You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Fanny Huang and Version 3 by Fanny Huang.
Studies have demonstrated that individuals with complex regional pain syndrome (CRPS) often exhibit dysbiosis, with imbalances in beneficial and pathogenic gut bacteria. Dysbiosis can lead to increased gut permeability and systemic inflammation, contributing to the chronic pain experienced in CRPS. B, an essential trace element, has shown promise in modulating the gut microbiome positively and exerting anti-inflammatory effects. Preclinical and clinical studies suggest that B supplementation may alleviate neuropathic pain and improve CRPS symptoms by restoring microbiota balance and reducing inflammation.
- complex regional pain syndrome
- microbiota
- prebiotic boron
1. Introduction
Complex regional pain syndrome (CRPS), also known as reflex sympathetic dystrophy or causalgia, is a debilitating and persistent neuroinflammatory disorder that typically emerges in the aftermath of stressful events, such as surgery or trauma. CRPS can manifest in two forms: CRPS-I affects individuals without confirmed nerve injury, while CRPS-II affects those with associated nerve damage [1]. Both types of CRPS are diagnoses reached by ruling out other potential causes, and they involve pain that is disproportionate to the initial injury, accompanied by the presence of signs in at least two and symptoms in at least three of the following categories: sensory, vasomotor, sudomotor, and motor or trophic [2].
CRPS patients commonly experience decreased mobility, an inability to work, a diminished quality of life (QoL), as well as depression, anxiety, and long-term reliance on opioids [3]. Therefore, it is crucial to provide an early diagnosis and initiate treatment promptly to curb disease progression and enhance patients’ QoL. Conservative approaches, such as medication use, physical therapy interventions, or injections, are among the available treatment options for CRPS. While there is ongoing debate regarding this matter, early symptoms of CRPS are widely believed to be influenced by sympathetic activity, resulting in the adoption of sympathetic blocks for symptom management purposes [4]. Experimental treatments like intravenous immunoglobulin have yielded unsatisfactory results thus far. Tragically, 20–50% of individuals with less than one year’s worth of symptoms do not respond well enough to conservative strategies and may consequently necessitate chronic opioid use or implantation procedures involving neuromodulation devices like spinal cord stimulators or dorsal root ganglion stimulators [5]. As CRPS progresses into its chronic stage, observed efficacy from established treatment methods diminishes even further [2].
The lack of satisfactory treatment for CRPS is due to the incomplete understanding of its root causes. Despite years of research, there is still only a partial understanding of how exactly CRPS develops. The condition involves an abnormal reaction of tissues to injury as well as enhanced sensitivity in both peripheral and central nervous systems (CNS), accompanied by inflammation and autonomic dysfunction. Furthermore, it is thought that genetic factors and psychological influences play a role in the progression of CRPS. Studies have primarily concentrated on the human leukocyte antigen system due to significant alterations in gene expression within this system [1].
CRPS is defined as an immune reaction that promotes inflammations and impairs neuropeptide signaling [6]. This activation of the innate immune system leads to the proliferation of keratinocytes and the release of proinflammatory cytokines, such as interleukin (IL)-6, IL-1, and tumor necrosis factor-alpha (TNF-α) [7]. These cytokines initiate a cascade within the immune system, causing histamine-induced vasodilation, which results in redness, swelling, pain, and warmth during the acute phase of CRPS [8]. Moreover, these proinflammatory cytokines stimulate osteoblasts and osteoclasts, resulting in accelerated bone remodeling and the distinctive osteoporotic alterations observed in chronic CRPS cases [9][10]. Additionally, neuropathic inflammation is thought to be a key contributor to the onset of CRPS. The activation of peripheral nociceptors situated on C-fibers leads to the transmission of pain signals to the dorsal ganglia and the affected tissue [11]. This retrograde transmission produces proinflammatory neuropeptides, such as substance P (SP) and calcitonin gene-related peptide (CGRP) [11].
The correlation between the GM and painful conditions has garnered considerable research interest. Extensive evidence suggests that GM plays a vital role in maintaining human physiological balance, impacting systemic inflammation, immunity, circadian rhythm, and hormone regulation—all of which have been linked to pain. The GM has shown associations with various types of pain, including visceral pain, inflammatory pain, headache, neuropathic pain, chronic pain, and opioid tolerance. The overall genomic material from intestinal microbes has been estimated to be greater than 100 times the size of the human genome. Most gut microbiota bacteria in healthy adults are derived from two phyla, Firmicutes and Bacteroidetes. These populations are believed to be influenced by various factors at personal, interpersonal, environmental, and geographical levels that shape the composition of the microbiome [12].
