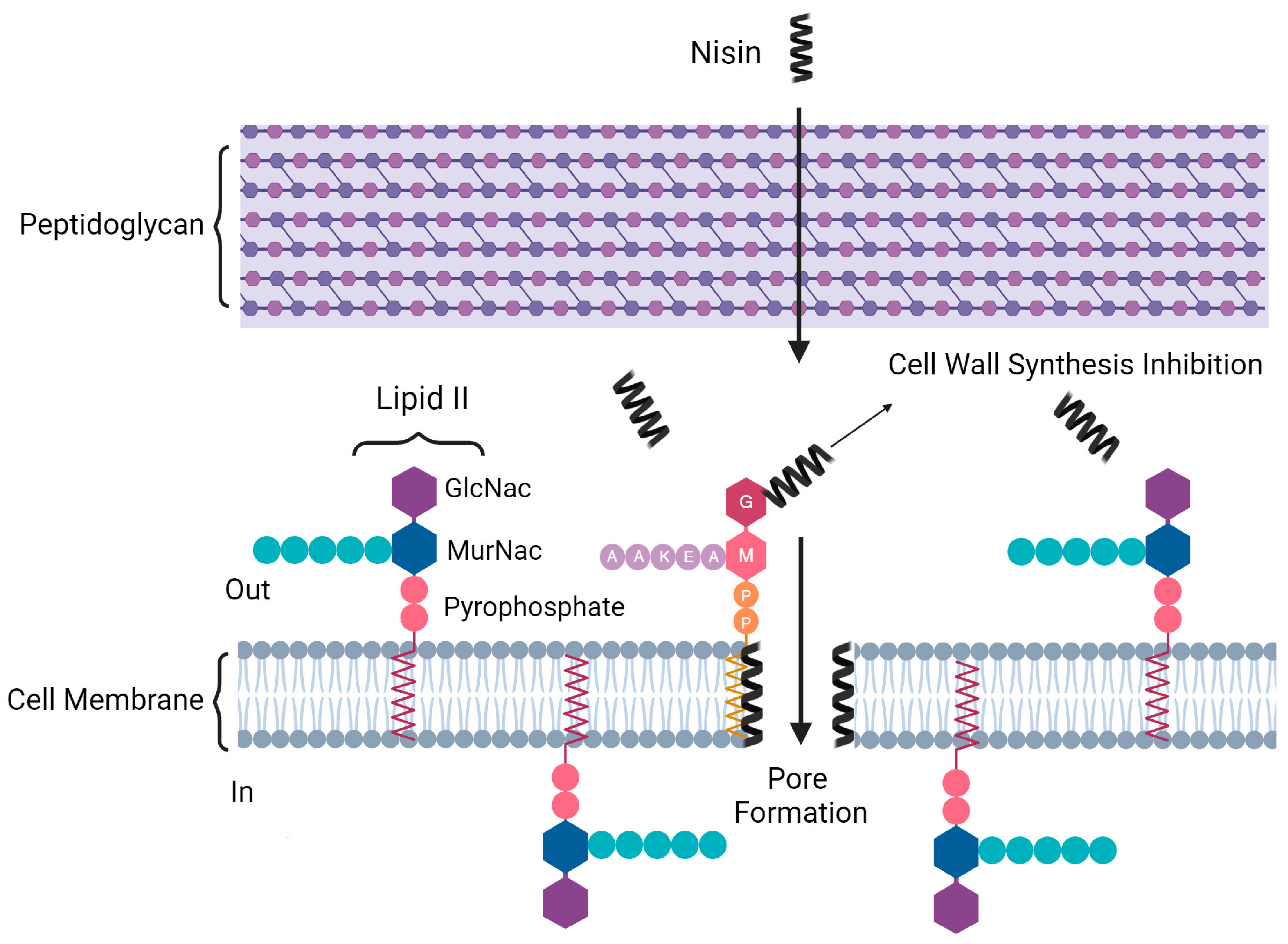
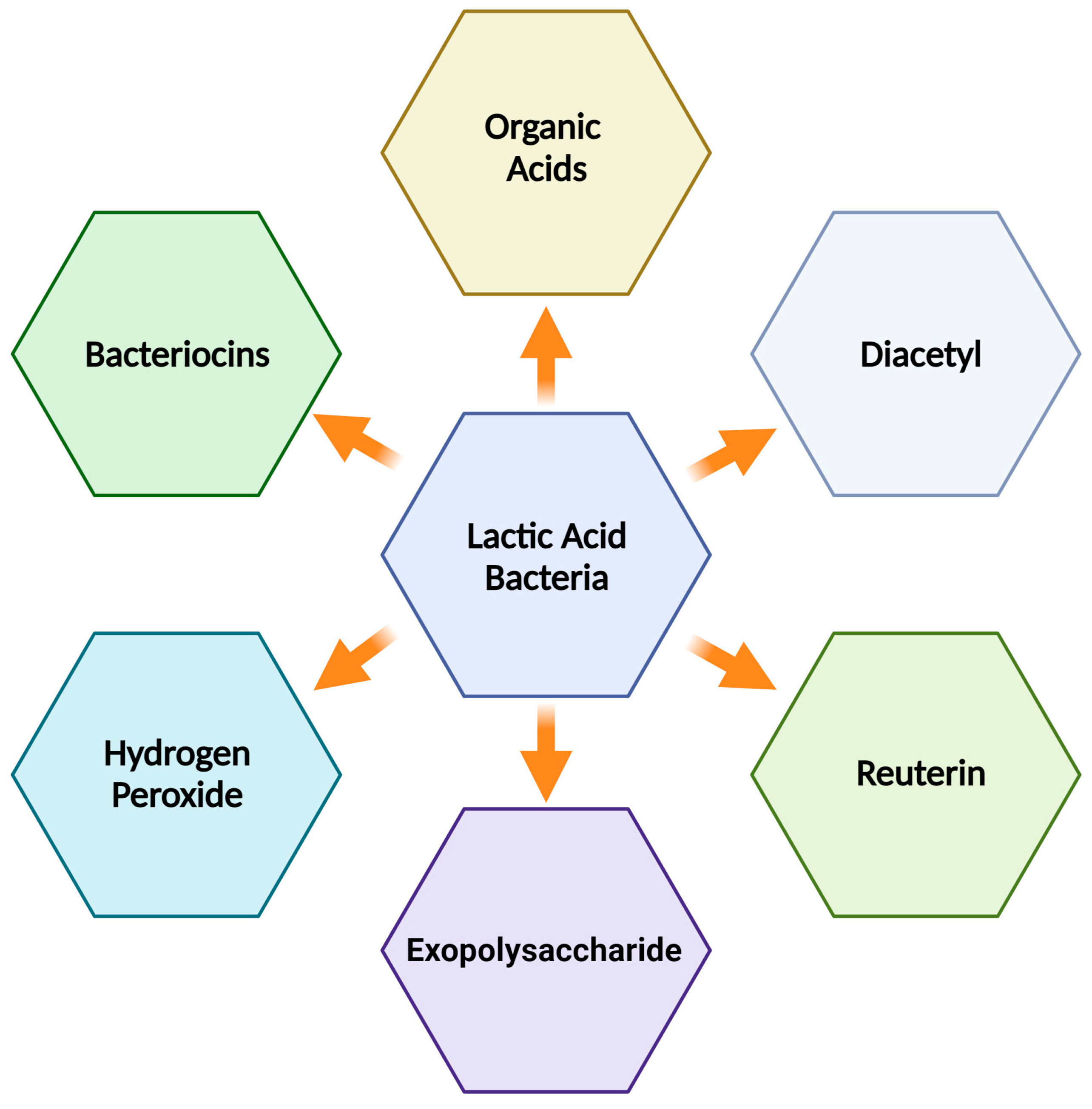
Lactic acid bacteria (LAB) are regarded as ‘Generally Recognized as Safe’ (GRAS) and are commonly used in the dairy industry and also form part of the microbiota of the human intestine. LAB play a significant role in biopreservation because they produce a variety of antimicrobial metabolites during the development and fermentation processes. The use of antimicrobial-producing LAB in the production of dairy products, which can be incorporated into fermented or nonfermented dairy products, implies a processing advantage to improve the safety and quality of dairy products, providing an additional barrier against foodborne diseases. Among the most common antimicrobials are bacteriocins, which are ribosomally produced antimicrobial peptides. They can kill or inhibit undesirable bacterial strains, whether closely related or not, without harming themselves. This ability is especially relevant in the food industry. Notably, many LAB bacteriocins, including those derived from such bacteria, have shown efficacy against Listeria monocytogenes, a significant concern in traditional cheeses made from raw milk.
