Kim, J.-H.; Lee, M.-C.; Lee, H.-J.; Cho, K.-H. Overexpression of caveolin-1 attenuates brain edema by inhibiting tight junction degradation. Oncotarget 2016, 7, 67857–67867.
- Jasmin, J.-F.; Malhotra, S.; Dhallu, M.S.; Mercier, I.; Rosenbaum, D.M.; Lisanti, M.P. Caveolin-1 Deficiency Increases Cerebral Ischemic Injury. Circ. Res. 2007, 100, 721–729.
- Song, L.; Ge, S.; Pachter, J.S. Caveolin-1 regulates expression of junction-associated proteins in brain microvascular endothelial cells. Blood 2006, 109, 1515–1523.
- Head, B.P.; Peart, J.N.; Panneerselvam, M.; Yokoyama, T.; Pearn, M.L.; Niesman, I.R.; Bonds, J.A.; Schilling, J.M.; Miyanohara, A.; Headrick, J.; et al. Loss of Caveolin-1 Accelerates Neurodegeneration and Aging. PLoS ONE 2010, 5, e15697.
- Ha, T.-Y.; Choi, Y.R.; Noh, H.R.; Cha, S.-H.; Kim, J.-B.; Park, S.M. Age-related increase in caveolin-1 expression facilitates cell-to-cell transmission of α-synuclein in neurons. Mol. Brain 2021, 14, 122.
- Williams, M.C. Alveolar Type I Cells: Molecular Phenotype and Development. Annu. Rev. Physiol. 2003, 65, 669–695.
- Gil, J. Number and distribution of plasmalemmal vesicles in the lung. Fed. Proc. 1983, 42, 2414–2418.
- Stoeber, M.; Schellenberger, P.; Siebert, C.A.; Leyrat, C.; Helenius, A.; Grünewald, K. Model for the architecture of caveolae based on a flexible, net-like assembly of Cavin1 and Caveolin discs. Proc. Natl. Acad. Sci. USA 2016, 113, E8069–E8078.
- Lannes-Costa, P.S.; Pimentel, B.A.d.S.; Nagao, P.E. Role of Caveolin-1 in Sepsis—A Mini-Review. Front. Immunol. 2022, 13, 902907.
- Wang, X.M.; Kim, H.P.; Song, R.; Choi, A.M. Caveolin-1 confers antiinflammatory effects in murine macrophages via the MKK3/p38 MAPK pathway. Am. J. Respir. Cell Mol. Biol. 2006, 34, 434–442.
- Jiang, R.; Cai, J.; Zhu, Z.; Chen, D.; Wang, J.; Wang, Q.; Teng, Y.; Huang, Y.; Tao, M.; Xia, A.; et al. Hypoxic trophoblast HMGB1 induces endothelial cell hyperpermeability via the TRL-4/caveolin-1 pathway. J. Immunol. 2014, 193, 5000–5012.
- Tsai, T.H.; Tam, K.; Chen, S.F.; Liou, J.Y.; Tsai, Y.C.; Lee, Y.M.; Huang, T.Y.; Shyue, S.K. Deletion of caveolin-1 attenuates LPS/GalN-induced acute liver injury in mice. J. Cell. Mol. Med. 2018, 22, 5573–5582.
- Cai, L.; Yi, F.; Dai, Z.; Huang, X.; Zhao, Y.D.; Mirza, M.K.; Xu, J.; Vogel, S.M.; Zhao, Y.-Y. Loss of caveolin-1 and adiponectin induces severe inflammatory lung injury following LPS challenge through excessive oxidative/nitrative stress. Am. J. Physiol. Cell. Mol. Physiol. 2014, 306, L566–L573.
- Kettele, J.; Klein, D. Caveolin-1, cancer and therapy resistance. Int. J. Cancer 2018, 143, 2092–2104.
- Ludwig, A.; Nichols, B.J.; Sandin, S. Architecture of the caveolar coat complex. J. Cell Sci. 2016, 129, 3077–3083.
- Weibel, E.R.; West, J.B.; Quirk, J.D.; Sukstanskii, A.L.; Woods, J.C.; Lutey, B.A.; Conradi, M.S.; Gierada, D.S.; Yusen, R.D.; Castro, M.; et al. Morphological basis of alveolar-capillary gas exchange. Physiol. Rev. 1973, 53, 419–495.
