Fungal pathogens pose a major threat to food production worldwide. Traditionally, chemical fungicides have been the primary means of controlling these pathogens, but many of these fungicides have recently come under increased scrutiny due to their negative effects on the health of humans, animals, and the environment. Furthermore, the use of chemical fungicides can result in the development of resistance in populations of phytopathogenic fungi. Therefore, new environmentally friendly alternatives that provide adequate levels of disease control are needed to replace chemical fungicides—if not completely, then at least partially. A number of alternatives to conventional chemical fungicides have been developed, including plant defence elicitors (PDEs); biological control agents (fungi, bacteria, and mycoviruses), either alone or as consortia; biochemical fungicides; natural products; RNA interference (RNAi) methods; and resistance breeding.
- plant defence elicitors
- fungal disease management
- plants
1. Introduction
1.1. Chemical Fungicides
1.2. The Disadvantages of Chemical Fungicides: Environmental Toxicity and Resistance Development
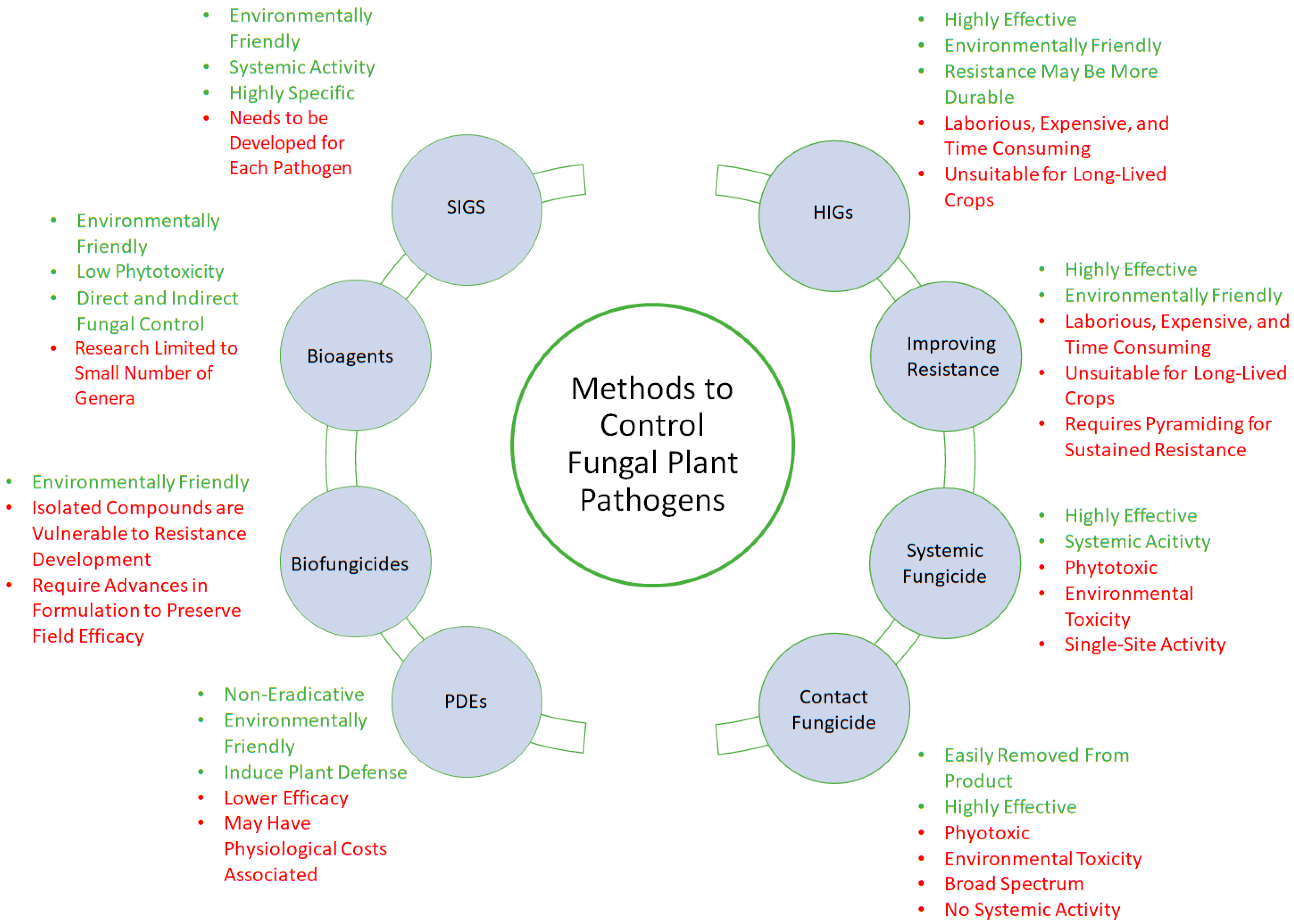
2. Alternative Management of Fungal Diseases
2.1. Agronomic Practices and Cultivation Methods
2.2. Improving Plants’ Genetic Resistance through the Use of R and S Genes
References
- Gullino, M.; Leroux, P.; Smith, C. Uses and challenges of novel compounds for plant disease control. Crop Prot. 2000, 19, 1–11.
