1. Introduction
Originally identified in viruses half a century ago
[1], circular RNAs (circRNAs) were initially regarded as byproducts of aberrant splicing events, casting a cloud of uncertainty over their biological relevance until 2012. This viewpoint underwent a marked shift when circRNAs were found in archaea playing a specific biological function
[2]. In recent years, due to advancements in RNA sequencing methodologies, an increased number of reports have delineated the structural architecture and functional roles of circRNAs in the metabolic machinery of various organisms, notably including humans
[3][4][5][3,4,5].
The structure of circRNA comprises a circular single-stranded RNA molecule wherein the 3′ and 5′ ends are connected through a covalent phosphodiester bond
[5][6][5,6]. This circular configuration predominantly arises from a process termed back-splicing of exons and/or introns
[7][8][7,8]. The absence of free 3′ and 5′ ends, common targets of exonucleolytic degradation, confer to circRNAs exceptional stability, reflected in a half-life that surpasses 48 h
[9]. This significantly contrasts with the approximately 10 h half-life of linear transcripts
[10].
2. Biogenesis and Functions of circRNAs
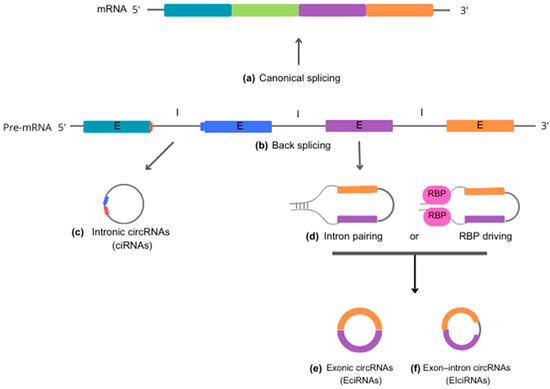
According to the molecular mechanisms underlying biogenesis, circRNAs can be classified into three primary categories: (i) intronic circRNAs (ciRNAs), (ii) exonic circRNAs (EcircRNAs), and (iii) exon-intron circRNAs (EiciRNAs). Each category represents a unique mode of circularization and has its own distinct biogenesis mechanisms
[11][21] (
Figure 1).
Figure 1. Schematic representation of circRNA biogenesis. Precursor mRNA (pre-mRNA) is the primary RNA transcript synthesized on a DNA template in the cell nucleus. Pre-mRNA can follow two distinct cellular pathways. In the upward direction, pre-mRNA gives rise to 5′-3′ mRNA sequences through canonical splicing (a), which removes introns and generates a molecule to be exported to the cytoplasm for guiding protein synthesis. In the alternate downward direction, pre-mRNA produces circRNAs through back-splicing reactions (b). Intronic circRNAs (ciRNA) (c) are formed by a lariat-driven cyclization process that exclusively involves introns. Specific sequence elements adjacent to an exon, rich in guanine (G) and uracil (U) (depicted in blue), bind to an 11-nucleotide cytosine (C)-rich sequence (shown in red) near another exon. This binding allows evasion of the debranching reaction and prevents degradation by exonucleases. Reverse complementary sequences and/or RNA-binding proteins (RBP) (d) act to bring the nucleotide sequences into close proximity. This process facilitates the back-splicing reaction, leading to the generation of exonic circRNA (EciRNA) (e) or exon-intron circRNA (EIciRNA) (f). Abbreviations: mRNA, messenger RNA; pre-mRNA, precursor mRNA; ciRNA, intronic circRNA; EciRNA, exonic circRNA; EIciRNA, exon-intron circRNA; E, exon; I, intron; RBP, RNA-binding proteins.
Intronic circular RNAs (ciRNAs) are generated through a lariat-driven cyclization process that involves only introns and takes place within the nucleus. In the standard splicing process, introns are typically excised from precursor RNA through splicing reactions and are ultimately degraded by exonucleases following a debranching reaction. However, in the case of ciRNAs, specific sequence elements adjacent to exons inhibit the removal of introns by the splicing machinery. These sequences, which are rich in guanine (G) and uracil (U) near one exon and feature an 11-nucleotide cytosine (C)-rich sequence near another exon, promote intron circularization. In this process, introns containing these unusual sequence elements evade debranching and instead form a circular structure, resulting in the formation of stable ciRNAs
[6][12][6,22].
Exonic circRNAs (EcircRNAs) predominantly originate from one or multiple exons of pre-mRNA transcripts. They are abundant in the cytoplasm and may also be found in exosomes. The formation of EcircRNAs involves a process called intron-pairing-driven cyclization, in which complementary sequences within the same or different introns base-pair with each other, ultimately producing a circular RNA molecule composed entirely of exonic sequences. EcircRNAs represent a substantial proportion—more than 80%—of all circRNAs
[11][13][21,23].