Recent research has sparked great interest in the possible involvement of the gut microbiota in pain, as discussed in several well-regarded reviews [13][14]. Animal studies have revealed that a healthy population of gut bacteria is essential for normal perception of visceral pain, while an imbalanced composition can contribute to the development of conditions including irritable bowel syndrome (IBS), neuropathic pain, and inflammatory pain [15][16][17][18]. In human clinical studies, alterations in bacterial diversity or dysbiosis within the GM have been linked to various painful conditions, such as visceral pain, fibromyalgia (FM), and knee arthritis [19][20][21]. It is worth noting that there have been multiple instances where patients with CRPS experienced improvement following the use of antibiotics known to impact the microbiome [22][23]. In these cases, the individuals had been dealing with CRPS for a significant amount of time before being prescribed antibiotics for unrelated reasons. These reports suggest a potential connection between microbiota and long-term management of CRPS [2].
Additionally, in his review titled “Neurogenic neuroinflammation in fibromyalgia and complex regional pain syndrome”, Geoffrey Littlejohn suggests that neuroinflammation may have a “neurogenic” basis, possibly influenced by pain and stress [11]. However, it is premature to solely attribute neuroinflammation and central sensitization to a primary neurogenic origin without considering the well-established presence of coexisting factors, such as small intestinal bacterial overgrowth (SIBO), mitochondrial dysfunction, and vitamin D deficiency associated with gastrointestinal (GI) dysbiosis [24].
Recently, there has been evidence of SIBO in individuals with FM, which refers to the presence of colonic bacteria in the distal small bowel [25]. Furthermore, patients with CRPS have shown impaired intestinal permeability (IP) [26][27][28]. It is suggested that defective immune cell function plays a role in the pathophysiology of both FM and CRPS [29][30][31]. Peripheral blood mononuclear cells from patients with FM and CRPS exhibited reduced messenger ribonucleic acid (mRNA) expression for anti-inflammatory cytokines, indicating an increased inflammatory response [29][30]. It could be hypothesized that a compromised gut barrier may contribute to specific immunological reactions associated with these syndromes. There is evidence suggesting that restoring normal IP could potentially improve disease activity in certain human conditions [32]. Another possible explanation for the elevated IP in both FM and CRPS patients might be related to distress caused by their pain. It is well-established that pain can induce stress, although the level of distress does not necessarily correlate directly with the intensity of pain [32]. Research conducted on animal models and human mucosal investigations supports the role of stress in altering IP [33][34]. One hypothesis could be that pain-related distress independently contributes to changes in IP. Altered IP may arise from various factors, such as SIBO, gut infections, gut inflammation, medication usage, stress levels, trauma, or the use of non-steroidal anti-inflammatory drugs (NSAIDs) [35].
Neuroinflammation plays a role in FM and CRPS as much as GI dysbiosis, vitamin D deficiency, and mitochondrial dysfunction. These independent factors often occur together, and each contributes to the development of both peripheral and central hyperalgesia. The consistent pain relief seen with interventions targeting intestinal dysbiosis (antibiotics), vitamin D deficiency (supplementation), and mitochondrial dysfunction (ubiquinone) demonstrates that these painful conditions have multiple underlying causes that can be addressed [24]. Thus, the timely initiation of therapy is crucial for improving patient prognosis in cases of CRPS, as the symptoms can vary over time. The main goals of treatment are to restore limb functionality, reduce pain, and enhance QoL. This typically involves a multidisciplinary approach that includes patient education, physical and occupational therapy, psychiatric support, and interventions from pain medicine specialists through medications or surgical procedures.
2. Perspectives to Use the Prebiotic Boron-Containing Compounds in Complex Regional Pain Syndrome
MABCs hold promise as a potential therapeutic approach in CRPS, a debilitating chronic pain condition that affects a considerable number of individuals worldwide. Several studies have highlighted the role of the gut microbiota in the modulation of pain responses and the development of chronic pain conditions, such as FM and IBS.
Recent research has shown that alterations in gut microbiota composition and IP are associated with chronic pain conditions. This suggests that targeting the microbiota–gut–brain axis could be a potential path for therapeutic intervention in CRPS. MABCs have emerged as a potential therapeutic strategy due to their ability to modulate gut microbiota composition and restore gut homeostasis. These compounds have been shown to improve IP and reduce inflammation, which are key factors in the development of chronic pain.
Prebiotic B deficiency in the diet causes dysbiosis and degradation of the gel mucus in the intestinal tract. Damage in the gut mucus layer aggravates gut inflammation and infection. The intestine microbiota sends bidirectional signals to organs, affecting the metabolism of the whole body and is a contributing factor to metabolic illness. A damaged gut mucosal barrier can be an essential interface between the host and flora. PBCs also improve immunity, have antioxidant and anti-inflammatory actions on the microbiome [36], and determine the health of the body through the main “axes” defined in the scientific literature: “microbiota–gut–brain axis”, “microbiota–gut–bone axis”, “microbiota–gut–thyroid axis”, “microbiota–gut–cartilage axis”, and “microbiota–gut–heart axis” [37]. Subsequently, PBCs have a major role in the avoidance of certain illnesses, such as OA, OP, RA, cardiovascular inflammation, depression, obesity, T2D, and thyroid diseases [38].
