Plant metabolomics is a rapidly advancing field of plant sciences and systems biology. It involves comprehensive analyses of small molecules (metabolites) in plant tissues and cells. These metabolites include a wide range of compounds, such as sugars, amino acids, organic acids, secondary metabolites (e.g., alkaloids and flavonoids), lipids, and more. Metabolomics allows an understanding of the functional roles of specific metabolites in plants’ physiology, development, and responses to biotic and abiotic stresses. It can lead to the identification of metabolites linked with specific traits or functions. Plant metabolic networks and pathways can be better understood with the help of metabolomics. Researchers can determine how plants react to environmental cues or genetic modifications by examining how metabolite profiles change under various crop stages. Metabolomics plays a major role in crop improvement and biotechnology.
- metabolomics
- mass spectrometry
- plant metabolomics
- crop improvement
- abiotic stresses
1. Introduction
2. Metabolomic Platforms and Large-Scale Metabolite Databases
Metabolomics is a dynamic and developing area that comprehensively understands the metabolic characteristics of biological systems. Metabolomics is the systematic study of the metabolome of cells, biofluids, tissues, or organisms, utilizing high-throughput analytical techniques to identify and measure the changes in metabolites linked with diseases. Multiple analysis techniques are required due to the complexity of the metabolome and the vast range of physiochemical properties of the metabolites. Mass spectrometry, NMR, LC-MS, and GC-MS are the most often utilized analytical platforms. These approaches enable extensive data generation and enhanced chemometric analysis, which provide basic information about the metabolites (Figure 1). In contrast to NMR, mass spectrometry’s higher sensitivity enables researchers to systematically cover the metabolome data. Due to this, researchers were able to find novel metabolic biomarkers and molecules that can aid the reconstruction of metabolic networks. Recent developments in ionization technologies, such as air pressure chemical ionization (APCI), electrospray ionization (ESI), and MALDI-TOF, have improved the accuracy of mass spectrometry [1]. Due to the large sample requirement of NMR and its lower sensitivity, its capacities to identify physical properties of ligands, binding sites on the protein, direct binding of the target protein, and the detection of protein–ligand complex structures continue to be its advantages over MS.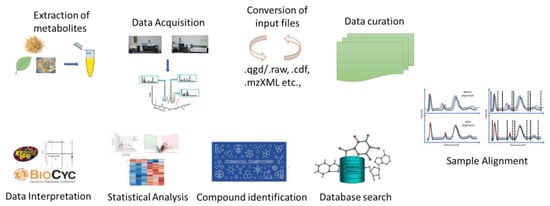
3. Role of Metabolomics in Crop Improvement
Metabolomics is a promising approach to the understanding of abiotic stress tolerance in plant species. The use of metabolomics can help in designing novel strategies to direct metabolism towards crop improvement. Metabolomics has recently been used to seek unique metabolites in plants throughout their life cycles. Crop yield loss is significantly affected by biotic and abiotic stresses [12]. The identification of specific events that activate immune sensors in plants to provide resistance, such as effector-triggered immunity, pattern-triggered immunity, and pattern recognition receptors, is necessary for the detection of invasive species. The plant produces phytohormones to provide stress resistance as soon as abiotic stress occurs. Stomatal conductance is disrupted by oxidative stress, which also activates a number of signaling systems [13]. Overall, a specific plant species with a unique gene expression profile reflects the precise composition of its metabolites. The activation of a specific metabolic network results in the synthesis of a novel bioactive compound [14]. The general steps involved, from diagnostics to metabolomics-assisted breeding for crop improvement, are shown in Figure 1.4. Metabolomics and Its Regulations in Abiotic Stresses