- lactic acid bacteria
- dairy foods
- antimicrobial compounds
1. Bacteriocins in Dairy Foods
2. Organic Acids and Their Antimicrobial Properties
3. Antimicrobial Compounds Diversity in Dairy Foods
References
- Yang, S.-C.; Lin, C.H.; Sung, C.T.; Fang, J.Y. Antibacterial activities of bacteriocins: Application in foods and pharmaceuticals. Front. Microbiol. 2014, 5, 241.
- Mercado, V.; Olmos, J. Bacteriocin Production by Bacillus Species: Isolation, Characterization, and Application. Probiotics Antimicrob. Proteins 2022, 14, 1151–1169.
- Cotter, P.D.; Hill, C.; Ross, R.P. Bacteriocins: Developing innate immunity for food. Nat. Rev. Microbiol. 2005, 3, 777–788.
- Balciunas, E.M.; Martinez, F.A.C.; Todorov, S.D.; de Melo Franco, B.D.G.; Converti, A.; de Souza Oliveira, R.P. Novel biotechnological applications of bacteriocins: A review. Food Control 2012, 32, 134–142.
- Cintas, L.; Casaus, M.P.; Herranz, C.; Nes, I.F.; Hernández, P.E. Bacteriocins of lactic acid bacteria. Food Sci. Technol. Int. 2001, 7, 281–305.
- Sun, Z.; Wang, X.; Zhang, X.; Wu, H.; Zou, Y.; Li, P.; Sun, C.; Xu, W.; Liu, F.; Wang, D. Class III bacteriocin Helveticin-M causes sublethal damage on target cells through impairment of cell wall and membrane. J. Ind. Microbiol. Biotechnol. 2018, 45, 213–227.
- Thompson, J.; Collins, M.; Mercer, W. Characterization of a proteinaceous antimicrobial produced by Lactobacillus helveticus CNRZ450. J. Appl. Bacteriol. 1996, 80, 338–348.
- Nilsen, T.; Nes, I.F.; Holo, H. Enterolysin A, a cell wall-degrading bacteriocin from Enterococcus faecalis LMG 2333. Appl. Environ. Microbiol. 2003, 69, 2975–2984.
- Khorshidian, N.; Khanniri, E.; Mohammadi, M.; Mortazavian, A.M.; Yousefi, M. Antibacterial Activity of Pediocin and Pediocin-Producing Bacteria Against Listeria monocytogenes in Meat Products. Front. Microbiol. 2021, 12, 709959.
- O’Connor, P.M.; Ross, R.P.; Hill, C.; Cotter, P.D. Antimicrobial antagonists against food pathogens: A bacteriocin perspective. Curr. Opin. Food Sci. 2015, 2, 51–57.
- Yi, Y.; Li, P.; Zhao, F.; Zhang, T.; Shan, Y.; Wang, X.; Liu, B.; Chen, Y.; Zhao, X.; Lü, X. Current status and potentiality of class II bacteriocins from lactic acid bacteria: Structure, mode of action and applications in the food industry. Trends Food Sci. Technol. 2022, 120, 387–401.
- Garsa, A.K.; Kumariya, R.; Kumar, A.; Lather, P.; Kapila, S.; Sood, S. Industrial cheese whey utilization for enhanced production of purified pediocin PA-1. LWT 2014, 59, 656–665.
- Egan, K.; Field, D.; Rea, M.C.; Ross, R.P.; Hill, C.; Cotter, P.D. Bacteriocins: Novel solutions to age old spore-related problems? Front. Microbiol. 2016, 7, 461.
- Coelho, M.; Silva, C.; Ribeiro, S.; Dapkevicius, M.; Rosa, H. Control of Listeria monocytogenes in fresh cheese using protective lactic acid bacteria. Int. J. Food Microbiol. 2014, 191, 53–59.
- Ayivi, R.D.; Gyawali, R.; Krastanov, A.; Aljaloud, S.O.; Worku, M.; Tahergorabi, R.; Da Silva, R.; Ibrahim, S.A. Lactic Acid Bacteria: Food Safety and Human Health Applications. Dairy 2020, 1, 202–232.