The indigestible PBC species are microbiota-accessible and cause an increase in the level of volatile fatty acids due to the increase in the activity of commensal bacteria, especially the level of butyrate producers [39]. Most human butyrate producers belong to the Firmicutes phylum, including species such as Butyrivibrio fibrisolvens, Clostridium butyricum, C. kluyveri, Eubacterium limosum, and Faecalibacterium prausnitzii [40]. In addition, other bacteria produce butyrate; Anaerostipes spp., Bifidobacterium spp., and E. hallii. Bifidobacterium spp. generate butyrate from lactate (a product of glucose metabolism) and acetate. All primary butyrate-producing bacteria are anaerobic, meaning they can only grow in low-oxygen environments. Because oxygen levels in a healthy colon are extremely low, these organisms thrive in this ecosystem. The Firmicutes phylum has recently been shown to increase intestinal levels of AI-2 and butyrate. Acetate and propionate are the main products of the Bacteroidetes phylum. Thus, the increase in AI-2 levels favored the expansion of Firmicutes in the microbiota treated with antibiotics and inhibited Bacteroidetes, therefore counteracting the dysbiosis induced by antibiotic treatment [41][42].
Researchers proposed the MoA of PBCs as a mediator for the production of butyrate in the colon (Figure 1). Thus, the AI-2B level increases and stimulates the growth of butyrate-producing bacteria (mainly Firmicutes) [43]. AI-2B transfers the borate anion to mucins stimulated by butyrate and, through the high level of AI-2B, inhibits pathogenic bacteria (such as Vibrio spp.) [44][45][46]. The reverse process of decreasing prebiotic borate intake leads to the inhibition of butyrate-producing bacteria and the growth of pathogenic bacteria that are stimulated by low AI-2B. In addition, the lack of PBCs in the diet accelerates the loss of B from the mucus in the feces by complexing, mainly with fucose and sialic acid (Figure 1). Subsequently, PBCs are the ones that have an essential impact on host–microbial symbiosis in health, and mainly on butyrate-producing bacteria and AI-2 signaling molecules, according to the model shown in Figure 1, in two directions: microbiota–gut–immune system and microbiota–gut–musculoskeletal system.
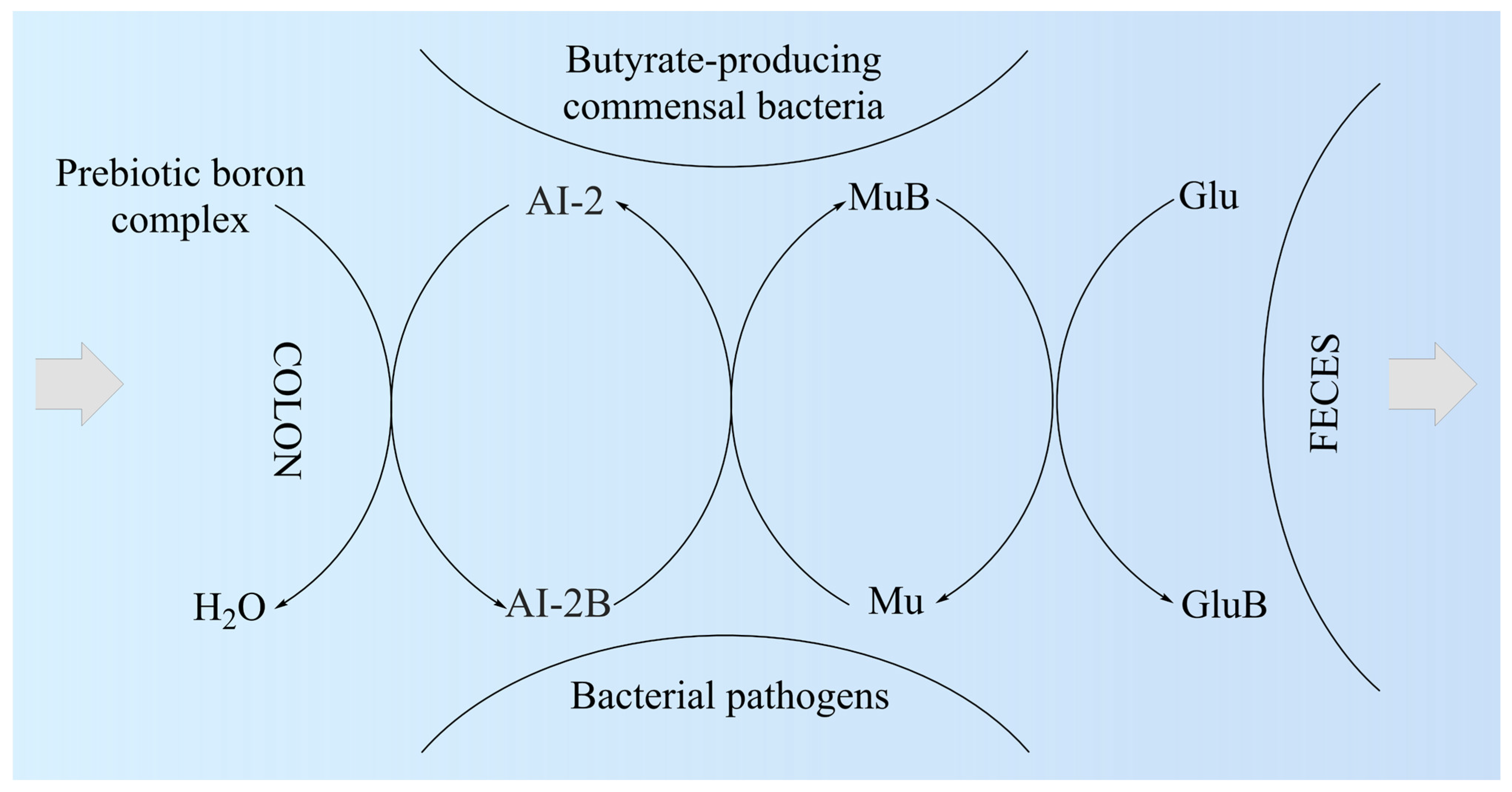
Figure 1. The proposed mechanism of action of prebiotic boron complex as a mediator for AI-2B and MuB production. AI-2: Autoinducer-2; AI-2B: Autoinducer-2–furanosyl borate diester; Glu: Monosaccharide (mainly fucose and sialic acid); GluB: Monosaccharide–boron complex; Mu: Mucin gel; MuB: Mucin gel–borate complex.
