You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 1 by George E. Zakynthinos and Version 3 by Mona Zou.
New-onset atrial fibrillation (NOAF) is the most frequently encountered cardiac arrhythmia observed in patients with COVID-19 infection, particularly in Intensive Care Unit (ICU) patients. The proposed mechanisms that could contribute to the development of Atrial fibrillation (AF) in these patients include myocardial damage resulting from direct virus-induced cardiac injury, potentially leading to perimyocarditis; a cytokine crisis and heightened inflammatory response; hypoxemia due to acute respiratory distress; disturbances in acid-base and electrolyte levels; as well as the frequent use of adrenergic drugs in critically ill patients.
- new-onset atrial fibrillation
- COVID-19
- ICU
- critical illness
- trigger factors
1. Incidence of New-Onset Atrial Fibrillation in Patients with Severe COVID-19
Cardiovascular diseases have emerged as prominent contributors to clinical deterioration and unfavorable outcomes in COVID-19 patients [1][26]. The correlation between COVID-19 and cardiac arrhythmias has also been highlighted [2][11]. In a cohort study involving 137 patients admitted to tertiary hospitals in China, 7.3% of them initially presented with heart palpitations [3][27]. Atrial fibrillation (AF) is the most prevalent form of atrial arrhythmia in COVID-19 patients, with the prevalence of NOAF ranging from 3% to 10% in non-ICU patients [2][4][10,11].
In an initial study conducted by Wang et al., it was observed that arrhythmias were more frequent among ICU patients with COVID-19 (44.4%) compared to non-ICU patients (6.9%); however, the specific nature of these arrhythmias was not detailed [5]. In a retrospective multicenter study by Kantasamy et al., which encompassed 109 consecutive patients admitted to the ICU with confirmed COVID-19 pneumonia and a definitive outcome (death or discharge), 14.6% developed NOAF during their ICU stay. This risk was more pronounced in older patients with underlying chronic heart failure and chronic kidney disease. It is worth noting that three-fourths of the population had two or more comorbidities [6][28].
In a study conducted by Colon et al., which involved 115 COVID-19 patients admitted to the hospital, including 69 in the medical ICU and 46 in a general medicine ward, a tachyarrhythmia, primarily NOAF, that was not present upon admission was identified in 19 patients (16.5%). All these cases were among ICU patients (27.5% of ICU patients), while no such arrhythmias were reported among patients admitted to general wards [7][13]. A multicenter cohort study covering critically ill adult patients admitted to ICUs in five hospitals in Saudi Arabia found that 10.7% developed NOAF during their ICU stay [8][29].
In another retrospective study encompassing ICU-admitted COVID-19 patients, the incidence of NOAF was 14.9%, with the median age of the NOAF group being 79.0 years. The risk of NOAF correlated with older age and the presence of comorbidities [9][30]. Similarly, among COVID-19 patients admitted to two hospitals in New York City, atrial arrhythmias (without specifying the type) were reported in 17.7% of the patients who received mechanical ventilation, in contrast to 1.9% of patients without mechanical ventilation [10][14].
In their meta-analysis of 31 studies exploring NOAF in hospitalized COVID-19 patients, Romiti et al. found that NOAF was present in 8% of cases, suggesting that the actual prevalence of atrial fibrillation could be as high as 27%, considering the substantial heterogeneity among the studies. AF in COVID-19 patients was more likely to occur in older individuals with conditions such as hypertension, diabetes, concurrent coronary artery disease, congestive heart failure, and critical clinical status [11][31]. It may reflect a direct viral invasion or a result of RV dysfunction, yet NOAF is frequently encountered in nonCOVID-19 ARDS ICU patients.
NOAF is a common complication of critical illness, emerging as the predominant cardiac arrhythmia in non-COVID-19 ICUs [12][13][39,40], and significantly impacting management and prognosis [14][15][41,42].