- Ahmad, V.; Khan, M.S.; Jamal, Q.M.S.; Alzohairy, M.A.; Al Karaawi, M.A.; Siddiqui, M.U. Antimicrobial potential of bacteriocins: In therapy, agriculture and food preservation. Int. J. Antimicrob. Agents 2017, 49, 1–11.
- Fessard, A.; Remize, F. Why are Weissella spp. not used as commercial starter cultures for food fermentation? Fermentation 2017, 3, 38.
- Krishnamoorthi, R.; Srinivash, M.; Mahalingam, P.U.; Malaikozhundan, B.; Suganya, P.; Gurushankar, K. Antimicrobial, anti-biofilm, antioxidant and cytotoxic effects of bacteriocin by Lactococcus lactis strain CH3 isolated from fermented dairy products—An in vitro and in silico approach. Int. J. Biol. Macromol. 2022, 220, 291–306.
- Perez, R.H.; Zendo, T.; Sonomoto, K. Novel bacteriocins from lactic acid bacteria (LAB): Various structures and applications. Microb. Cell Factories 2014, 13, S3.
- Abo-amer, A.E. Characterization of a Bacteriocin-like Inhibitory Substance Produced by Lactobacillus plantarum Isolated from Egyptian Home-Made Yogurt. Sci. Asia 2007, 33, 313–319.
- Ribeiro, S.C.; Ross, R.P.; Stanton, C.; Silva, C.C.G.; Vandera, E.; Tsirka, G.; Kakouri, A.; Koukkou, A.-I.; Samelis, J.; Silva, S.P.M. Characterization and Application of Antilisterial Enterocins on Model Fresh Cheese. J. Food Prot. 2017, 80, 1303–1316.
- Mitra, S.; Mukhopadhyay, B.; Biswas, S. Potential application of the nisin Z preparation of Lactococcus lactis W8 in preservation of milk. Lett. Appl. Microbiol. 2011, 53, 98–105.
- Dal Bello, B.; Cocolin, L.; Zeppa, G.; Field, D.; Cotter, P.D.; Hill, C. Technological characterization of bacteriocin producing Lactococcus lactis strains employed to control Listeria monocytogenes in Cottage cheese. Int. J. Food Microbiol. 2012, 153, 58–65.
- Chen, W. Lactic Acid Bacteria: Bioengineering and Industrial Applications; Springer: Berlin/Heidelberg, Germany, 2019.
- Ibarra-Sánchez, L.A.; El-Haddad, N.; Mahmoud, D.; Miller, M.J.; Karam, L. Invited review: Advances in nisin use for preservation of dairy products. J. Dairy Sci. 2020, 103, 2041–2052.
- Cui, H.Y.; Wu, J.; Li, C.Z.; Lin, L. Anti-listeria effects of chitosan-coated nisin-silica liposome on Cheddar cheese. J. Dairy Sci. 2016, 99, 8598–8606.
- Balay, D.R.; Dangeti, R.V.; Kaur, K.; McMullen, L.M. Purification of leucocin A for use on wieners to inhibit Listeria monocytogenes in the presence of spoilage organisms. Int. J. Food Microbiol. 2017, 255, 25–31.
- Trinetta, V.; Floros, J.D.; Cutter, C.N. Sakacin a-containing pullulan film: An active packaging system to control epidemic clones of Listeria monocytogenes in ready-to-eat foods. J. Food Saf. 2010, 30, 366–381.
- Pinilla, C.M.B.; Brandelli, A. Antimicrobial activity of nanoliposomes co-encapsulating nisin and garlic extract against Gram-positive and Gram-negative bacteria in milk. Innov. Food Sci. Emerg. Technol. 2016, 36, 287–293.
- Meng, F.; Zhu, X.; Zhao, H.; Nie, T.; Lu, F.; Lu, Z.; Lu, Y. A class Ⅲ bacteriocin with broad-spectrum antibacterial activity from Lactobacillus acidophilus NX2-6 and its preservation in milk and cheese. Food Control 2020, 121, 107597.
