Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Eduardo Tomanik and Version 3 by Catherine Yang.
Graphene and graphene-based materials are relatively novel 2D materials with great tribological potential. Graphene is inherently low-friction, very high stiffness, and its thermal conductivity may reduce friction and wear.
- friction
- wear
- lubricants
- greases
- mechanical efficiency
1. Graphene-Based Materials
Graphene is part of a broad family of carbon nano allotropes and graphene itself has a family, consisting primarily of sp2 carbon atoms arranged in a hexagonal network [1][2][3][39,40,41], as represented in Figure 11. The National Physical Laboratory (NPL), in collaboration with international partners, through the ISO/TS 21356-1:2021 [4][1], has been working in terminology and definitions that provide a common and consistent understanding of the different types of graphene worldwide, Table 12. Nevertheless, despite their efforts, there is still no unambiguous nomenclature on different publications and, therefore, in this paper, the original nomenclature used in the reference will be cited, with some comments when necessary.
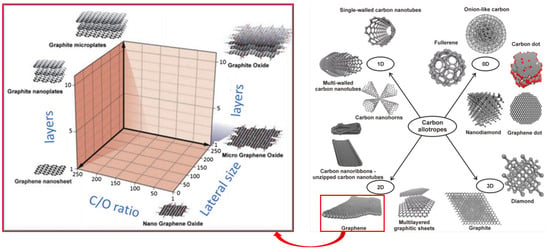
Table 12.
Nano-carbo and graphene-based materials.
| Terms * | Definitions * |
|---|---|
| Graphene Graphene layer Single-layer graphene Monolayer graphene |
A single layer of carbon atoms with each atom bound to three neighbors in a honeycomb structure. Can be abbreviated as 1LG to distinguish from bilayer graphene (2LG) and few-layer graphene (FLG). It has edges and can have defects and grain boundaries where the bonding is disrupted. |
| Bilayer graphene 2LG |
Two-dimensional material consisting of two well-defined stacked graphene layers. |
| Few-layer graphene FLG |
Two-dimensional material consisting of three to ten well-defined stacked graphene layers. |
| Graphene nanoplate Graphene nanoplatelet GNP |
Nanoplate consisting of graphene layers. Typically have thicknesses of between 1 nm and 3 nm and lateral dimensions ranging from approximately 100 nm to 100 µm. |
| Graphene oxide GO |
Chemically modified graphene prepared by oxidation and exfoliation of graphite, causing extensive oxidative modification of the basal plane. Graphene oxide is a single-layer material with a high oxygen content, typically characterized by C/O atomic ratios of approximately 2, depending on the method of synthesis. The functional groups found include hydroxyl (OH), carboxyl (COOH), and epoxide (COC). ** |
| Reduced graphene oxide rGO |
Reduced oxygen content for graphene oxide. If graphene oxide was fully reduced, then graphene would be the product. However, in practice, some oxygen-containing functional groups remain and not all sp3 bonds will return back to the sp2 configuration. Different reducing agents will lead to different carbon-to-oxygen ratios and different chemical compositions in reduced graphene oxide. It can take the form of several morphological variations such as platelets and worm-like structures. |
| Carbon NanoTubes (CNTs) | Carbon with a diameter of nanometers and a length of micrometers (where the length-to-diameter ratio exceeds 1000) |
Additionally, other carbon variants are being investigated as lubricant additives. Wolk 2018 [5][42] used graphene quantum dots (GQD), covalently functionalized with dodecyl amine and obtained a reduction in friction coefficient from 0.17 to 0.11 on the macro scale in addition to significantly inhibiting corrosion. Graphene types can be transformed before or during usage. See Figure 12 from Georgakilas, 2015 [2][40]. As mentioned, one should be careful when reading that a kind of graphene was used in the experiment since the original graphene type could suffer exfoliation, degradation, and transformations during the tribological process. It can be, for instance, wrapped up to form 0D fullerenes, rolled up to establish the cylindrical structure of 1D carbon nanotubes, or stacked to form 3D graphite.