The most promising technique for understanding the regulation of abiotic and biotic stress tolerance in plant species is metabolomics (Figure 2). In metabolomics studies, a plethora of sophisticated MS-based instruments are widely utilized to enhance the comprehension of plants’ ability to withstand abiotic stress [15]. In general, plant metabolic profiling under abiotic stressors can be performed using GC-MS. Time of flight–mass spectrometry allows for the quick and efficient discrimination and detection of a variety of metabolites in mixed samples, which is beneficial for the identification of abiotic stress-regulated metabolites [16,17,18][16][17][18]. Abiotic stresses drastically change plant growth and development, severely restricting plant distribution and lowering the agricultural productivity [17]. Plants experience osmotic stress as a result of altered ion concentration and homeostasis under drought and salinity stress [19]. All fundamental metabolites, including sugars, sugar alcohols, and amino acids are difficult to synthesize in plants under abiotic stressors [20]. Eight wheat cultivars were subjected to GC-MS metabolic profiling in order to gain insights into the mechanism of drought tolerance. Under drought stress, an elevated amino acid concentration was observed [21]. In 2018, Yang and colleagues [22] applied RP/UPLC-MS to conduct metabolic profiling of drought-stressed maize. The results indicated increased lipid and carbohydrate metabolism, along with an accelerated glutathione cycle. Metabolic profiling using LC-MS and GC-MS data also supported the difference in metabolite accumulation between young and mature leaves [23,24][23][24]. A GC-MS technique was used to detect increased synthesis of 4-hydroxycinnamic acid, ferulic acid, stearic acid, and xylitol in rice under drought conditions [6] GC-MS-based metabolic profiling of rice seedlings under salt stress revealed the hyperaccumulation of key amino acids such as leucine, isoleucine, valine, and proline [25]. Comparative metabolic profiling using GC-TOF-MS in salinity-tolerant and susceptible genotypes of rice revealed higher concentrations of amino acids [26]. GC-MS based profiling under salinity stress conditions revealed elevated levels of proline, sucrose, xylose, maltose, and organic acids [27]. Another investigation on rice grown under salt stress found that it possessed less shikimate and quinate [28]. In rice, using jasmonate has been demonstrated to reduce salt damage. The jasmonate pathway is a crucial hormonal mechanism of great relevance [29]. Furthermore, metabolomics technologies have been used to investigate changes in the metabolic profiles of numerous crop plants. Furthermore, various metabolomics tools have been used to investigate changes in the metabolic profiles of numerous crop plants, including tomato, maize, barley, and wheat [30,31,32][30][31][32]. The synthesis of secondary metabolites is impacted by heat stress [33]. LC-MS/MS-HPLC profiling of wheat grains revealed higher amounts of sucrose during heat stress [34]. Comparative metabolic profiling of heat-tolerant and susceptible soybean genotypes showed higher concentrations of carbohydrates in the heat-tolerant genotype. Many metabolites, including arabitol, pinitol, and erythritol, were also produced in lower concentrations by these tolerant genotypes [35]. In order to observe the impacts of heat stress, metabolomics studies were also carried out for other significant crops, including tomato, maize, and wheat [36,37,38][36][37][38]. According to metabolic fingerprinting, tomato plants under N stress have lower concentrations of organic and amino acids [39]. A metabolic profiling technique based on UHPLC revealed that barley underwent nutrient stress-induced synthesis of organic acids, amino acids, and S-responsive metabolites [40]. Metabolic profiling of P-deficient barley exhibits lower amounts of various organic acids [41]. Similarly, P stress in nodules and roots was examined by common bean metabolic profiling [42], and low nitrogen levels in wheat were also studied [43].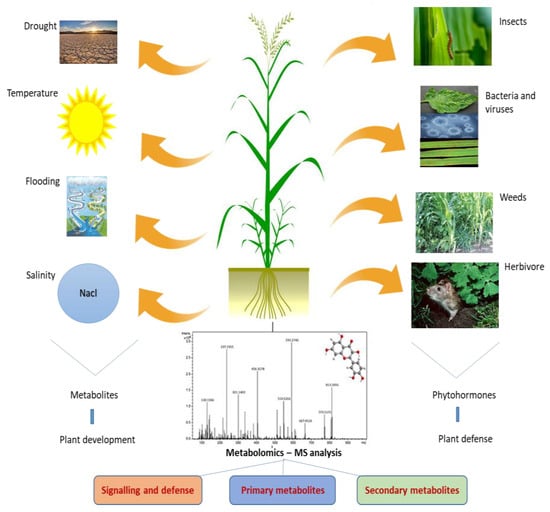
References
- Issaq, H.J.; Van, Q.N.; Waybright, T.J.; Muschik, G.M.; Veenstra, T.D. Analytical and statistical approaches to metabolomics research. J. Sep. Sci. 2009, 32, 2183–2199.