Exon-intron (EiciRNAs) are predominantly found in the nucleus, suggesting that they play a role in nuclear functions and processes. These particular circRNAs form as a result of intron retention between exons. The biosynthesis of EiciRNAs is driven by RNA-binding proteins (RBPs) that recognize and bind to specific gene sequences within the introns. This facilitates the creation of splicing sites at both ends of adjacent exons, which may occur through protein interactions or the formation of RBP dimers. Thereby, the interaction leads to the formation of covalent links between the splicing acceptor and donor sites, triggering the cyclization process and leading to the formation of EiciRNAs
[8][14][8,24].
These three primary categories of circRNAs have been found to have specific functions and regulatory roles within cells. These roles can vary depending on the type and cellular context. Additionally, new categories of circRNAs have been identified, including fusion circRNAs (f-circRNAs), read-through circRNAs (rt-circRNAs), and mitochondria-encoded circRNAs (mecciRNAs)
[11][21].
3. Function of circRNAs in the Cellular Metabolism
Although the understanding of the role of circular RNAs (circRNAs) in biological processes is still in its infancy, significant findings have been reported. CircRNAs may regulate gene expression by interacting with DNA (via interaction with RNA polymerase II or through methylation) and RNA (via mRNA trapping, acting as miRNA sponges, and influencing mRNA stability)
[5][15][5,25]. Moreover, circRNAs can modulate protein interactions, act as protein sponges, and even synthesize short peptides or proteins
[16][17][18][26,27,28].
4. CircRNAs Regulating Gene Expression—Interaction with DNA
4.1. Direct Regulation of Gene Expression
The majority of circRNAs are located in the cytoplasm, with a smaller portion found in the nucleus
[19][29]. Within the nucleus, these circRNAs interact with RNA polymerase II at the promoter regions of host genes, thereby impacting transcription modulation
[20][30].
4.2. Regulation of DNA Methylation
A recently discovered mechanism by which circRNAs modulate gene regulation involves altering the DNA methylation of downstream genes. For instance, circRNA ACR activates PTEN-induced putative kinase 1 (Pink1) expression by directly interacting with DNA methyltransferase 3 beta and inhibiting its role in DNA methylation at the Pink1 promoter
[21][31].
4.3. Retrotransposon
Dong et al. (2016) have demonstrated the capacity of circRNA to form pseudogenes. While pseudogenes derived from linear mRNA maintain exon sequences consistent with their normal counterparts, those derived from circRNA possess exon connection sequences that are reversed in orientation compared to common genes. This finding suggests a potential role for circRNAs as reverse transposons, contributing to alterations in genome structure and the regulation of gene expression
[22][32].
5. CircRNAs Regulating Gene Expression—Interaction with RNA
5.1. Regulation of Gene Expression
CircRNAs play a crucial role in the regulation of gene expression by actively influencing the transcription of linear RNA. Ashwal-Fluss et al. (2014) and Sinha et al. (2016) propose that when pre-mRNA contains a translation initiation site and undergoes nonlinear splicing, resulting in cyclization, it leads to a reduction in mRNA transcription. This decrease subsequently leads to a decline in downstream protein production for translation, a phenomenon known as the “mRNA trap”
[7]. The competitive relationship between back-splicing and linear splicing may serve as a general mechanism for regulating mRNA processing connected to corresponding host genes
[7][23][7,33].
5.2. Regulation of mRNA Stability
CircRNAs have been found to regulate mRNA stability in certain cases. Hansen et al. (2013) discovered that the circular RNA derived from the cerebellar-degeneration-related protein 1 (CDR1) gene forms a double-stranded structure with CDR1 mRNA, thereby enhancing its stability
[24][34]. Additionally, in murine macrophages, Circ-Ras-GEF domain family member 1B (Ras-GEF1B) increases the stability of intercellular cell adhesion molecule-1 (ICAM-1) mRNA, leading to its heightened expression in the lipopolysaccharide (LPS)/toll-like receptor 4 (TLR4) inflammatory signaling pathway
[25][35]. Garikipati et al. (2019) reported that CircFndc3b stabilizes the mRNA fused in sarcoma (FUS), as circFndc3b is primarily localized in the cytoplasm. These findings highlight the diverse roles circRNAs play in regulating gene expression
[26][36].
5.3. Sponges of miRNAs
CircRNAs possess specific interaction sites, referred to as miRNA response elements (MREs), which enable them to function as sponges for miRNAs, drawing miRNAs away from their target mRNAs and thereby influencing gene expression and protein synthesis. This mechanism is one of the ways circRNAs can regulate cellular functions and contribute to physiological and pathological processes. Li et al. (2023) demonstrated that a single circRNA might have multiple and efficient miRNA-binding sites, conferring upon them a significant regulatory role in miRNA biology
[27][37].