In an early study, ICU physicians observed NOAF in almost one out of three critically ill patients [16][43]. This percentage remains consistent in subsequent studies as well. The incidence of NOAF in mixed ICUs, encompassing both surgical and medical cases, varies widely in the literature, ranging between 19% and 35% [12][13][14][15][17][18][19][20][17,18,19,20,39,40,41,42].
2. Pathophysiological Mechanisms Assumed to Be Involved in the Appearance of NOAF
In the available literature, the specific types and underlying mechanisms of reported arrhythmias related to critically ill patients with COVID-19 have not yet been thoroughly elucidated.
There are numerous proposed pathogenetic mechanisms, including direct viral myocardial injury (myocarditis), imbalances in myocardial oxygen supply and demand, hypoxemia due to acute respiratory distress, an increased inflammatory response, elevated right ventricular afterload, diffuse endothelialitis, procoagulant activity, acid-base imbalances, and electrolyte abnormalities (Figure 1) [21][22][23][4,47,48].
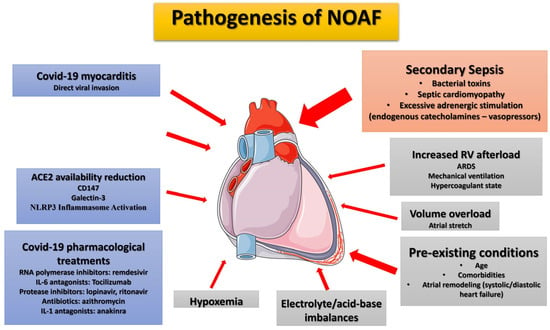
Figure 1.
Proposed pathophysiological mechanisms and risk factors for new-onset atrial fibrillation in ICU COVID-19 ARDS patients. During SARS-CoV-2 infection, chronic factors (age, pre-existing comorbidities), COVID-19-specific factors (inflammation, cytokine storm, direct viral invasion, treatments received)) and ICU risk factors (secondary sepsis, associated with adrenergic-endogenous/exogenous-overstimulation, right ventricular overload: ARDS/mechanical ventilation, hypoxemia, electrolyte imbalances, and acid-base disturbances) may contribute to NOAF emergence. The most significant factor in ICU patients, in the authors’ opinion, is secondary sepsis (orange box) which typically occurs relatively late in the course since SARS-CoV-2 infection. The arrow width indicates the degree of contribution of each factor in NOAF emergence. ACE2, angiotensin converting enzyme 2; ARDS, acute respiratory distress syndrome; COVID-19, Corona Virus Disease 2019; IL-1, interleucin-1; IL-6, interleukin-6; NLRP3, NOD-like receptor pyrin domain-containing 3; NOAF, new-onset atrial fibrillation; RNA, rivonucleic acid; RV, right ventricle.
Myocarditis could reasonably serve as a primary explanation for new-onset arrhythmias in COVID-19 patients who undergo cardiac injury, leading to the development of arrhythmias. A study revealed that the prevalence of heart failure was 23% among patients with COVID-19 [24][49]. However, it remains unclear whether the new cardiomyopathy (potentially due to myocarditis) or the exacerbation of underlying myocardial dysfunction could account for the high prevalence of heart failure reported in a few studies within this population [25][26][50,51].
Undoubtedly, the COVID-19 virus invades the myocardium. An initial post-mortem pathological study discovered myocardial tissue positive for SARS-CoV-2 through reverse transcription polymerase chain reaction (RTPCR) and electron microscopy in one out of four patients examined [27][52]. Nevertheless, cardiac involvement is primarily based on mildly increased troponin levels in most studies [5][28][29][30][5,6,7,8]. More detailed echocardiographic examinations are relatively scarce [31][32][53,54], with conflicting results concerning the correlation between increased troponin levels and cardiac function impairment [33][34][55,56].