- Soltani, S.; Zirah, S.; Rebuffat, S.; Couture, F.; Boutin, Y.; Biron, E.; Subirade, M.; Fliss, I. Gastrointestinal Stability and Cytotoxicity of Bacteriocins from Gram-Positive and Gram-Negative Bacteria: A Comparative In Vitro Study. Front. Microbiol. 2022, 12, 780355.
- Ageni, L.; Ajibade, G.A.; Yerima, B.; Appah, J. Shelf life extension study of ogi and fufu using bacteriocin isolated from Lactobacillus acidophilus of fermented dairy products. Afr. J. Microbiol. Res. 2017, 11, 1286–1293.
- Moigani, N.; Amirinia, C. Kinetics of Growth and Bacteriocin production in Lactobacillus casei RN 78 isolated from a dairy sample in Iran. Int. J. Dairy Sci. 2007, 5, 1–12.
- Castillo Martinez, F.A.; Balciunas, E.M.; Salgado, J.M.; Domínguez González, J.M.; Converti, A.; Oliveira, R.P.D.S. Lactic acid properties, applications and production: A review. Trends Food Sci. Technol. 2013, 30, 70–83.
- Gänzle, M.G. Lactic metabolism revisited: Metabolism of lactic acid bacteria in food fermentations and food spoilage. Curr. Opin. Food Sci. 2015, 2, 106–117.
- Leyva Salas, M.; Mounier, J.; Valence, F.; Coton, M.; Thierry, A.; Coton, E. Antifungal Microbial Agents for Food Biopreservation—A Review. Microorganisms 2017, 5, 37.
- Garnier, L.; Valence, F.; Mounier, J. Diversity and Control of Spoilage Fungi in Dairy Products: An Update. Microorganisms 2017, 5, 42.
- Delavenne, E.; Mounier, J.; Déniel, F.; Barbier, G.; Le Blay, G. Biodiversity of antifungal lactic acid bacteria isolated from raw milk samples from cow, ewe and goat over one-year period. Int. J. Food Microbiol. 2012, 155, 185–190.
- Fernandez, B.; Vimont, A.; Desfossés-Foucault, E.; Daga, M.; Arora, G.; Fliss, I. Antifungal activity of lactic and propionic acid bacteria and their potential as protective culture in cottage cheese. Food Control 2017, 78, 350–356.
- Xu, R.; Sa, R.; Jia, J.; Li, L.; Wang, X.; Liu, G. Screening of Antifungal Lactic Acid Bacteria as Bioprotective Cultures in Yogurt and a Whey Beverage. J. Food Prot. 2021, 84, 953–961.
- Axel, C.; Zannini, E.; Arendt, E.K. Mold spoilage of bread and its biopreservation: A review of current strategies for bread shelf life extension. Crit. Rev. Food Sci. Nutr. 2017, 57, 3528–3542.
- Dagnas, S.; Gauvry, E.; Onno, B.; Membré, J.-M. Quantifying Effect of Lactic, Acetic, and Propionic Acids on Growth of Molds Isolated from Spoilesd Bakery Products. J. Food Prot. 2015, 78, 1689–1698.
- Cabo, M.L.; Braber, A.F.; Koenraad, P.M.F.J. Apparent Antifungal Activity of Several Lactic Acid Bacteria against Penicillium discolor Is Due to Acetic Acid in the Medium. J. Food Prot. 2002, 65, 1309–1316.
- Garnier, L.; Penland, M.; Thierry, A.; Maillard, M.-B.; Jardin, J.; Coton, M.; Salas, M.L.; Coton, E.; Valence, F.; Mounier, J. Antifungal activity of fermented dairy ingredients: Identification of antifungal compounds. Int. J. Food Microbiol. 2020, 322, 108574.
- Garrote, G.L.; Abraham, A.G.; De Antoni, G.L.; Garrote, A.G.A.G.L.; Kim, D.-H.; Chon, J.-W.; Kang, I.-B.; Kim, H.; Kim, H.-S.; Song, K.-Y.; et al. Inhibitory Power of Kefir: The Role of Organic Acids. J. Food Prot. 2000, 63, 364–369.
- Lind, H.; Jonsson, H.; Schnürer, J. Antifungal effect of dairy propionibacteria—Contribution of organic acids. Int. J. Food Microbiol. 2005, 98, 157–165.