Butyrate is known to be a key metabolite of the GM that mediates the effects on the immune system cells (T-cells, antigen-presenting cells, monocytes, and neutrophils) and plays a key function in maintaining gut immune homeostasis, but also has potential future therapeutic for a spectrum of intestine and systemic illness [47][48][49][50][51][52]. Recent studies have found that butyrate-producing microbes protect against or are associated with less severe symptoms of a long list of conditions related to chronic inflammation: allergies, IBS, RA, PD, high blood pressure, insomnia, anxiety, and T2D [47]. Moreover, butyrate is involved in boosting the mechanical and immune barrier of the intestine and promotes a healthy intestinal barrier, preventing the “leaky gut” syndrome [53], and has been reported to be an inhibitor of histone deacetylases (HDACs). A growing number of studies have found that butyrate may exert protective effects on atherosclerosis, hypertension, and vascular health [47].
Subsequently, butyrate-producing bacteria of Firmicutes filum can have a key role in the healthy symbiosis of the human colon as a major energy source for the colonic mucosa, increasing mucin secretion, and as an important modulator of gene expression, inflammation, differentiation, and apoptosis in host cells with a function in improving intestinal barrier function [54][55]. B stimulates butyrate-producing bacteria through AI-2B. Butyrate stimulates the de novo synthesis of mucins, ensures the transfer of B to them, and catalyzes the incorporation of B-stabilized mucins into mucus (Figure 1). Mucins are the growth substrate for butyrate-producing Firmicutes, and because mucosal butyrate producers release butyrate close to the epithelium, they may increase the bioavailability of butyrate to the host [56].
Firmicutes are the main butyrate-producing bacteria in the human colon, especially E. rectale, Clostridium leptum, Roseburia spp., and F. prausnitzii [57][58]. Furthermore, butyrate plays a role in controlling the synthesis of cathelicidins, which are polycationic peptides involved in the innate immune system of mammals and demonstrate wide-ranging antimicrobial capabilities against potential intestinal pathogens. Conversely, a decreased presence of butyrate-producing organisms in the intestinal environment leads to the proliferation of aerobic bacteria from the Enterobacteriaceae family, which is commonly associated with signs of intestinal dysbiosis [59].
The number of articles which study the in vitro and in vivo effects of B on butyrate and SCFA production is limited. There are a few studies that show that B as a feed additive can improve ruminal microbial fermentation and promote SCFA formation, especially an increase in butyrate by 40%, while total SCFA increases by over 60% [39][60]. A recent study proved that B improved the disordered gut morphology, and combined treatments with probiotics reduced both oxidative damage and inflammatory processes [61]. The latest studies have noticed that there are some close correlations between B intake, AI-2, the Firmicutes phylum, and the production of butyrate [62][63]. In addition, chlorogenic acid, the ligand in the DCB complex, and other phenolic compounds, such as caffeic acid and rutin, are also reported to increase microbial butyrate [64]. Therefore, DCB, like PBCs, has in its composition both a prebiotic part of phenolic acid and B as an essential nutritional element for butyrate-producing bacteria [65]. Thus, many of the functions of butyrate on health status are also found in the case of prebiotic B ingestion in both animals and humans (Figure 2).