- Ralston-Hooper, K.J.; Adamec, J.; Jannash, A.; Mollenhauer, R.; Ochoa-Acuña, H.; Sepúlveda, M.S. Use of GC × GC/TOF-MS and LC/TOF-MS for metabolomic analysis of Hyalella azteca chronically exposed to atrazine and its primary metabolite, desethylatrazine. J. Appl. Toxicol. 2011, 31, 399–410.
- Turner, M.F.; Heuberger, A.L.; Kirkwood, J.S.; Collins, C.C.; Wolfrum, E.J.; Broeckling, C.D.; Prenni, J.E.; Jahn, C.E. Non-targeted metabolomics in diverse sorghum breeding lines indicates primary and secondary metabolite profiles are associated with plant biomass accumulation and photosynthesis. Front. Plant Sci. 2016, 7, 953.
- Ramautar, R.; de Jong, G.J. Recent developments in liquid-phase separation techniques for metabolomics. Bioanalysis 2014, 6, 1011–1026.
- Zrodnikov, Y.; Davis, C.E. The highs and lows of FAIMS: Predictions and future trends for high field asymmetric waveform ion mobility spectrometry. J. Nanomed. Nanotechnol. 2012, 3, 109e.
- Ma, X.; Xia, H.; Liu, Y.; Wei, H.; Zheng, X.; Song, C.; Chen, L.; Liu, H.; Luo, L. Transcriptomic and metabolomic studies disclose key metabolism pathways contributing to well-maintained photosynthesis under the drought and the consequent drought-tolerance in rice. Front. Plant Sci. 2016, 7, 1886.
- Misra, B.B.; van der Hooft, J.J. Updates in metabolomics tools and resources: 2014–2015. Electrophoresis 2016, 37, 86–110.
- Perez de Souza, L.; Naake, T.; Tohge, T.; Fernie, A.R. From chromatogram to analyte to metabolite. How to pick horses for courses from the massive web resources for mass spectral plant metabolomics. Gigascience 2017, 6, gix037.
- Johnson, C.H.; Ivanisevic, J.; Benton, H.P.; Siuzdak, G. Bioinformatics: The next frontier of metabolomics. Anal. Chem. 2015, 87, 147–156.
- Tsugawa, H.; Cajka, T.; Kind, T.; Ma, Y.; Higgins, B.; Ikeda, K.; Kanazawa, M.; VanderGheynst, J.; Fiehn, O.; Arita, M. MS-DIAL: Data-independent MS/MS deconvolution for comprehensive metabolome analysis. Nat. Methods 2015, 12, 523–526.
- Xie, W.; Lv, X.; Ye, L.; Zhou, P.; Yu, H. Construction of lycopene-overproducing Saccharomyces cerevisiae by combining directed evolution and metabolic engineering. Metab. Eng. 2015, 30, 69–78.
- Hein, J.A.; Sherrard, M.E.; Manfredi, K.P.; Abebe, T. The fifth leaf and spike organs of barley (Hordeum vulgare L.) display different physiological and metabolic responses to drought stress. BMC Plant Biol. 2016, 16, 248.
- García-Cristobal, J.; García-Villaraco, A.; Ramos, B.; Gutierrez-Mañero, J.; Lucas, J. Priming of pathogenesis related-proteins and enzymes related to oxidative stress by plant growth promoting rhizobacteria on rice plants upon abiotic and biotic stress challenge. J. Plant Physiol. 2015, 188, 72–79.
- Han, Y.Y.; Li, A.X.; Li, F.; Zhao, M.R.; Wang, W. Characterization of a wheat (Triticum aestivum L.) expansin gene, TaEXPB23, involved in the abiotic stress response and phytohormone regulation. Plant Physiol. Biochem. 2012, 54, 49–58.
- Mhlongo, M.I.; Piater, L.A.; Madala, N.E.; Labuschagne, N.; Dubery, I.A. The chemistry of plant–microbe interactions in the rhizosphere and the potential for metabolomics to reveal signaling related to defense priming and induced systemic resistance. Front. Plant Sci. 2018, 9, 112.