- Kelman, A. Introduction: The importance of research on the control of postharvest diseases of perishable food crops. Phytopathology 1989, 79, 1374.
- Ragsdale, N.N.; Sisler, H.D. Social and political implications of managing plant diseases with decreased availability of fungicides in the United States. Annu. Rev. Phytopathol. 1994, 32, 545–557.
- Steinhauer, N.; Kulhanek, K.; Antúnez, K.; Human, H.; Chantawannakul, P.; Chauzat, M.P.; vanEngelsdorp, D. Drivers of colony losses. Curr. Opin. Insect. Sci. 2018, 26, 142–148.
- Millardet, P.M.A. The Discovery of Bordeaux Mixture; The American Phytopathological Society: St. Paul, MN, USA, 2018.
- Baibakova, E.; Nefedjeva, E.; Suska-Malawska, M.; Wilk, M.; Sevriukova, G.; Zheltobriukhov, V. Modern Fungicides: Mechanisms of Action, Fungal Resistance and Phytotoxic Effects. Annu. Res. Rev. Biol. 2019, 32, 1–16.
- Tamm, L.; Thuerig, B.; Apostolov, S.; Blogg, H.; Borgo, E.; Corneo, P.E.; Fittje, S.; de Palma, M.; Donko, A.; Experton, C.; et al. Use of Copper-Based Fungicides in Organic Agriculture in Twelve European Countries. Agronomy 2022, 12, 673.
- Lamichhane, J.R.; Osdaghi, E.; Behlau, F.; Köhl, J.; Jones, J.B.; Aubertot, J.-N. Thirteen decades of antimicrobial copper compounds applied in agriculture. A review. Agron. Sustain. Dev. 2018, 38, 28.
- Karuppuchamy, P.; Venugopal, S. Chapter 21—Integrated Pest Management. In Ecofriendly Pest Management for Food Security; Omkar, Ed.; Academic Press: San Diego, CA, USA, 2016; pp. 651–684.
- Anna La, T.; Valeria, I.; Federica, C. Copper in plant protection: Current situation and prospects. Phytopathol. Mediterr. 2018, 57, 201–236.
- Oziengbe, E.O.; Osazee, J.O. Antifungal Activity of Copper Sulphate Against Colletotrichum Gloeosporioides. J. Asian Sci. Res. 2012, 2, 835–839.
- Oliver, R.; Hewitt, H.G. Fungicides in Crop Protection: Second Edition; CABI: Egham, UK, 2014; pp. 1–190.
- Dias, M. Phytotoxicity: An Overview of the Physiological Responses of Plants Exposed to Fungicides. J. Bot. 2012, 2012, 135479.
- Petit, A.N.; Fontaine, F.; Vatsa, P.; Clément, C.; Vaillant-Gaveau, N. Fungicide impacts on photosynthesis in crop plants. Photosynth. Res. 2012, 111, 315–326.
- Kromann, P.; Taipe, A.; Perez, W.; Forbes, G. Rainfall Thresholds as Support for Timing Fungicide Applications in the Control of Potato Late Blight in Ecuador and Peru. Plant Dis. 2009, 93, 142–148.
- Vicent, A.; Armengol, J.; García-Jiménez, J. Rain Fastness and Persistence of Fungicides for Control of Alternaria Brown Spot of Citrus. Plant Dis. 2007, 91, 393–399.
- Garcia, P.; Rivero, R.; Ruiz, J.; Romero, L. The Role of Fungicides in the Physiology of Higher Plants: Implications for Defense Responses. Bot. Rev. 2003, 69, 162–172.
- Klittich, C.J.R. Fungicide Mobility and the Influence of Physical Properties. In Retention, Uptake, and Translocation of Agrochemicals in Plants; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2014; Volume 1171, pp. 95–109.
- Klittich, C.J.; Ray, S.L. Effects of physical properties on the translaminar activity of fungicides. Pestic. Biochem. Physiol. 2013, 107, 351–359.