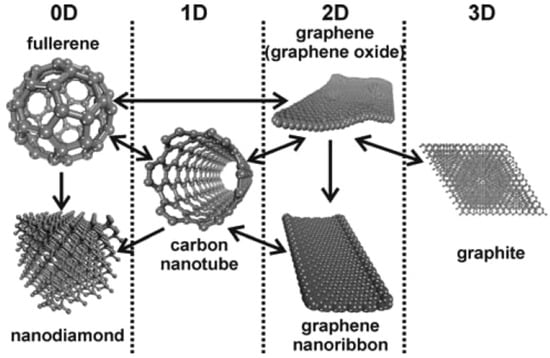
Figure 12. Scheme showing interconversions among various carbon nanoallotropes. From Georgakilas [2]. Reproduced with permission.
Scheme showing interconversions among various carbon nanoallotropes. From Georgakilas [40]. Reproduced with permission.
Any amount of graphene-based material for industrial applications will contain particles with variation in terms of size, number of layers, etc. Even if the sample contains mostly graphene particles with 1 to 6 layers, the relative share of such small particles in area and volume will be much lower than the number of particles. Figure 13, reproduced from [6][43], shows the relative share of the number of particles, area, and volume for two samples having mostly particles with few layers. Despite containing mostly particles of few layers, the volume share on both samples is dominated by particles of six or more layers. The reader should be aware that when along this resviearchw a given reference is said to use a given type of graphene, the reported effect may be caused by another graphene type contained in the study sample. The contribution of the different sub-populations on tribological applications still needs to be studied and probably is different for different properties, e.g., friction and wear being influenced more by the nanoparticles area and volume while electrical and thermal conductivity by the number of such nanoparticles.
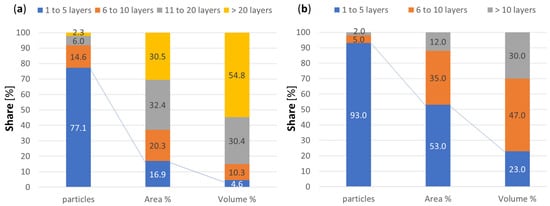
Figure 13.
Number of layers, area, volume. (
a
) Graphene of few layers, data from the MGgrafeno. (
2. Functionalization of Graphene-Based Materials for Tribological Applications
Graphene-based materials, as already mentioned in this paper, are very promising for tribological applications due to their self-lubricating lamellar structure and film-forming abilities, providing excellent anti-wear and friction-reducing performances. Nevertheless, despite those features, there are challenges to be overcome, such as the great propensity to aggregation, before they fully achieve the advertised properties [7][6], Penkov 2014 [8][44], Chouhan 2020 [9][45] and as e.g., to make it hydrophilic, Kinoshita 2014 [10][46]. A very studied way to solve that problem is by modifying the graphene surface through functionalization with different functional groups (oxygen-based, nitrogen-based, sulfur-based, halogen-based, etc.); ions; molecules; or particles (Hu 2018 [11][47], Wang 2021 [12][48], Guo 2021 [13][49], Rabchinskii 2020 [14][50]. As summarized in Figure 14, the physicochemical properties of graphene can be tailored via a covalent or non-covalent approach. Non-covalent functionalization preserves the conjugated π system of graphene, maintaining a low degree of defects in the sheets and they are usually easier to process than covalent functionalization; however, the bond between the coupling agent and graphene is relatively weaker, which can affect the stability of the obtained material. Covalent functionalization on the other hand, although it promotes changes in the conjugated π system, by the covalent bonds formed in the basal plane and edges, allows edge-selective reactions (Shi Q, 2020 [15][51]). That is relevant because the obtained material will add the beneficial properties of the coupling agent, but it may preserve most of the intrinsic properties of graphene [16][26]. Regardless of the approach, it is essential that the interactions/bonds formed be (1) properly selected, to render, for instance, dispersibility in polar and/or non-polar fluids; (2) stable; (3) selective; and (4) in enough extension to achieve the desired property.