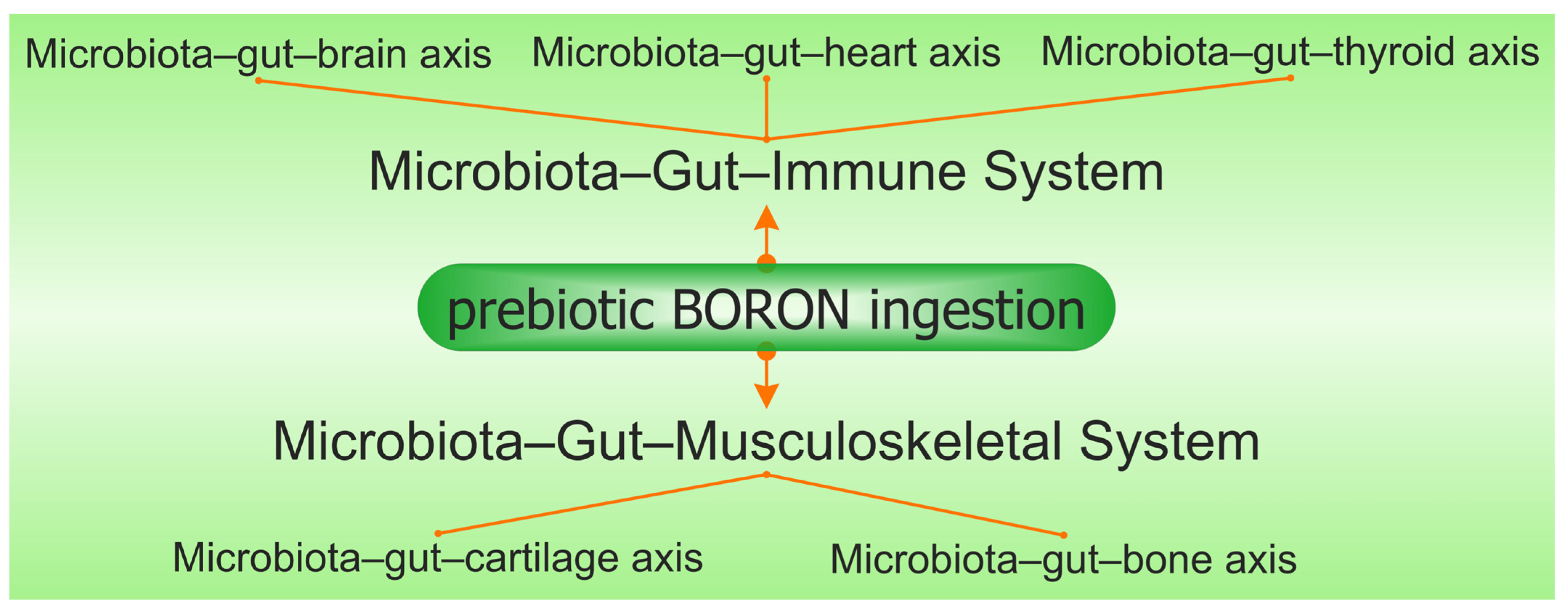
Figure 2. The synergy involving prebiotic boron, the microbiota–gut–immune system, and the microbiota–gut–musculoskeletal system is mediated via the microbiota–gut–organ axis.
References
- Taylor, S.S.; Noor, N.; Urits, I.; Paladini, A.; Sadhu, M.S.; Gibb, C.; Carlson, T.; Myrcik, D.; Varrassi, G.; Viswanath, O. Complex regional pain syndrome: A comprehensive review. Pain Ther. 2021, 10, 875–892.
- Crock, L.W.; Baldridge, M.T. A role for the microbiota in complex regional pain syndrome? Neurobiol. Pain 2020, 8, 100054.
- Birklein, F.; Ajit, S.K.; Goebel, A.; Perez, R.S.G.M.; Sommer, C. Complex regional pain syndrome—Phenotypic characteristics and potential biomarkers. Nat. Rev. Neurol. 2018, 14, 272–284.
- Birklein, F.; Dimova, V. Complex regional pain syndrome—Up-to-date. Pain Rep. 2017, 2, e624.
- Mekhail, N.; Deer, T.R.; Poree, L.; Staats, P.S.; Burton, A.W.; Connolly, A.T.; Karst, E.; Mehanny, D.S.; Saweris, Y.; Levy, R.M. Cost-effectiveness of dorsal root ganglion stimulation or spinal cord stimulation for complex regional pain syndrome. Neuromodulation 2021, 24, 708–718.
- Li, W.W.; Guo, T.Z.; Shi, X.; Birklein, F.; Schlereth, T.; Kingery, W.S.; Clark, J.D. Neuropeptide regulation of adaptive immunity in the tibia fracture model of complex regional pain syndrome. J. Neuroinflamm. 2018, 15, 105.
- Dirckx, M.; Stronks, D.L.; van Bodegraven-Hof, E.A.M.; Wesseldijk, F.; Groeneweg, J.G.; Huygen, F.J.P.M. Inflammation in cold complex regional pain syndrome. Acta Anaesthesiol. Scand. 2015, 59, 733–739.
- Sommer, C.; Leinders, M.; Üçeyler, N. Inflammation in the pathophysiology of neuropathic pain. Pain 2018, 159, 595–602.
- Baronio, M.; Sadia, H.; Paolacci, S.; Prestamburgo, D.; Miotti, D.; Guardamagna, V.A.; Natalini, G.; Sullivan, S.G.B.; Bertelli, M. Molecular aspects of regional pain syndrome. Pain Res. Manag. 2020, 2020, 7697214.