- Warneke, B.; Thiessen, L.; Mahaffee, W. Effect of Fungicide Mobility and Application Timing on the Management of Grape Powdery Mildew. Plant Dis. 2019, 104, 1167–1174.
- Ayesha, M.S.; Suryanarayanan, T.S.; Nataraja, K.N.; Prasad, S.R.; Shaanker, R.U. Seed Treatment With Systemic Fungicides: Time for Review. Front. Plant Sci. 2021, 12, 654512.
- Deising, H.; Reimann, S.; Pascholati, S. Mechanisms and significance of fungicide resistance. Braz. J. Microbiol. Publ. Braz. Soc. Microbiol. 2008, 39, 286–295.
- Mohandoss, J.; Suryanarayanan, T. Effect of fungicide treatment on foliar fungal endophyte diversity in mango. Sydowia 2009, 61, 11–24.
- Leyronas, C.; Mériaux, B.; Raynal, G. Chemical Control of Neotyphodium spp. Endophytes in Perennial Ryegrass and Tall Fescue Seeds. Crop Sci. 2006, 46, 98–104.
- Kalia, A.; Gosal, S.K. Effect of pesticide application on soil microorganisms. Arch. Agron. Soil Sci. 2011, 57, 569–596.
- Murphy, B.R.; Doohan, F.M.; Hodkinson, T.R. A seed dressing combining fungal endophyte spores and fungicides improves seedling survival and early growth in barley and oat. Symbiosis 2017, 71, 69–76.
- Lloyd, A.W.; Percival, D.; Yurgel, S.N. Effect of Fungicide Application on Lowbush Blueberries Soil Microbiome. Microorganisms 2021, 9, 1366.
- Lloyd, A.W.; Percival, D.; Langille, M.G.I.; Yurgel, S.N. Changes to Soil Microbiome Resulting from Synergetic Effects of Fungistatic Compounds Pyrimethanil and Fluopyram in Lowbush Blueberry Agriculture, with Nine Fungicide Products Tested. Microorganisms 2023, 11, 410.
- Kahle, M.; Buerge, I.J.; Hauser, A.; Müller, M.D.; Poiger, T. Azole Fungicides: Occurrence and Fate in Wastewater and Surface Waters. Environ. Sci. Technol. 2008, 42, 7193–7200.
- Bereswill, R.; Golla, B.; Streloke, M.; Schulz, R. Entry and toxicity of organic pesticides and copper in vineyard streams: Erosion rills jeopardise the efficiency of riparian buffer strips. Agric. Ecosyst. Environ. 2012, 146, 81–92.
- Zubrod, J.P.; Bundschuh, M.; Arts, G.; Brühl, C.A.; Imfeld, G.; Knäbel, A.; Payraudeau, S.; Rasmussen, J.J.; Rohr, J.; Scharmüller, A.; et al. Fungicides: An Overlooked Pesticide Class? Environ. Sci. Technol. 2019, 53, 3347–3365.
- Kiaune, L.; Singhasemanon, N. Pesticidal copper (I) oxide: Environmental fate and aquatic toxicity. Rev. Env. Contam. Toxicol. 2011, 213, 1–26.
- Belsky, J.; Joshi, N.K. Effects of Fungicide and Herbicide Chemical Exposure on Apis and Non-Apis Bees in Agricultural Landscape. Front. Environ. Sci. 2020, 8, 81.
- Weisenburger, D.D. Human health effects of agrichemical use. Hum. Pathol. 1993, 24, 571–576.
- Habig, M.; Lorrain, C.; Feurtey, A.; Komluski, J.; Stukenbrock, E.H. Epigenetic modifications affect the rate of spontaneous mutations in a pathogenic fungus. Nat. Commun. 2021, 12, 5869.
- Hermann, D.; Stenzel, K. FRAC Mode-of-action Classification and Resistance Risk of Fungicides. In Modern Crop Protection Compounds; Wiley-VCH: Hoboken, NJ, USA, 2019; pp. 589–608.
- Katan, J. Cultural approaches for disease management: Present status and future prospects. J. Plant Pathol. 2010, 92, S7–S9.
- Conway, K.E. An overview of the influence of sustainable agricultural systems on plant diseases. Crop Prot. 1996, 15, 223–228.
- Palti, J. Cultural Practices and Infectious Crop Diseases; Springer Science & Business Media: Berlin, Germany, 2012; Volume 9.
- Fischer, I.H.; Soares-Colletti, A.R.; Palharini, M.C.d.A.; Parisi, M.C.M.; Amorim, L. Temporal progress and spatial patterns of quiescent diseases in guava influenced by sanitation practices. Sci. Agric. 2017, 74, 68–76.