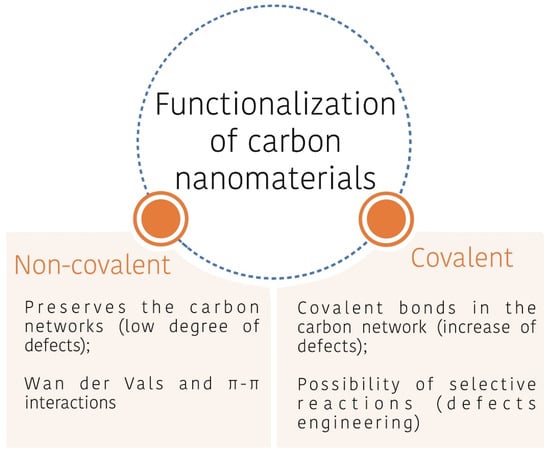
Figure 14.
Main routes for the functionalization of nanomaterials.
The most common methods for graphene functionalization described in the literature are summarized in Figure 15. The chemical reactions make use of well-established synthesis routes from organic chemistry (radical reactions, cycloadditions, nucleophilic additions, and substitutions) to manipulate the graphene’s properties. Electrochemical reactions enable the functionalization and exfoliation to take place simultaneously, in addition to not using aggressive reagents, and are easily scalable. Regarding irradiation, among the available routes, microwave and ultraviolet-assisted reactions deserve mention.
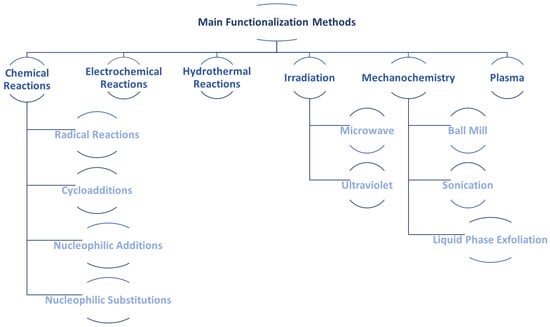
Figure 15.
Main routes of covalent routes for functionalization of nanomaterials.
3. Graphene Functionalization for Use in Lubricants
Additives with enhanced dispersibility and stability enable formulators to increase their concentration in the lubricant without inducing aggregation and sedimentation, which is important for shelf-life and, therefore, for real-life applications ([7][17][6,55]). As a derivative, GO inherits many physical and chemical properties from graphene, making it an attractive base material for the synthesis of lubricant additives with enhanced dispersibility. The abundant oxygen-containing functional groups provide uniform water dispersion to its nanosheets. However, it is challenging to produce straight GO-based oil dispersions (Gao, 2022 [18][9]). That is why approaches such as the GO modification with a long organic chain have been studied, i.e., to promote homogeneous and long-term dispersion of graphene in oil-based lubricants. Nevertheless, the commonly used graphene modifiers, such as oleic and stearic acid, tend to decompose given the heating generated during the friction process. That lead Bao et al. [19][56] to use a reflux reaction to covalently modify the GO with polyisobutylene succinimide (PIBS) and evaluate the tribology properties of the resultant material. The GO-T154/oil dispersion showed stability over one year and provided a wear rate and coefficient of friction of less than 60% and 54%, respectively, compared to those of base oil. Moreover, GO-T154 exhibited higher heat stability. Such results were attributed to a synergistic interaction between the precursors, which led to the formation of uniform and continuous carbon film on the contacting surface. See Figure 16.