- Xu, S.; Xue, X.; You, K.; Fu, J. Caveolin-1 regulates the expression of tight junction proteins during hyperoxia-induced pulmonary epithelial barrier breakdown. Respir. Res. 2016, 17, 50.
- Salvo Romero, E.; Alonso Cotoner, C.; Pardo Camacho, C.; Casado Bedmar, M.; Vicario, M. The intestinal barrier function and its involvement in digestive disease. Rev. Esp. Enferm. Dig. 2015, 107, 686–696.
- Turner, J.R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 2009, 9, 799–809.
- Groschwitz, K.R.; Hogan, S.P. Intestinal barrier function: Molecular regulation and disease pathogenesis. J. Allergy Clin. Immunol. 2009, 124, 3–20.
- Halpern, M.D.; Denning, P.W. The role of intestinal epithelial barrier function in the development of NEC. Tissue Barriers 2015, 3, e1000707.
- Anand, R.J.; Leaphart, C.L.; Mollen, K.P.; Hackam, D.J. The Role of The Intestinal Barrier in The Pathogenesis of Necrotizing Enterocolitis. Shock 2007, 27, 124–133.
- Bruewer, M.; Utech, M.; Ivanov, A.I.; Hopkins, A.M.; Parkos, C.A.; Nusrat, A. Interferon-gamma induces internalization of epithelial tight junction proteins via a macropinocytosis-like process. Faseb J. 2005, 19, 923–933.
- Clayburgh, D.R.; Barrett, T.A.; Tang, Y.; Meddings, J.B.; Van Eldik, L.J.; Watterson, D.M.; Clarke, L.L.; Mrsny, R.J.; Turner, J.R. Epithelial myosin light chain kinase-dependent barrier dysfunction mediates T cell activation-induced diarrhea in vivo. J. Clin. Investig. 2005, 115, 2702–2715.
- Ivanov, A.I.; Nusrat, A.; Parkos, C.A. Endocytosis of Epithelial Apical Junctional Proteins by a Clathrin-mediated Pathway into a Unique Storage Compartment. Mol. Biol. Cell 2004, 15, 176–188.
- Matsuda, M.; Kubo, A.; Furuse, M.; Tsukita, S. A peculiar internalization of claudins, tight junction-specific adhesion molecules, during the intercellular movement of epithelial cells. J. Cell Sci. 2004, 117, 1247–1257.
- Schwarz, B.T.; Wang, F.; Shen, L.; Clayburgh, D.R.; Su, L.; Wang, Y.; Fu, Y.; Turner, J.R. LIGHT Signals Directly to Intestinal Epithelia to Cause Barrier Dysfunction via Cytoskeletal and Endocytic Mechanisms. Gastroenterology 2007, 132, 2383–2394.
- Shen, L.; Turner, J.R.; Van Itallie, C.M.; Tietgens, A.J.; Anderson, J.M.; Nusrat, M.E.A.; Lu, R.; Dalgalan, D.; Mandell, E.K.; Parker, S.S.; et al. Actin Depolymerization Disrupts Tight Junctions via Caveolae-mediated Endocytosis. Mol. Biol. Cell 2005, 16, 3919–3936.
- Wroblewski, L.E.; Shen, L.; Ogden, S.; Romero–Gallo, J.; Lapierre, L.A.; Israel, D.A.; Turner, J.R.; Peek, R.M. Helicobacter pylori Dysregulation of Gastric Epithelial Tight Junctions by Urease-Mediated Myosin II Activation. Gastroenterology 2009, 136, 236–246.
- Zeissig, S.; Bürgel, N.; Günzel, D.; Richter, J.; Mankertz, J.; Wahnschaffe, U.; Kroesen, A.J.; Zeitz, M.; Fromm, M.; Schulzke, J.D. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn’s disease. Gut 2007, 56, 61–72.
- Marchiando, A.M.; Shen, L.; Graham, W.V.; Weber, C.R.; Schwarz, B.T.; Austin, J.R., II; Raleigh, D.R.; Guan, Y.; Watson, A.J.; Montrose, M.H.; et al. Caveolin-1–dependent occludin endocytosis is required for TNF-induced tight junction regulation in vivo. J. Cell Biol. 2010, 189, 111–126.
- Leiva, T.D.; Cai, X.; Liebe, H.; Golubkova, A.; Schlegel, C.B.; Hunter, C.M. Caveolin-1 and Tight Junction Expression in Human Intestine as a Susceptibility Marker of Necrotizing Enterocolitis. J. Am. Coll. Surg. 2022, 235, S173–S174.