Even fewer studies present echocardiographic data in critically ill COVID-19 patients, especially without differentiating between mechanically ventilated (MV) and non-MV patients, which represent the more severe COVID-19 cases. Furthermore, when these data are presented, they often lack information regarding the presence of arrhythmias, including NOAF, and their association with heart function. However, it appears that both the right and left ventricles are significantly impaired in COVID-19 patients.
The ECHO-COVID study, a multicenter research effort across European ICUs employing conventional echocardiography, reported that among the 69% of MV patients, 34.5% exhibited right ventricular (RV) dysfunction, and 22% displayed left ventricular (LV) systolic dysfunction, based on rough visual estimation. However, 30% of these patients had a previous history of cardiomyopathy and were not excluded from the study [35][57].
Notably, speckle-tracking echocardiography seems to be a more reliable method for assessing myocardial damage. This approach showed severe impairment in most COVID-19 patients, even when the ejection fraction (EF) seemed normal. For instance, Janus et al. discovered a severely depressed global longitudinal strain of the left ventricle (LV-LS) at −11.8% in 31 COVID-19 patients, which is severely depressed although concerned COVID -19 patients hospitalized in the general ward, obviously without severe ARDS [36][58]. Similarly, Van den Heuvel et al. assessed LV function using strain imaging in 51 patients, 17 of whom required MV. Among them, 14 patients (27%) displayed impaired LV function, with 11 having a low LV-LS and a normal EF [33][55]. In a multicenter study by Karagodin et al., in a large multicenter study around the world, the mean LV-LS was reported at −17.9% in ICU patients. However, only a subset of these patients required mechanical ventilation (15% in the whole cohort) [37][59].
During the pandemic, the right ventricle has been widely recognized as one of the most frequently affected cardiovascular areas in COVID-19 [23][38][39][48,60,61]. This, as already reported in the ECHO-COVID study, employing only conventional echocardiography, has been noted [35][57]. The reported prevalence of right ventricular dysfunction ranges from 6% to almost 50%, the wide variability being explained by differences in the severity of the included population and the definitions used for ventricular dysfunction [40][41][62,63]. Current COVID-19 data using comprehensive echocardiography, especially in the most severe subset of patients, indicate a significant impairment of the right ventricular strain in more severe patients. Specifically, the RV longitudinal strain (RV-LS) has been found to be lower in patients with COVID-19-related ARDS compared to those without ARDS (−21.3% vs. −24.6%), in patients in the ICU compared to those not in the ICU (−17.5% vs. −19.8%), and in non-survivors compared to survivors (−19% vs. −14%) [42][43][44][64,65,66]. However, the mechanical ventilator settings and respiratory system mechanics were not reported, which prevents conclusive remarks on the effects of mechanical ventilation on the observed outcomes. Moreover, an association between right ventricular dysfunction and increased mortality has also been proposed [41][45][63,67].
The involvement of the right ventricle in COVID-19 ARDS is likely multifactorial, possibly more than the left ventricle. This is due to factors such as the disease itself, increased afterload resulting from lung impairment, pulmonary vascular microthrombosis, and the potential impact of mechanical ventilator settings. It is widely acknowledged that mechanical ventilation can notably affect both RV myocardial performance and RV afterload. When airway pressure is not restricted, it often leads to pulmonary arterial hypertension [46][68]. Notably, Schmitt et al. have pointed out that positive end-expiratory pressure (PEEP) obstructs RV outflow and influences RV systolic function, possibly by escalating pulmonary vascular resistance [47][69]. This phenomenon is likely exacerbated in COVID-19 ARDS, where there is an increased occurrence of dead space formation and relatively preserved compliance of the respiratory system [48][49][70,71]. Recent findings indicate that in COVID-19 ARDS patients with mostly preserved respiratory system mechanics, PEEP impairs RV function and has an amplified effect on RV afterload by inducing non-zone three conditions [50][72].
Pericardial effusion serves as a direct indicator of cardiac involvement in COVID-19 patients. It might also reflect the severity of infection, as it has been more frequently reported in ICU patients compared to non-ICU patients (23.2% vs. 16.3%) [37][59].