- Han, X.; Li, L.; Wei, C.; Zhang, J.; Bao, J. Facilitation of l-Lactic Acid Fermentation by Lignocellulose Biomass Rich in Vitamin B Compounds. J. Agric. Food Chem. 2019, 67, 7082–7086.
- Wang, C.; Sun, J.; Lassabliere, B.; Yu, B.; Zhao, F.; Zhao, F.; Chen, Y.; Liu, S.Q. Potential of lactic acid bacteria to modulate coffee volatiles and effect of glucose supplementation: Fermentation of green coffee beans and impact of coffee roasting. J. Sci. Food Agric. 2018, 99, 409–420.
- Milesi, M.M.; Vinderola, G.; Sabbag, N.; Meinardi, C.A.; Hynes, E. Influence on cheese proteolysis and sensory characteristics of non-starter lactobacilli strains with probiotic potential. Food Res. Int. 2009, 42, 1186–1196.
- Cubas-Cano, E.; González-Fernández, C.; Ballesteros, M.; Tomás-Pejó, E. Biotechnological advances in lactic acid production by lactic acid bacteria: Lignocellulose as novel substrate. Biofuels Bioprod. Biorefin. 2018, 12, 290–303.
- Hibbing, M.E.; Fuqua, C.; Parsek, M.R.; Peterson, S.B. Bacterial competition: Surviving and thriving in the microbial jungle. Nat. Rev. Microbiol. 2010, 8, 15–25.
- Hathout, A.S.; Aly, S.E. Role of lactic acid bacteria as a biopreservative agent of Talbina. J. Am. Sci. 2010, 6, 889–898.
- Sharma, H.; Ozogul, F.; Bartkiene, E.; Rocha, J.M. Impact of lactic acid bacteria and their metabolites on the techno-functional properties and health benefits of fermented dairy products. Crit. Rev. Food Sci. Nutr. 2021, 63, 4819–4841.
- Ayad, E.; Nashat, S.; El-Sadek, N.; Metwaly, H.; El-Soda, M. Selection of wild lactic acid bacteria isolated from traditional Egyptian dairy products according to production and technological criteria. Food Microbiol. 2004, 21, 715–725.
- Peng, K.; Koubaa, M.; Bals, O.; Vorobiev, E. Recent insights in the impact of emerging technologies on lactic acid bacteria: A review. Food Res. Int. 2020, 137, 109544.
- Bintsis, T. Lactic acid bacteria as starter cultures: An update in their metabolism and genetics. AIMS Microbiol. 2018, 4, 665–684.
- Langa, S.; Martín-Cabrejas, I.; Montiel, R.; Landete, J.; Medina, M.; Arqués, J. Combined antimicrobial activity of reuterin and diacetyl against foodborne pathogens. J. Dairy Sci. 2014, 97, 6116–6121.
- Axelsson, L.; Chung, T.C.; Dobrogosz, W.J.; Lindgren, S.E. Production of a broad spectrum antimicrobial substance by Lactobacillus reuteri. Microb. Ecol. Health Dis. 1989, 2, 131–136.
- Xu, Y.; Wang, Y.; Ding, X.; Wang, J.; Zhan, X. Inhibitory effects of reuterin on biofilm formation, quorum sensing and virulence genes of Clostridium perfringens. LWT 2022, 162, 113421.
- Ling, L.; Pang, M.; Luo, H.; Cheng, W.; Jiang, K.; Wang, Y. Antifungal activity of diacetyl, a volatile organic compound, on Trichoderma lixii F2 isolated from postharvest Lanzhou lily bulbs. Food Biosci. 2023, 52, 102365.
- Sun, M.-C.; Hu, Z.-Y.; Li, D.-D.; Chen, Y.-X.; Xi, J.-H.; Zhao, C.-H. Application of the Reuterin System as Food Preservative or Health-Promoting Agent: A Critical Review. Foods 2022, 11, 4000.
- Ortiz-Rivera, Y.; Sánchez-Vega, R.; Gutiérrez-Méndez, N.; León-Félix, J.; Acosta-Muñiz, C.; Sepulveda, D. Production of reuterin in a fermented milk product by Lactobacillus reuteri: Inhibition of pathogens, spoilage microorganisms, and lactic acid bacteria. J. Dairy Sci. 2017, 100, 4258–4268.