6. CircRNAs Regulating Cellular Metabolism—Interaction with Protein
6.1. Protein Translation
Gene translation into proteins typically necessitates the recognition of the 5′ cap structure of mRNAs
[28][38]. However, recent studies have revealed that circRNAs can also be translated into proteins, given the presence of internal ribosome entry site (IRES) elements within their structure
[16][26]. The initial discovery of a circRNA functioning as a protein translator was reported in the hepatitis C virus; this single-stranded circRNA produced a protein consisting of 122 amino acids
[29][39].
The circRNAs, with their versatile regulatory capacity, significantly contribute to orchestrating the intricate network of cellular metabolism. These molecules play a crucial role in cellular function, particularly in brain tissue. Thus, exploring the role of circRNAs in neurodegenerative diseases, such as PD, could bring new therapeutic strategies to interrupt disease progression
[16][30][26,40].
6.2. Sponges and Proteins
In addition to MREs, circRNAs also possess protein interaction sites within their structure. Recent research by Ulshöfer et al. (2021) and Aufiero et al. (2019) has found that these sites function as protein sponges, impacting protein expression, biogenesis, and pathophysiological progression. This expands
theour understanding of the multifaceted regulatory capabilities of circRNAs in gene expression and their influence on cellular processes
[31][32][41,42].
7. CircRNAs Relationship between Alpha-Synuclein and microRNAs in the Parkinson’s Disease
Alpha-synuclein (SNCA) is a cytosolic protein that is abundantly expressed in a healthy brain and plays crucial roles in neurotransmission through interactions with synaptic vesicles and proteins involved in exocytosis
[33][43]. In cells, SNCA functions by regulating protein ubiquitination, chaperone activity, kinase-dependent pathways, and dopamine metabolism, which is a neurotransmitter depleted in Parkinson’s disease (PD)
[34][44]. Exposure to cellular stressors triggers structural changes in SNCA, leading to the formation of fibrillar aggregates called Lewy Bodies and Lewy neurites
[35][45]. These SNCA inclusions, prominently observed in nigral dopaminergic neurons, are hallmarks of both sporadic and familial forms of PD
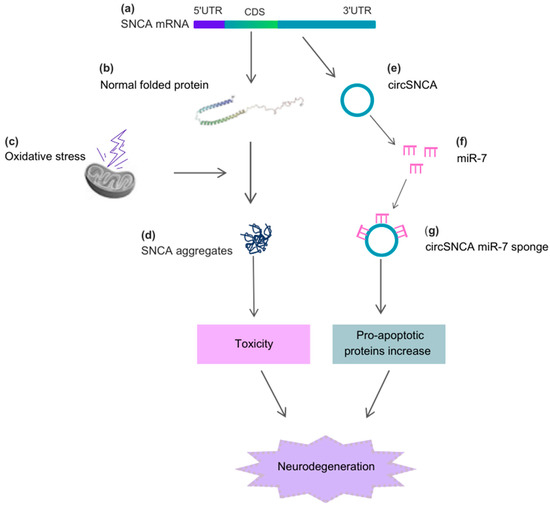
[36][37][38][46,47,48]. Circular RNA (circRNA) can also be derived from the proximal 3′UTR of SNCA mRNA, containing the high-affinity site for miR-7, and can efficiently sponge miR-7 in cell culture
[39][49]. When human neuroblastoma SH-SY5Y cells are treated with the dopamine agonist pramipexole, circSNCA content decreases, leading to increased miR-7 levels and a subsequent decrease in SNCA protein levels. Furthermore, higher circSNCA expression has been reported to be associated with increased expression of pro-apoptotic proteins such as CASP3, BAX, PTEN, and P53, as well as decreased content of autophagy-associated protein LC3B-II
[39][49] (
Figure 2).
Figure 2. Alpha-synuclein (SNCA), circSNCA, and the cellular stressors acting on the neurodegenerative process of PD. (a) The CDS region of SNCA mRNA originates the (b) normal folded SNCA protein, which, under (c) oxidative stress, causes the formation of (d) SNCA aggregates. These aggregates induce toxicity and further neurodegeneration. The 3′UTR region of SNCA mRNA originates (e) circSNCA, which acts as a sponge for miR-7 (f,g), leading to an increase in pro-apoptotic proteins and neurodegeneration. Abbreviations: 5′UTR, 5′untranslated region; 3′UTR, 3′untranslated region; CDS, coding sequence.
One of the best-characterized circular RNAs is the Cerebellar Degeneration-Related Protein 1 Antisense RNA (CDR1as), which is highly expressed in the mammalian brain. It is part of a regulatory network of non-coding RNAs, including miR-7, miR-671, and the cyrano-long RNA
[24][40][34,50]. CDR1as contains over 70 binding sites for miR-7
[24][34]. Recent research indicates that knockout of CDR1as results in miR-7 depletion, associated with synaptic dysfunction and significant sensorimotor alterations
[41][51]. CDR1as has a site that tightly binds miR-671, exhibiting nearly perfect complementarity to the mature sequence of the microRNA. This interaction allows miR-671 to induce CDR1as cleavage, catalyzed by Argonaute 2
[24][41][34,51]. As proposed by Piwecka et al. (2017), in terms of regulation, CDR1as manages miR-7 levels, while miR-671 controls cellular CDR1as content
[41][51].