Considering the aforementioned factors and the significant impact on cardiac function during the COVID-19 era, especially in critically ill patients, one would expect an increased incidence of NOAF in such cases. Conversely, patients in the ICU who experience NOAF may demonstrate more compromised cardiac function, which could be evident through troponin levels, echocardiography, and other examinations.
Indeed, NOAF is increased in critically ill patients, especially those on mechanical ventilation, when compared to less severe patients, as previously mentioned [2][7][11,13]. However, there is no strong association between heart impairment and NOAF.
Therefore, Kanthasamy et al. [8][29] found that over 90% of severe COVID-19 patients in their study had elevated troponin levels above the normal range. However, they did not observe a significant association with NOAF or left ventricular systolic function (Table 1).
The meta-analysis of Romiti et al. [11][31], which included a large number of COVID-19 patients with an increased incidence of NOAF, did not find any difference in new heart function impairment.
Most studies reporting NOAF in critically ill patients fail to address potential secondary conditions and the timing of NOAF onset during the course of COVID-19. Although increased inflammatory markers and the need for vasopressors were reported during AF episodes, they do not specify whether the appearance of AF coincided with a secondary infection episode [2][7][21][4,11,13]. Similarly, studies confirming the heightened incidence of NOAF in ICU COVID-19 patients do not specify whether the virus or other factors often present in critically ill patients are associated with its occurrence [2][4][7][10][51][52][53][54][9,10,11,12,13,14,15,16]. Ergun et al., who included only critically ill patients with severe illness, also found that NOAF was predominantly detected in patients who developed secondary bacterial infections during ICU follow-ups [9][30].
Furthermore, there have been consistent reports of troponin elevation in bacterial sepsis, indicating altered cardiomyocyte permeability or some degree of necrosis, either with or without evident cardiac dysfunction [55][56][74,75]. Additionally, sepsis-induced myocardial dysfunction is highly prevalent among ICU patients, attributed to heightened circulating levels of catecholamines and cytokines, commonly found in cases of severe sepsis and septic shock [57][58][76,77], thereby justifying an increased occurrence of arrhythmias. Nonetheless, reduced systemic vascular resistance might obscure the altered myocardial performance. Sepsis-induced vasoplegia could account for a seemingly preserved LV Ejection Fraction in such instances [59][60][21,78]. In researchers' study, markers of inflammation, the requirement for vasopressors, and lactate levels exhibited a gradual increase leading up to the onset of NOAF, while hypoxemia notably improved in NOAF patients on the day AF manifested. However, apparent dysfunction of the RV or LV was not evident (though sepsis cardiomyopathy cannot be ruled out since both LV and RV myocardial strain were significantly impaired). Moreover, troponin levels exhibited a significant increase on the day of AF compared to admission, further supporting the connection between NOAF and secondary sepsis [59][21].
Consistent with the above, patients who underwent invasive mechanical ventilation were more prone to requiring vasopressor support (95.4% vs. 1.5%) and experiencing other complications, including arrhythmias [10][14], while cardiac arrests and arrhythmias are likely the consequence of systemic illness and not solely the direct effects of COVID-19 infection [2][11].
In the general ICU, NOAF occurs more frequently among septic patients receiving vasopressor agents, those with electrolyte imbalances, and those with more severe disease states [17][61][62][63][17,79,80,81]. Hypokalemia and alterations in autonomic activity balance due to vasopressors can modify ion channel behavior and cellular automaticity, increasing susceptibility to AF [64][65][82,83]. Particularly, epinephrine has chronotropic effects that can lead to increased atrial ectopic discharges, thus triggering more NOAF episodes [64][82]. Greater illness severity is also linked to a higher risk of NOAF development, likely due to increased release of catecholamines and progressively severe autonomic dysfunction [15][62][66][42,80,84]. However, phenomena such as the cytokine storm and oxidative stress, possibly contributing to atrial remodeling, are among the potential triggers for AF onset, particularly in individuals with a predisposition [13][40]. Elevated serum levels of inflammatory mediators, including tumor necrosis factor-alpha (TNF-α) and interleukins (IL)-1β, IL-6, IL-8, and IL-10, have been observed in non-COVID-19 patients with NOAF, showing a correlation with AF duration and severity [67][85]. Consequently, treatments aimed at reducing inflammatory responses and oxidative stress have shown promising results in mitigating atrial structural and electrical remodeling [68][69][86,87].