- Kronstein, R.; Seebach, J.; Großklaus, S.; Minten, C.; Engelhardt, B.; Drab, M.; Liebner, S.; Arsenijevic, Y.; Abu Taha, A.; Afanasieva, T.; et al. Caveolin-1 opens endothelial cell junctions by targeting catenins. Cardiovasc. Res. 2011, 93, 130–140.
- Ares, G.; Buonpane, C.; Sincavage, J.; Yuan, C.; Wood, D.R.; Hunter, C.J. Caveolin 1 is Associated with Upregulated Claudin 2 in Necrotizing Enterocolitis. Sci. Rep. 2019, 9, 4982.
- Garg, P.M.; Tatum, R.; Ravisankar, S.; Shekhawat, P.S.; Chen, Y.-H. Necrotizing enterocolitis in a mouse model leads to widespread renal inflammation, acute kidney injury, and disruption of renal tight junction proteins. Pediatr. Res. 2015, 78, 527–532.
- Günzel, D.; Yu, A.S.L. Claudins and the Modulation of Tight Junction Permeability. Physiol. Rev. 2013, 93, 525–569.
- Quest, A.F.G.; Gutierrez-Pajares, J.L.; Torres, V.A. Caveolin-1: An ambiguous partner in cell signalling and cancer. J. Cell. Mol. Med. 2008, 12, 1130–1150.
- Ni, K.; Wang, C.; Carnino, J.; Jin, Y. The Evolving Role of Caveolin-1: A Critical Regulator of Extracellular Vesicles. Med. Sci. 2020, 8, 46.
- Luissint, A.-C.; Artus, C.; Glacial, F.; Ganeshamoorthy, K.; Couraud, P.-O. Tight junctions at the blood brain barrier: Physiological architecture and disease-associated dysregulation. Fluids Barriers CNS 2012, 9, 23.
- Su, L.; Shen, L.; Clayburgh, D.R.; Nalle, S.C.; Sullivan, E.A.; Meddings, J.B.; Abraham, C.; Turner, J.R. Targeted Epithelial Tight Junction Dysfunction Causes Immune Activation and Contributes to Development of Experimental Colitis. Gastroenterology 2009, 136, 551–563.
- Boscher, C.; Nabi, I.R. CAVEOLIN-1: Role in Cell Signaling. Adv. Exp. Med. Biol. 2012, 729, 29–50.
- Couet, J.; Li, S.; Okamoto, T.; Ikezu, T.; Lisanti, M.P. Identification of peptide and protein ligands for the caveolin-scaffolding domain: Implications for the interaction of caveolin with caveolae-associated proteins. J. Biol. Chem. 1997, 272, 6525–6533.
- Mineo, C.; James, G.L.; Smart, E.J.; Anderson, R.G. Localization of Epidermal Growth Factor-stimulated Ras/Raf-1 Interaction to Caveolae Membrane. J. Biol. Chem. 1996, 271, 11930–11935.
- Venema, V.J.; Zou, R.; Ju, H.; Marrero, M.B.; Venema, R.C. Caveolin-1 Detergent Solubility and Association with Endothelial Nitric Oxide Synthase Is Modulated by Tyrosine Phosphorylation. Biochem. Biophys. Res. Commun. 1997, 236, 155–161.
- Yamamoto, M.; Toya, Y.; Schwencke, C.; Lisanti, M.P.; Myers, M.G.; Ishikawa, Y. Caveolin Is an Activator of Insulin Receptor Signaling. J. Biol. Chem. 1998, 273, 26962–26968.
- Fielding, P.E.; Chau, P.; Liu, D.; Spencer, T.A.; Fielding, C.J. Mechanism of Platelet-Derived Growth Factor-Dependent Caveolin-1 Phosphorylation: Relationship to Sterol Binding and the Role of Serine-80. Biochemistry 2004, 43, 2578–2586.
- Schlegel, A.; Arvan, P.; Lisanti, M.P. Caveolin-1 binding to endoplasmic reticulum membranes and entry into the regulated secretory pathway are regulated by serine phosphorylation: Protein sorting at the level of the endoplasmic reticulum. J. Biol. Chem. 2001, 276, 4398–4408.
- Yamada, E. The fine structure of the gall bladder epithelium of the mouse. J. Biophys. Biochem. Cytol. 1955, 1, 445–458.
More