- Guo, R.; Shi, L.; Jiao, Y.; Li, M.; Zhong, X.; Gu, F.; Liu, Q.; Xia, X.; Li, H. Metabolic responses to drought stress in the tissues of drought-tolerant and drought-sensitive wheat genotype seedlings. AoB Plants 2018, 10, ply016.
- Kang, Z.; Babar, M.A.; Khan, N.; Guo, J.; Khan, J.; Islam, S.; Shrestha, S.; Shahi, D. Comparative metabolomic profiling in the roots and leaves in contrasting genotypes reveals complex mechanisms involved in post-anthesis drought tolerance in wheat. PLoS ONE 2019, 14, e0213502.
- Khan, F.; Fuentes, D.; Threthowan, R.; Mohammad, F.; Ahmad, M. Comparative metabolite profiling of two wheat genotypes as affected by nitrogen stress at seedling stage. JAPS J. Anim. Plant Sci. 2019, 29, 260–268.
- Blum, A. Osmotic adjustment is a prime drought stress adaptive engine in support of plant production. Plant Cell Environ. 2017, 40, 4–10.
- Krasensky, J.; Jonak, C. Drought, salt, and temperature stress-induced metabolic rearrangements and regulatory networks. J. Exp. Bot. 2012, 63, 1593–1608.
- Yadav, A.K.; Carroll, A.J.; Estavillo, G.M.; Rebetzke, G.J.; Pogson, B.J. Wheat drought tolerance in the field is predicted by amino acid responses to glasshouse-imposed drought. J. Exp. Bot. 2019, 70, 4931–4948.
- Yang, L.; Fountain, J.C.; Ji, P.; Ni, X.; Chen, S.; Lee, R.D.; Kemerait, R.C.; Guo, B. Deciphering drought-induced metabolic responses and regulation in developing maize kernels. Plant Biotechnol. J. 2018, 16, 1616–1628.
- Skirycz, A.; Inzé, D. More from less: Plant growth under limited water. Curr. Opin. Biotechnol. 2010, 21, 197–203.
- Nam, K.-H.; Kim, D.Y.; Kim, H.J.; Pack, I.-S.; Kim, H.J.; Chung, Y.S.; Kim, S.Y.; Kim, C.-G. Global metabolite profiling based on GC–MS and LC–MS/MS analyses in ABF3-overexpressing soybean with enhanced drought tolerance. Appl. Biol. Chem. 2019, 62, 15.
- Gayen, D.; Barua, P.; Lande, N.V.; Varshney, S.; Sengupta, S.; Chakraborty, S.; Chakraborty, N. Dehydration-responsive alterations in the chloroplast proteome and cell metabolomic profile of rice reveals key stress adaptation responses. Environ. Exp. Bot. 2019, 160, 12–24.
- Gupta, P.; De, B. Metabolomics analysis of rice responses to salinity stress revealed elevation of serotonin, and gentisic acid levels in leaves of tolerant varieties. Plant Signal. Behav. 2017, 12, e1335845.
- Shelden, M.C.; Dias, D.A.; Jayasinghe, N.S.; Bacic, A.; Roessner, U. Root spatial metabolite profiling of two genotypes of barley (Hordeum vulgare L.) reveals differences in response to short-term salt stress. J. Exp. Bot. 2016, 67, 3731–3745.
- Chang, J.; Cheong, B.E.; Natera, S.; Roessner, U. Morphological and metabolic responses to salt stress of rice (Oryza sativa L.) cultivars which differ in salinity tolerance. Plant Physiol. Biochem. 2019, 144, 427–435.
- Kurotani, K.-I.; Hayashi, K.; Hatanaka, S.; Toda, Y.; Ogawa, D.; Ichikawa, H.; Ishimaru, Y.; Tashita, R.; Suzuki, T.; Ueda, M. Elevated levels of CYP94 family gene expression alleviate the jasmonate response and enhance salt tolerance in rice. Plant Cell Physiol. 2015, 56, 779–789.
- Zörb, C.; Geilfus, C.-M.; Mühling, K.H.; Ludwig-Müller, J. The influence of salt stress on ABA and auxin concentrations in two maize cultivars differing in salt resistance. J. Plant Physiol. 2013, 170, 220–224.