Nyholm and Espallargas [7][6] in their review discussed the steric stabilization and electrostatic stabilization mechanisms, see Figure 17, by which the surface functionalization can enhance dispersion stability and inhibit agglomeration of carbon nanostructures with potential application as lubricant additives. Steric stabilization prevents agglomeration mainly via the steric effect, a result of the repulsive forces induced by long-chain functional groups, typically polymers, attached to the surface of the nanostructure. Those groups can form a brush-like layer that shields the particles from attractive interactions. This concept was studied by Yu 2023 [20][57] from DFT calculations performed with different Organic Friction Modifiers (OFM), and amphiphilic surfactant molecules, combined with graphene (Spikes, 2015 [21][58], Ouyang, 2021 [22][59], Cyriac, 2021 [23][60]). The authors observed that the materials that presented lower friction coefficients in the tribological tests also presented high adsorption energy, meaning stronger adsorption on the substrate surface. Under load and shear force, the hydrocarbon tail from OFM-moiety can easily slip between opposite polar groups, resulting in a decrease in friction coefficient. Additionally, during the friction process, the adsorbed layer will inevitably be destroyed, but the molecules with higher adsorption energy can recompose themselves more quickly to recover the brush structure to keep the friction coefficient low throughout the whole friction process. Thus, from the experimental data, the authors inferred that lubricant molecules with those features can conduct tribochemical reactions during the friction process to form protective tribofilm leading to a low wear rate [20][57]. Electrostatic stabilization relates to the functionalization of the nanocarbon surface with electrostatically repulsing functional groups that increase the apparent surface charge and the double layer thickness of the structure, so that when the particles get closer, their electrostatic repulsion may overcome the attractive van der Waals forces. Electrostatic and steric stabilization mechanisms can also be combined in a so-called electrosteric approach, by selecting large sterically hindering groups with functional terminations that repel each other, such as a polyelectrolyte. In aqueous systems, both mechanisms prevail, while in non-aqueous systems, steric stabilization is dominant.
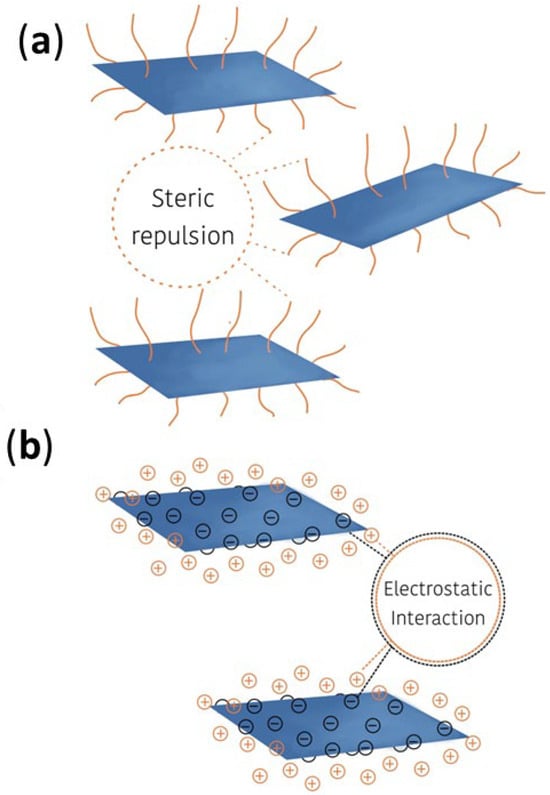
Figure 17.
Functionalization scheme: (
a
) steric stabilization and (
b
) electrostatic stabilization.
The authors also argued about a highly advanced strategy for surface modification. That involves a specific selection of functional groups to intentionally undergo tribochemical reactions under harsh operating conditions, acting as a precursor to the formation of a tribofilm with improved friction-reducing and anti-wear characteristics. That approach is illustrated in Figure 18 [7][6]. For example, Zhao 2018 [24][61], investigating the lubrication properties of graphene additives with different degrees of exfoliation, and Liu 2021 [25][62], from the study of graphene/N-butyl pyridinium tetrafluoroborate ionic liquid, have reported nano-structural evolution and the formation of tribochemically active functional groups, respectively, on tribofilms characterized after tribological testing performed with functionalized graphenes.