- Assari, F.; Mojgani, N.; Sanjabi, M.R.; Mirdamadi, S.; Jahandar, H. Technological Assessment of Autochthonous Lactic Acid Bacteria and Their Antibacterial Activities against Food borne Pathogens in Goat Milk Lactic Cheese. Appl. Food Biotechnol. 2023, 10, 61–71.
- Tulini, F.L.; Bíscola, V.; Choiset, Y.; Hymery, N.; Le Blay, G.; De Martinis, E.C.P.; Chobert, J.-M.; Haertlé, T. Evaluation of the proteolytic activity of Enterococcus faecalis FT132 and Lactobacillus paracasei FT700, isolated from dairy products in Brazil, using milk proteins as substrates. Eur. Food Res. Technol. 2015, 241, 385–392.
- Al-Baarri, A.N. Enhanced antibacterial activity of lactoperoxidase-thiocyanate-hydrogen peroxide system in reduced-lactose milk whey. Int. J. Food Sci. 2019, 2019, 8013402.
- Yousefi, M.; Nematollahi, A.; Shadnoush, M.; Mortazavian, A.M.; Khorshidian, N. Antimicrobial Activity of Films and Coatings Containing Lactoperoxidase System: A Review. Front. Nutr. 2022, 9, 828065.
- Jurášková, D.; Ribeiro, S.C.; Silva, C.C.G. Exopolysaccharides Produced by Lactic Acid Bacteria: From Biosynthesis to Health-Promoting Properties. Foods 2022, 11, 156.
- Costa, O.Y.A.; Raaijmakers, J.M.; Kuramae, E.E. Microbial Extracellular Polymeric Substances: Ecological Function and Impact on Soil Aggregation. Front. Microbiol. 2018, 9, 1636.
- Angelin, J.; Kavitha, M. Exopolysaccharides from probiotic bacteria and their health potential. Int. J. Biol. Macromol. 2020, 162, 853–865.
- Johansen, P.; Jespersen, L. Impact of quorum sensing on the quality of fermented foods. Curr. Opin. Food Sci. 2017, 13, 16–25.
- Lima, E.M.F.; Quecán, B.X.; da Cunha, L.R.; de Melo Franco, B.D.; Pinto, U.M. Cell-Cell Communication in Lactic Acid Bacteria: Potential Mechanisms, in Lactic Acid Bacteria; CRC Press: Boca Raton, FL, USA, 2020; pp. 1–14.
- Declerck, N.; Bouillaut, L.; Chaix, D.; Rugani, N.; Slamti, L.; Hoh, F.; Lereclus, D.; Arold, S.T. Structure of PlcR: Insights into virulence regulation and evolution of quorum sensing in Gram-positive bacteria. Proc. Natl. Acad. Sci. USA 2007, 104, 18490–18495.
- LaSarre, B.; Federle, M.J. Exploiting Quorum Sensing to Confuse Bacterial Pathogens. Microbiol. Mol. Biol. Rev. 2013, 77, 73–111.
- Kareb, O.; Aïder, M. Quorum Sensing Circuits in the Communicating Mechanisms of Bacteria and Its Implication in the Biosynthesis of Bacteriocins by Lactic Acid Bacteria: A Review. Probiotics Antimicrob. Proteins 2019, 12, 5–17.
- Park, H.; Shin, H.; Lee, K.; Holzapfel, W. Autoinducer-2 properties of kimchi are associated with lactic acid bacteria involved in its fermentation. Int. J. Food Microbiol. 2016, 225, 38–42.
- Quintieri, L.; Caputo, L.; Brasca, M.; Fanelli, F. Recent Advances in the Mechanisms and Regulation of QS in Dairy Spoilage by Pseudomonas spp. Foods 2021, 10, 3088.
- Yi, L.; Dong, X.; Grenier, D.; Wang, K.; Wang, Y. Research progress of bacterial quorum sensing receptors: Classification, structure, function and characteristics. Sci. Total. Environ. 2020, 763, 143031.
- Abisado, R.G.; Benomar, S.; Klaus, J.R.; Dandekar, A.A.; Chandler, J.R. Bacterial quorum sensing and microbial community interactions. mBio 2018, 9, e02331-17.