- Borrelli, G.M.; Fragasso, M.; Nigro, F.; Platani, C.; Papa, R.; Beleggia, R.; Trono, D. Analysis of metabolic and mineral changes in response to salt stress in durum wheat (Triticum turgidum ssp. durum) genotypes, which differ in salinity tolerance. Plant Physiol. Biochem. 2018, 133, 57–70.
- Rouphael, Y.; Raimondi, G.; Lucini, L.; Carillo, P.; Kyriacou, M.C.; Colla, G.; Cirillo, V.; Pannico, A.; El-Nakhel, C.; De Pascale, S. Physiological and metabolic responses triggered by omeprazole improve tomato plant tolerance to NaCl stress. Front. Plant Sci. 2018, 9, 249.
- Thomason, K.; Babar, M.A.; Erickson, J.E.; Mulvaney, M.; Beecher, C.; MacDonald, G. Comparative physiological and metabolomics analysis of wheat (Triticum aestivum L.) following post-anthesis heat stress. PLoS ONE 2018, 13, e0197919.
- Wang, X.; Hou, L.; Lu, Y.; Wu, B.; Gong, X.; Liu, M.; Wang, J.; Sun, Q.; Vierling, E.; Xu, S. Metabolic adaptation of wheat grain contributes to a stable filling rate under heat stress. J. Exp. Bot. 2018, 69, 5531–5545.
- Chebrolu, K.K.; Fritschi, F.B.; Ye, S.; Krishnan, H.B.; Smith, J.R.; Gillman, J.D. Impact of heat stress during seed development on soybean seed metabolome. Metabolomics 2016, 12, 28.
- Sun, C.; Gao, X.; Li, M.; Fu, J.; Zhang, Y. Plastic responses in the metabolome and functional traits of maize plants to temperature variations. Plant Biol. 2016, 18, 249–261.
- Paupière, M.J.; Müller, F.; Li, H.; Rieu, I.; Tikunov, Y.M.; Visser, R.G.; Bovy, A.G. Untargeted metabolomic analysis of tomato pollen development and heat stress response. Plant Reprod. 2017, 30, 81–94.
- Qi, X.; Xu, W.; Zhang, J.; Guo, R.; Zhao, M.; Hu, L.; Wang, H.; Dong, H.; Li, Y. Physiological characteristics and metabolomics of transgenic wheat containing the maize C4 phosphoenolpyruvate carboxylase (PEPC) gene under high temperature stress. Protoplasma 2017, 254, 1017–1030.
- Sung, J.; Lee, S.; Lee, Y.; Ha, S.; Song, B.; Kim, T.; Waters, B.M.; Krishnan, H.B. Metabolomic profiling from leaves and roots of tomato (Solanum lycopersicum L.) plants grown under nitrogen, phosphorus or potassium-deficient condition. Plant Sci. 2015, 241, 55–64.
- Ghosson, H.; Schwarzenberg, A.; Jamois, F.; Yvin, J.-C. Simultaneous untargeted and targeted metabolomics profiling of underivatized primary metabolites in sulfur-deficient barley by ultra-high performance liquid chromatography-quadrupole/time-of-flight mass spectrometry. Plant Methods 2018, 14, 62.
- Huang, C.Y.; Roessner, U.; Eickmeier, I.; Genc, Y.; Callahan, D.L.; Shirley, N.; Langridge, P.; Bacic, A. Metabolite profiling reveals distinct changes in carbon and nitrogen metabolism in phosphate-deficient barley plants (Hordeum vulgare L.). Plant Cell Physiol. 2008, 49, 691–703.
- Hernández, G.; Valdés-López, O.; Ramírez, M.; Goffard, N.; Weiller, G.; Aparicio-Fabre, R.; Fuentes, S.I.; Erban, A.; Kopka, J.; Udvardi, M.K. Global changes in the transcript and metabolic profiles during symbiotic nitrogen fixation in phosphorus-stressed common bean plants. Plant Physiol. 2009, 151, 1221–1238.
- Zhang, Y.; Ma, X.-M.; Wang, X.-C.; Liu, J.-H.; Huang, B.-Y.; Guo, X.-Y.; Xiong, S.-P.; La, G.-X. UPLC-QTOF analysis reveals metabolomic changes in the flag leaf of wheat (Triticum aestivum L.) under low-nitrogen stress. Plant Physiol. Biochem. 2017, 111, 30–38.