All these phenomena are also commonly reported, and indeed, more exaggerated, in COVID-19 patients [70][88]. COVID-19 has been characterized from the outset as an inflammatory condition [71][89], and targeting the dysregulated immune response has been proposed as a promising therapeutic strategy [72][73][74][75][76][90,91,92,93,94]. A link between AF and COVID-19 might be explained by the heightened burden of systemic inflammation, at least in the initial disease stage. Similarly, like in non-COVID-19 patients, higher levels of C-reactive protein and interleukin-6 were observed in COVID-19 patients with NOAF compared to those without AF [77][95]. Moreover, COVID-19 patients exhibited elevated levels of proinflammatory cytokines, such as interleukin (IL)-1, IL-6, interferon gamma (IFN-g), IFN-inducible protein-10 (IP-10), and monocyte chemoattractant protein-1 (MCP-1), possibly driving the activated T-helper-1 cell response [78][96]. Additionally, it was reported that patients requiring ICU admission had higher concentrations of granulocyte colony-stimulating factor (GCSF), IP10, MCP-1, macrophage inflammatory protein-1A (MIP-1A), and tumor necrosis factor-alpha (TNF-a) compared to non-ICU patients [79][97]. Inflammation may also trigger arrhythmias through determining a more severe clinical course of COVID-19. The inflammatory burden of a cytokine storm during COVID-19 was associated with critical illness, greater disease severity, and outcome [78][79][80][96,97,98].
It is worth noting that recent reports have observed an increased incidence of secondary infections in COVID-19 ARDS patients on mechanical ventilation, particularly after the seventh day in the ICU [81][82][99,100]. This may be partially attributed to the use of corticosteroids, Tocilizumab, and Anakinra in COVID-19 ARDS treatment [81][82][99,100]. Accordingly, sepsis appears to be a primary triggering factor for NOAF in non-COVID-19 ICU patients as well [12][13][17][19][20][17,19,20,39,40]. Therefore, the increased sepsis incidence in COVID-19 ICU patients may partly justify the increased NOAF incidence compared to non-COVID-19 ICU patients. Additionally, the increased burden of systemic inflammation may result in increased NOAF incidence in non-ICU COVID-19 patients, as increased levels of systemic inflammation are mainly encountered at the initial disease stages.
The aforementioned data support the notion that a degree of myocardial injury is present in severe COVID-19 patients admitted to the ICU. Taken collectively, these findings may be interpreted as outcomes of a complex interplay between systemic inflammation—mainly secondary sepsis when patients are already in the ICU, which typically occurs relatively late in the course of the initial COVID-19 disease—clinical status, and the onset of AF.
In an attempt to outline the pathophysiology of COVID-19-related NOAF, several other presumed mechanisms have been proposed at the cellular level, which, however, may play a role in the early presentation of NOAF, before the progression to severe ARDS, mechanical ventilation initiation, and admission to ICU (Figure 1) [69][87]. They include:
- (a)
-
Reduced availability of angiotensin-converting enzyme (ACE) 2: Atrial ACE2 catabolizes transforming growth factor-β1 (TGF-β1), the principal pro-fibrotic cytokine [83][101]. This may underlie atrial arrhythmogenesis and potentially increase susceptibility to atrial fibrillation (AF) in COVID-19 patients [84][102].