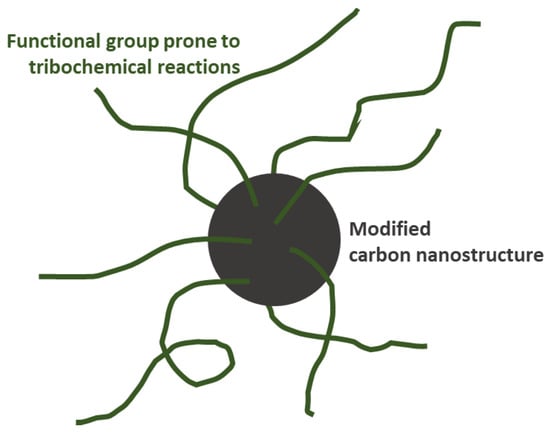
Figure 18. Functional groups can act as precursors for active tribofilm formation. Adapted from Nyholm [7].
4. Tribological Mechanisms of Graphene-Based Materials
The effect of 2D materials, in particular graphene, on tribological performance may be due to different mechanisms. Graphene can act as a protective tribofilm, as a viscous modifier, etc. To ease this review organization, the main mechanisms were divided according to Figure 19 and will be discussed in separate sub-topics. The reader must keep in mind that more than one mechanism may have occurred in the case described in the reference as well as in the real-life applications.
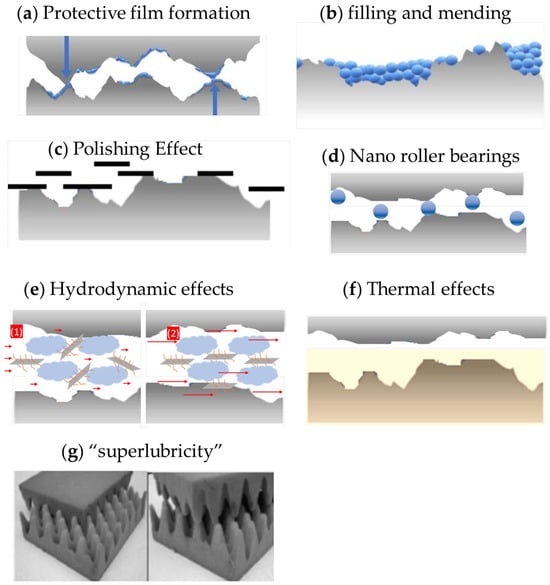
Figure 19. Graphene’s tribological mechanisms. (a) Typical FM tribofilm (b) Surface filling and mending (c) Polishing effect (d) nano roller bearings (e) Hydrodynamics at (1) low shear rate, (2) high shear rate. (f) Thermal effects (g) Superlubricity, incommensurable contact.
Other graphene-tribological-related applications such as dry lubricant, coating, etc. are discussed in separate chapters. Table 23 and Table 34 summarize the main references of using graphene as an oil and grease additive and the proposed mechanism for the reported benefits. The use of graphene on aqueous solutions or as metalworking fluids are not covered in this research.
Table 23.
Carbon-based materials as an additive to oil.