- Wehmeyer, C.; Pap, T.; Buckley, C.D.; Naylor, A.J. The role of stromal cells in inflammatory bone loss. Clin. Exp. Immunol. 2017, 189, 1–11.
- Littlejohn, G. Neurogenic neuroinflammation in fibromyalgia and complex regional pain syndrome. Nat. Rev. Rheumatol. 2015, 11, 639–648.
- Tonelli Enrico, V.; Vo, N.; Methe, B.; Morris, A.; Sowa, G. An unexpected connection: A narrative review of the associations between gut microbiome and musculoskeletal pain. Eur. Spine J. 2022, 31, 3603–3615.
- Dworsky-Fried, Z.; Kerr, B.J.; Taylor, A.M.W. Microbes, microglia, and pain. Neurobiol. Pain 2020, 7, 100045.
- Guo, T.Z.; Wei, T.; Tajerian, M.; Clark, J.D.; Birklein, F.; Goebel, A.; Li, W.W.; Sahbaie, P.; Escolano, F.L.; Herrnberger, M.; et al. Complex regional pain syndrome patient immunoglobulin M has pronociceptive effects in the skin and spinal cord of tibia fracture mice. Pain 2020, 161, 797–809.
- Luczynski, P.; Tramullas, M.; Viola, M.; Shanahan, F.; Clarke, G.; O’Mahony, S.; Dinan, T.G.; Cryan, J.F. Microbiota regulates visceral pain in the mouse. eLife 2017, 6, e25887.
- Ramakrishna, C.; Corleto, J.; Ruegger, P.M.; Logan, G.D.; Peacock, B.B.; Mendonca, S.; Yamaki, S.; Adamson, T.; Ermel, R.; McKemy, D.; et al. Dominant role of the gut microbiota in chemotherapy induced neuropathic pain. Sci. Rep. 2019, 9, 20324.
- Shen, S.; Lim, G.; You, Z.; Ding, W.; Huang, P.; Ran, C.; Doheny, J.; Caravan, P.; Tate, S.; Hu, K.; et al. Gut microbiota is critical for the induction of chemotherapy-induced pain. Nat. Neurosci. 2017, 20, 1213–1216.
- Yang, C.; Fang, X.; Zhan, G.; Huang, N.; Li, S.; Bi, J.; Jiang, R.; Yang, L.; Miao, L.; Zhu, B.; et al. Key role of gut microbiota in anhedonia-like phenotype in rodents with neuropathic pain. Transl. Psychiatry 2019, 9, 57.
- Guo, T.Z.; Shi, X.; Li, W.W.; Wei, T.; Clark, J.D.; Kingery, W.S. Passive transfer autoimmunity in a mouse model of complex regional pain syndrome. Pain 2017, 158, 2410–2421.
- Clos-Garcia, M.; Andrés-Marin, N.; Fernández-Eulate, G.; Abecia, L.; Lavín, J.L.; van Liempd, S.; Cabrera, D.; Royo, F.; Valero, A.; Errazquin, N.; et al. Gut microbiome and serum metabolome analyses identify molecular biomarkers and altered glutamate metabolism in fibromyalgia. eBioMedicine 2019, 46, 499–511.
- Boer, C.G.; Radjabzadeh, D.; Medina-Gomez, C.; Garmaeva, S.; Schiphof, D.; Arp, P.; Koet, T.; Kurilshikov, A.; Fu, J.; Ikram, M.A.; et al. Intestinal microbiome composition and its relation to joint pain and inflammation. Nat. Commun. 2019, 10, 4881.
- Ware, M.A.; Bennett, G.J. Case report: Long-standing complex regional pain syndrome relieved by a cephalosporin antibiotic. Pain 2014, 155, 1412–1415.
- Weinstock, L.B.; Myers, T.L.; Walters, A.S.; Schwartz, O.A.; Younger, J.W.; Chopra, P.J.; Guarino, A.H. Identification and treatment of new inflammatory triggers for complex regional pain syndrome. A A Case Rep. 2016, 6, 272–276.
- Vasquez, A. Neuroinflammation in fibromyalgia and CRPS is multifactorial. Nat. Rev. Rheumatol. 2016, 12, 242.
- Pimentel, M.; Wallace, D.; Hallegua, D.; Chow, E.; Kong, Y.; Park, S.; Lin, H.C. A link between irritable bowel syndrome and fibromyalgia may be related to findings on lactulose breath testing. Ann. Rheum. Dis. 2004, 63, 450–452.
- MacDonald, T.T.; Monteleone, G. Immunity, inflammation, and allergy in the gut. Science 2005, 307, 1920–1925.
- Holden, W.; Orchard, T.; Wordsworth, P. Enteropathic arthritis. Rheum. Dis. Clin. N. Am. 2003, 29, 513–530.
- Maes, M.; Mihaylova, I.; Leunis, J.C. Increased serum IgA and IgM against LPS of enterobacteria in chronic fatigue syndrome (CFS): Indication for the involvement of Gram-negative enterobacteria in the etiology of CFS and for the presence of an increased gut–intestinal permeability. J. Affect. Disord. 2007, 99, 237–240.