- (b)
-
CD147 as an adjunctive player: CD147 facilitates SARS-CoV-2 invasion into host cells, including cardiomyocytes, by interacting with the viral spike protein [85][86][103,104]. In cardiomyocytes, CD147 strongly induces IL-18, activating matrix metalloproteinases (MMPs), and elevated circulating IL-18 levels are positively correlated with AF development [87][105]. Moreover, higher plasma levels of MMP-9 found in AF patients suggest that MMP-9 can serve as a marker of atrial remodeling [88][106]. Increased circulating MMP-9 has also been observed in COVID-19 patients [89][107]. CD147 also stimulates oxidative stress in cardiomyocytes and promotes negative ionotropic effects [90][108].
- (c)
-
Role of Galectin-3: Galectin-3 plays a role in the progression of atrial fibrosis and is involved in extracellular matrix formation. Elevated galectin-3 levels correlate with advanced AF and worse outcomes [91][109]. Notably, galectin-3 levels are increased in the serum of COVID-19 patients and correlate with COVID-19 severity [92][93][110,111].
- (d)
-
NLRP3 Inflammasome Activation: SARS-CoV-2, by binding to ACE2, purinergic receptors, and components of the complement-mediated pathway, stimulates the formation of the NOD-like receptor pyrin domain-containing 3 (NLRP3) inflammasome [94][112]. A causal link exists between NLRP3 inflammasome activation in atrial cardiomyocytes and the development of AF [67][95][96][85,113,114].
- (e)
-
Systemic Immune Cell Over-Activation: SARS-CoV-2 infection is characterized by systemic immune cell over-activation, leading to an imbalance between T-helper-1 and Th2 cells, elevated levels of various cytokines, including IL-1β, IL-2, IL-6, IL-7, interferons, TNF-α, monocyte chemoattractant protein-1, and macrophage inflammatory protein-1A [97][98][99][115,116,117]. At the cardiac level, pro-inflammatory cytokines, particularly IL-6, stimulate vascular smooth muscle proliferation, endothelial cell and platelet activation, and lead to apoptosis or necrosis of myocardial cells, which could mediate intra-atrial repolarization [100][118].
- (f)
-
Sympathetic Nervous System Activation: In viral infections like COVID-19, activation of the sympathetic nervous system [101][119] leads to increased Ca2+ influx and cardiomyocyte overload. Ca2+ release, along with the subsequent generation of delayed afterdepolarizations (DADs) and action potentials, increases the probability of AF events [102][120].
Another potential pathophysiological mechanism involves electrophysiological changes induced by pharmacological agents commonly used in COVID-19 treatment, which carry a risk of severe and potentially fatal cardiac dysfunction, such as torsades de pointes, ventricular tachycardia, and fibrillation (Figure 1) [103][32]. While drugs like hydroxychloroquine, lopinavir/ritonavir, and azithromycin (known to prolong the QT interval) have been largely abandoned, remdesivir is an authorized antiviral drug for COVID-19 treatment that requires attention [104][33]. COVID-19 patients with oxygen saturation levels below 94% receiving intravenous remdesivir have experienced new-onset atrial fibrillation (NOAF), which is more prevalent in patients undergoing mechanical ventilation. However, the interpretation remains inconclusive due to a small sample size, short follow-up duration, and the absence of a control group [105][34]. Further evidence indicates that remdesivir may act as a blocker of the atrioventricular node and could be pro-arrhythmic, especially in patients with structural heart disease [106][35]. Therefore, patients on remdesivir therapy, especially those with pre-existing risks, must be closely monitored.
Lastly, AF is an evolving age-related disease globally, influenced significantly by co-morbidities or lifestyle conditions such as hypertension, diabetes mellitus, obesity, chronic kidney disease, and inflammatory diseases (Figure 1) [17][107][108][17,36,37]. These factors also play a significant role in initiating new-onset atrial fibrillation (NOAF) in both COVID-19 and non-COVID-19 ICU patients [11][31].