| Lubricant | Graphene Type | Graphene Concentration | Dispersion/Surfactant | Benefit | Prop. Mechanism | Ref. | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grease | Graphene Type | Graphene Concentration | Dispersion/Surfactant | Benefit | Prop. Mechanism | Ref. | |||||||
| PAO4 | 3 GnP: 300, 600 and 750 m2/g (<2 nm, 1–2 µm lateral size) | 0.5 wt% | none | Increased wear resistance and thermal conductivity, especially in electric conditions | |||||||||
| Bentone Grease | Multilayer Graphene | [ | ? | 26 | ] | ethanol[63] | |||||||
| Higher dropping point. Lower wear and friction | tribofilm | [ | 49 | ] | [ | 83] | SAE 5W-30 | Graphene nanosheets | |||||
| N-dimethylformamide | |||||||||||||
| Grease | 0.03, 0.20, 0.40 and 0.6 wt% | Oleic acid | Lower friction and wear. Significant fuel saving | Tribofilm (self-healing) | Graphene (C 94%, O 6%) | [27][18] | |||||||
| 1 to 4% | none | CoF and wear reduction. 55% increase in Thermal conductivity | [ | 50 | ][84] | SAE 5W-30 | Powder (1.3 nm thick) and graphene nanoplates | 0.03 to 0.15 wt% | 15% lower wear, 35% lower friction. 77% higher TC, 30% viscosity | tribofilm | [ | ||
| Calcium grease | MWCNT and G nanosheets | 0.5, 1, 3 wt% | Up to 30% higher dropping point, 60% lower CoF, 74% lower wear | 28][64] | |||||||||
| [ | 51 | ] | [ | 85 | ] | HDD CH-4 20W-50 | Commercial N002-PDR | 0.5 to 3.0% | Lipophilic polymer, WinSperse 6020 | 20% higher TC | thermal | [29][ | |
| Cheavy-duty lithium grease | rGO, graphite, and MWCNT | 65 | 0.5, 1, 2, 3.5, 5 wt% | ] | |||||||||
| Increase in Timken test LCC, lower wear, friction, and temperature on rolling bearing tests. Increase in vibration damping. | [ | 52 | ] | [86] | SN-500 base oil | GO nanosheets | 0.02, 0.04, 0.06, 0.8 wt% | none | Reduced friction and wear on four-ball | Protective film | [30][66] | ||
| SN-150 base oil | |||||||||||||
| Base oil | Mixture of single and multilayer | poly isobutylene succinic imide (PIBSI) based | Reduced wear and friction | Tribofilm and thermal | [53][87] | GO functionalized with DtBHBA | 0.2 to 0.8 mg/mL | none | 40% lower CoF, 17% lower wear under rolling conditions | tribofilm | [9][45] | ||
| Lithium grease | 3D hierarchical porous Graphene | 0.1, 0.3 and 0.5 wt% | none | 52% lower wear, 20% lower friction | tribofilm | [54][88 | PAO 06 | GN and fluorinated graphene (FGN) | 0.005 to 0.020 wt% | T161 | Reduced friction | tribofilm | [31][67] |
| ] | |||||||||||||
| group II-III base oil | 3 variants of rGO | 0.01 wt% | Reduced wear and friction | [][89] | Engine oil | Graphene and GO | 0.02 to 0.06 mg/mL | none | Reduced friction | Mending and tribofilm | [32][68] | ||
| 20W-50 | MWCNT, Graphene Nanosheets, C nanoballs, and Fullerene Nanoparticles (C60) | 0.1 and 0.2% | |||||||||||
| Grease (and also as dry lubrication) | Graphene platelets, 2, 6–8, 11–15 nm | 1 wt% | Reduced friction and wear | tribofilm | [56][90] | 10% higher TC with C nanoballs | thermal | [33][ | |||||
| Ca and Li greases | 69 | ] | |||||||||||
| CNT | 7.5 wt% | MoS | 2 was also added to the grease | Reduced friction and wear | tribofilm | [57][91] | Mineral oil | GNS (grade C-750, 900, 407 Sigma Aldrich (St. Louis, MO, USA), <2 µm size) | 0.1, 0.5, 1.0 wt% | not informed | Reduced wear | [34] | |