- Üçeyler, N.; Valenza, R.; Stock, M.; Schedel, R.; Sprotte, G.; Sommer, C. Reduced levels of antiinflammatory cytokines in patients with chronic widespread pain. Arthritis Rheum. 2006, 54, 2656–2664.
- Üçeyler, N.; Eberle, T.; Rolke, R.; Birklein, F.; Sommer, C. Differential expression patterns of cytokines in complex regional pain syndrome. Pain 2007, 132, 195–205.
- Wallace, D.J.; Linker-Israeli, M.; Hallegua, D.; Silverman, S.; Silver, D.; Weisman, M.H. Cytokines play an aetiopathogenetic role in fibromyalgia: A hypothesis and pilot study. Rheumatology 2001, 40, 743–749.
- Hart, R.P.; Wade, J.B.; Martelli, M.F. Cognitive impairment in patients with chronic pain: The significance of stress. Curr. Pain Headache Rep. 2003, 7, 116–126.
- Wallon, C.; Yang, P.C.; Keita, A.V.; Ericson, A.C.; McKay, D.M.; Sherman, P.M.; Perdue, M.H.; Söderholm, J.D. Corticotropin-releasing hormone (CRH) regulates macromolecular permeability via mast cells in normal human colonic biopsies in vitro. Gut 2008, 57, 50–58.
- Buret, A.G. How stress induces intestinal hypersensitivity. Am. J. Pathol. 2006, 168, 3–5.
- Goebel, A.; Buhner, S.; Schedel, R.; Lochs, H.; Sprotte, G. Altered intestinal permeability in patients with primary fibromyalgia and in patients with complex regional pain syndrome. Rheumatology 2008, 47, 1223–1227.
- Turkez, H.; Arslan, M.E.; Tatar, A.; Mardinoglu, A. Promising potential of boron compounds against glioblastoma: In vitro antioxidant, anti-inflammatory and anticancer studies. Neurochem. Int. 2021, 149, 105137.
- Ahlawat, S.; Asha; Sharma, K.K. Gut–organ axis: A microbial outreach and networking. Lett. Appl. Microbiol. 2021, 72, 636–668.
- Córdova-Chávez, R.I.; Carrasco-Ruiz, M.F.; Rodríguez-Vera, D.; Pérez-Capistran, T.; Tamay-Cach, F.; Scorei, I.R.; Abad-García, A.; Soriano-Ursúa, M.A. Boron-containing compounds for prevention, diagnosis, and treatment of human metabolic disorders. Biol. Trace Elem. Res. 2023, 201, 2222–2239.
- Sizmaz, O.; Koksal, B.H.; Yildiz, G. Rumen microbial fermentation, protozoan abundance and boron availability in yearling rams fed diets with different boron concentrations. J. Anim. Feed Sci. 2017, 26, 59–64.
- Mayorga-Ramos, A.; Barba-Ostria, C.; Simancas-Racines, D.; Guamán, L.P. Protective role of butyrate in obesity and diabetes: New insights. Front. Nutr. 2022, 9, 1067647.
- Ji, Y.C.; Sun, Q.; Fu, C.Y.; She, X.; Liu, X.C.; He, Y.; Ai, Q.; Li, L.Q.; Wang, Z.L. Exogenous autoinducer-2 rescues intestinal dysbiosis and intestinal inflammation in a neonatal mouse necrotizing enterocolitis model. Front. Cell. Infect. Microbiol. 2021, 11, 694395.
- Bivar Xavier, K. Bacterial interspecies quorum sensing in the mammalian gut microbiota. Comptes Rendus Biol. 2018, 341, 297–299.
- Zhu, L.B.; Zhang, Y.C.; Huang, H.H.; Lin, J. Prospects for clinical applications of butyrate-producing bacteria. World J. Clin. Pediatr. 2021, 10, 84–92.
- Hsiao, A.; Ahmed, A.M.S.; Subramanian, S.; Griffin, N.W.; Drewry, L.L.; Petri, W.A., Jr.; Haque, R.; Ahmed, T.; Gordon, J.I. Members of the human gut microbiota involved in recovery from Vibrio cholerae infection. Nature 2014, 515, 423–426.
- Chen, J.; Vitetta, L. The role of butyrate in attenuating pathobiont-induced hyperinflammation. Immune Netw. 2020, 20, e15.
- Rettner, R.E.; Saier, M.H., Jr. The autoinducer-2 exporter superfamily. J. Mol. Microbiol. Biotechnol. 2010, 18, 195–205.
- Amiri, P.; Arefhosseini, S.; Bakhshimoghaddam, F.; Jamshidi Gurvan, H.; Hosseini, S.A. Mechanistic insights into the pleiotropic effects of butyrate as a potential therapeutic agent on NAFLD management: A systematic review. Front. Nutr. 2022, 9, 1037696.