In a model of cancer, by using a biotinylated circHIPK3 probe, Zeng et al. (2018) showed that miR-7 was pulled down by circHIPK3. These findings were corroborated with additional assays, and ectopic expression of circHIPK3 reversed miR-7 inhibition of its targets
[42][52].
Circzip-2, one of the two most highly expressed circRNAs in
C. elegans, was observed to be downregulated 18-fold in the SNCA-overexpression PD model when compared to the wild-type strain of
C. elegans. This circRNA potentially functions as a sponge for miR-60-3p, which suppresses mRNAs involved in the forkhead box O (FOXO) pathway. This pathway plays a protective role in the development of PD and aging
[43][53].
A recent study discovered 24 circRNAs with differential expression in substantia nigra (SN), medial temporal gyrus, and amygdala tissue samples from Parkinson’s disease (PD) patients. For instance, circSLC8A1 was found to be increased in the SN of PD patients. It contains seven binding sites for miR-128, which also show elevated levels in PD patients, suggesting a potential involvement of circSLC8A1 in regulating miR-128 function and/or activity. Furthermore, circSLC8A1 levels exhibited higher expression in cultured cells exposed to the oxidative stress-inducing agent paraquat, while its levels decreased following treatment with a neuroprotective antioxidant agent. This indicates a possible connection between circSLC8A1 and oxidative stress-related parkinsonism
[44][54]. Another study using a mice model intraperitoneally injected with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) identified differentially expressed circRNAs related to PD in the mice cerebellum through RNA-seq analysis
[45][55].
8. CircRNA in Neuroinflammation
A thorough study of the circRNA transcriptome in human brain glial cells, including astrocytes, microglia, and oligodendrocytes, highlights the unique nature of each transcriptome, pointing to their specific roles within the brain. The researchers found 265, 239, and 442 unique circRNAs in astrocytes, microglia, and oligodendrocytes, respectively. Intriguingly, the highest prevalence of circRNAs in these glial cell types comes from parent genes that predominantly express linear RNAs at relatively low levels, hinting at a preference for spliceosome activity targeting the back-splicing mechanism over the canonical splicing activity
[46][56].
CircPtk2, associated with neuroinflammation, has been found to act as a sponge for miR-29b in a cerebral ischemia model
[47][57]. When microglial culture-derived conditioned media were introduced to hippocampal neurons, apoptosis resulted but was alleviated by prior miR-29b overexpression in microglia. Changes in the expression of the miR-29 family were previously identified in the blood serum of patients with PD compared to a control group of healthy individuals (
n = 80 for each group). The findings revealed a significant decrease in components of the miR-29 family with increasing disease severity
[48][58].
CircRNAs are also linked to astrocytic activation
[49][50][59,60]. Studies indicate that methamphetamine-induced degeneration of dopaminergic neurons in the SNpc corresponds with widespread reactive astrogliosis in the striatum
[51][61]. By administering siRNA or lentiviral shRNA targeting circHIPK2, methamphetamine-induced astrocytic activation is noticeably suppressed due to the decrease of Sigma non-opioid intracellular receptor 1 (SIGMAR1) expression. SIGMAR1 is critical to astrocytic activation in vitro and in vivo
[49][50][59,60] and is a target of miR-124
[50][60]. circHIPK2 acts as an endogenous sponge for miR-124. Decreased expression of miR-124 is observed in PD patients
[52][53][62,63], and overexpression in PD mice leads to enhanced motor functions, reduced dopaminergic neuron loss, and diminished oxidative stress
[53][63]. Corroborating this notion, Yao et al. (2018) showed that miR-124 can suppress neuroinflammation in the MPTP-induced model of Parkinsonism
[54][64]. Recently, Zhang et al. (2023) showed that the expression of circHIPK3 in human serum and cerebral fluids of PD patients was significantly higher than in controls, followed by a significant reduction of miR-124 expression. The study also found that lipopolysaccharide (LPS)-treated BV2 cells exhibited higher expression of circHIPK3 and lower miR-124 expression. In addition, by using SH-SY5Y cells, these authors showed significantly impaired viability and elevated apoptotic rate, along with an upregulation of circHIPK3 and downregulation of miR-124 expression after treatment with conditionate medium (LPS-treated BV2 cells). The circHIPK3 enhances neuroinflammation by sponging miR-124 and regulating the miR-124-mediated STAT3/NRLP3 pathway in PD
[52][62].