| commercial lithium grease, mineral oil | rGO | 0.2, 0.4, 0.6 wt% | [ | 70 | ] | ||||||||
| 55 | toluene | CoF lower 30% for rolling, 20% for sliding-induced-rolling | tribofilm | [ | 58 | ][92] | GL-4EP90 (degraded) | Graphite, Graphene and [34][70] | Respectively 0.5, 0.5, 0.15 wt% | SiO2 for the GO, No dispersants | |||
| Li Grease | 16% lower wear | GN and Graphite | 0.2 to 2.0% | tribofilm | [ | 35][71] | |||||||
| tribofilm | [ | 59 | ] | [93] | 500N base oil | Expanded graphite and Potassium Borate | 0.03, 0.05, 0.10 wt% | - | 30% lower CoF, 36% lower wear | TC and Tribofilm | [ | ||
| Li-based grease | GN | 0.2 to 2.0% | Friction and wear reduction | Tribofilm and enhancement of FeO2 and LiO2 tribofilms | 36][72] | ||||||||
| [ | 60 | ] | [ | 94 | ] | Group lll base oil | Functionalized graphene (AGO-C(n)) | 0.005, 0.01, 0.02 wt% | - | ||||
| Lithium complex Grease | 22% lower CoF with 0.01 wt% | tribofilm | [ | FLG | 0.5, 1.0 and 2.0 wt% | 37][73] | |||||||
| 52% lower wear and 20% lower CoF | Tribofilm and ordered state of the graphene sheets (Hydro?) | [ | 61 | ] | [ | 95] | 5W-40 synthetic oil | RGO | 0.01 to 0.2 wt% | none | 5% lower CoF, 3% lower wear | tribofilm | |
| Polyurea Grease | FLG | [ | 0.5, 1.0 and 2.0 wt% | 38 | Reduced wear and friction. Improved rheology | Tribofilm][74] | |||||||
| [ | 62 | ] | [ | 96 | ] | PAO and PAO + additives | GN (3–8 layers) | 1 and 5% | tribofilm | [39][75] | |||
| HD-50 oil | Modified GNP | 0.005–0.1 wt% | Oleic acid and sodium dodecyl presulfate | Up to 35% lower wear | tribofilm | [40][76] | |||||||
| 20W50 SN/CF and SJ/CF | Graphene, thickness 8, 12, and 60 nm thick. | 0.01% | none | 70% higher Thermal conductivity, reduced wear | Thermal | [41][19] | |||||||
| Maritime engine oil | GO MXene-Nitrogen-doped | 0.01% | none | 7% oil thermo conductivity increase and reduced viscosity. | Thermal and Hydrodynamic | [42][32] | |||||||
| SN 150 oil | GO nanosheets | 0.1 wt% | |||||||||||
| Lithium hydroxide monohydrate, mineral oil (KN4010) | GFL 0.5 to 1.5 nm thick | 0.1, 0.5, 1.0 and 2.0 wt% | none | Reduced wear and friction. 1.6x higher welding point | [63][97] | polyisobutenyl succinic acid-polyamine ester | |||||||
| Li grease | NanoCarbon and GNPs | 0.2 wt% | Proprietary | Reduced friction and wear | [64][27] | Friction and wear reduction on boundary, mixed, and EHL lubricant regimes | tribofilm | [43][77] | |||||
| oil | G, rGO, MoS2, hBN | 0.4 wt% each and 0.2, 0.4 | None, stability improved by mixing process | Up 80% lower wear, 42% lower CoF | Filling and mending | [44][78] | |||||||
| pure paraffin liquid (PL) oil and with commercial additives | Graphene layered nanosheets | 0.1 wt% | mono-dispersed in silver (Ag) nanospheres | Reduction of Friction 40% and 36% on wear | Roller bearing and protective film | [45][79] | |||||||
| SAE 10W-30 | Gr by liquid exfoliation | 0.05 to 0.20 wt% | 40% lower CoF and 36% lower wear | Protective tribo film | [46][80] | ||||||||
| PAO 10 | GO, rGO, and graphene-like covalent-organic frameworks (GCF) nano-sheets | 0.002 to 0.08 wt% | [47][81] | ||||||||||
| Lubricant film | Graphene sheets | 0.02–0.06 wt% | The friction coefficient and wear scar diameter were reduced by 17% and 14%, respectively | [48][82] |
Table 34.
Carbon-based materials as an additive to grease.