- Bridgeman, S.C.; Northrop, W.; Melton, P.E.; Ellison, G.C.; Newsholme, P.; Mamotte, C.D.S. Butyrate generated by gut microbiota and its therapeutic role in metabolic syndrome. Pharmacol. Res. 2020, 160, 105174.
- Jourova, L.; Anzenbacherova, E.; Dostal, Z.; Anzenbacher, P.; Briolotti, P.; Rigal, E.; Daujat-Chavanieu, M.; Gerbal-Chaloin, S. Butyrate, a typical product of gut microbiome, affects function of the AhR gene, being a possible agent of crosstalk between gut microbiome, and hepatic drug metabolism. J. Nutr. Biochem. 2022, 107, 109042.
- Kyner, D.; Zabos, P.; Christman, J.; Acs, G. Effect of sodium butyrate on lymphocyte activation. J. Exp. Med. 1976, 144, 1674–1678.
- Kibbie, J.J.; Dillon, S.M.; Thompson, T.A.; Purba, C.M.; McCarter, M.D.; Wilson, C.C. Butyrate directly decreases human gut lamina propria CD4 T cell function through histone deacetylase (HDAC) inhibition and GPR43 signaling. Immunobiology 2021, 226, 152126.
- Siddiqui, M.T.; Cresci, G.A.M. The immunomodulatory functions of butyrate. J. Inflamm. Res. 2021, 14, 6025–6041.
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front. Immunol. 2019, 10, 277.
- Barbosa, S.J.A.; Oliveira, M.M.B.; Ribeiro, S.B.; de Medeiros, C.A.C.X.; Lima, M.L.S.; Guerra, G.C.B.; de Araújo Júnior, R.F.; de Sousa Junior, F.C.; Martins, A.A.; Paiva, D.F.F.; et al. The beneficial effects of Lacticaseibacillus casei on the small intestine and colon of Swiss mice against the deleterious effects of 5-fluorouracil. Front. Immunol. 2022, 13, 954885.
- Duncan, S.H.; Holtrop, G.; Lobley, G.E.; Calder, A.G.; Stewart, C.S.; Flint, H.J. Contribution of acetate to butyrate formation by human faecal bacteria. Br. J. Nutr. 2004, 91, 915–923.
- Van den Abbeele, P.; Belzer, C.; Goossens, M.; Kleerebezem, M.; De Vos, W.M.; Thas, O.; De Weirdt, R.; Kerckhof, F.M.; Van de Wiele, T. Butyrate-producing Clostridium cluster XIVa species specifically colonize mucins in an in vitro gut model. ISME J. 2013, 7, 949–961.
- Corrêa, T.A.F.; Rogero, M.M.; Hassimotto, N.M.A.; Lajolo, F.M. The two-way polyphenols–microbiota interactions and their effects on obesity and related metabolic diseases. Front. Nutr. 2019, 6, 188.
- Wang, X.; Qi, Y.; Zheng, H. Dietary polyphenol, gut microbiota, and health benefits. Antioxidants 2022, 11, 1212.
- Schlechte, J.; Zucoloto, A.Z.; Yu, I.L.; Doig, C.J.; Dunbar, M.J.; McCoy, K.D.; McDonald, B. Dysbiosis of a microbiota–immune metasystem in critical illness is associated with nosocomial infections. Nat. Med. 2023, 29, 1017–1027.
- Sizmaz, Ö.; Köksal, B.H.; Yildiz, G. Rumen fermentation characteristics of rams fed supplemental boric acid and humic acid diets. Ankara Üniv. Vet. Fak. Derg. 2022, 69, 337–340.
- Özkoç, M.; Can, B.; Şentürk, H.; Burukoğlu Dönmez, D.; Kanbak, G. Possible curative effects of boric acid and Bacillus clausii treatments on TNBS-induced ulcerative colitis in rats. Biol. Trace Elem. Res. 2023, 201, 1237–1251.
- Thompson, J.A.; Oliveira, R.A.; Djukovic, A.; Ubeda, C.; Xavier, K.B. Manipulation of the quorum sensing signal AI-2 affects the antibiotic-treated gut microbiota. Cell Rep. 2015, 10, 1861–1871.
- Thompson, J.A.; Oliveira, R.A.; Xavier, K.B. Chemical conversations in the gut microbiota. Gut Microbes 2016, 7, 163–170.
- Catalkaya, G.; Venema, K.; Lucini, L.; Rocchetti, G.; Delmas, D.; Daglia, M.; De Filippis, A.; Xiao, H.; Quiles, J.L.; Xiao, J.; et al. Interaction of dietary polyphenols and gut microbiota: Microbial metabolism of polyphenols, influence on the gut microbiota, and implications on host health. Food Front. 2020, 1, 109–133.
- Biţă, A.; Scorei, I.R.; Rangavajla, N.; Bejenaru, L.E.; Rău, G.; Bejenaru, C.; Ciocîlteu, M.V.; Dincă, L.; Neamţu, J.; Bunaciu, A.; et al. Diester chlorogenoborate complex: A new naturally occurring boron-containing compound. Inorganics 2023, 11, 112.
More