- Vincent, C.; Rancourt, B.; Carisse, O. Apple leaf shredding as a non-chemical tool to manage apple scab and spotted tentiform leafminer. Agric. Ecosyst. Environ. 2004, 104, 595–604.
- Mertely, J.C.; Chandler, C.K.; Xiao, C.L.; Legard, D.E. Comparison of Sanitation and Fungicides for Management of Botrytis Fruit Rot of Strawberry. Plant Dis. 2000, 84, 1197–1202.
- Meitz-Hopkins, J.C.; von Diest, S.G.; Koopman, T.A.; Tobutt, K.R.; Xu, X.; Lennox, C.L. Leaf shredding as an alternative strategy for managing apple scab resistance to demethylation inhibitor fungicides. Front. Hortic. 2023, 2, 1175168.
- Sturz, A.; Carter, M.; Johnston, H. A review of plant disease, pathogen interactions and microbial antagonism under conservation tillage in temperate humid agriculture. Soil Tillage Res. 1997, 41, 169–189.
- Page, K.; Dang, Y.; Dalal, R. Impacts of conservation tillage on soil quality, including soil-borne crop diseases, with a focus on semi-arid grain cropping systems. Australas. Plant Pathol. 2013, 42, 363–377.
- Palojärvi, A.; Kellock, M.; Parikka, P.; Jauhiainen, L.; Alakukku, L. Tillage System and Crop Sequence Affect Soil Disease Suppressiveness and Carbon Status in Boreal Climate. Front. Microbiol. 2020, 11, 534786.
- Kerdraon, L.; Laval, V.; Suffert, F. Microbiomes and Pathogen Survival in Crop Residues, an Ecotone Between Plant and Soil. Phytobiomes J. 2019, 3, 246–255.
- Bockus, W.W.; Shroyer, J.P. The impact of reduced tillage on soilborne plant pathogens. Annu. Rev. Phytopathol. 1998, 36, 485–500.
- Bziuk, N.; Maccario, L.; Douchkov, D.; Lueck, S.; Babin, D.; Sørensen, S.J.; Schikora, A.; Smalla, K. Tillage shapes the soil and rhizosphere microbiome of barley—But not its susceptibility towards Blumeria graminis f. sp. hordei. FEMS Microbiol. Ecol. 2021, 97, fiab018.
- Li, Y.; Wang, Z.; Li, T.; Zhao, D.; Han, J.; Liao, Y. Wheat rhizosphere fungal community is affected by tillage and plant growth. Agric. Ecosyst. Environ. 2021, 317, 107475.
- Ma, Z.; Guan, Z.; Liu, Q.; Hu, Y.; Liu, L.; Wang, B.; Huang, L.; Li, H.; Yang, Y.; Han, M.; et al. Chapter Four—Obstacles in continuous cropping: Mechanisms and control measures. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2023; Volume 179, pp. 205–256.
- Bennett, A.J.; Bending, G.D.; Chandler, D.; Hilton, S.; Mills, P. Meeting the demand for crop production: The challenge of yield decline in crops grown in short rotations. Biol. Rev. Camb. Philos. Soc. 2012, 87, 52–71.
- Scholte, K. Effect of crop rotation on the incidence of soil-borne fungal diseases of potato. Neth. J. Plant Pathol. 1992, 98, 93–101.
- Yuan, X.; Wang, B.; Hong, S.; Xiong, W.; Shen, Z.; Ruan, Y.; Li, R.; Shen, Q.; Dini-Andreote, F. Promoting soil microbial-mediated suppressiveness against Fusarium wilt disease by the enrichment of specific fungal taxa via crop rotation. Biol. Fertil. Soils 2021, 57, 1137–1153.
- Vargas Gil, S.; Meriles, J.M.; Haro, R.; Casini, C.; March, G.J. Crop rotation and tillage systems as a proactive strategy in the control of peanut fungal soilborne diseases. BioControl 2008, 53, 685–698.
- Lemańczyk, G.; Wilczewski, E.; Węglarz, W. Effect of catch crop and type of ploughed-in biomass on the health status of stem base and roots of spring wheatOddziaływanie międzyplonów ścierniskowych i rodzaju przyoranej biomasy na zdrowotność podstawy źdźbła i korzeni pszenicy jarej. Prog. Plant Prot. 2016, 56, 19–24.
- Trinchera, A.; Migliore, M.; Warren Raffa, D.; Ommeslag, S.; Debode, J.; Shanmugam, S.; Dane, S.; Babry, J.; Kivijarvi, P.; Kristensen, H.L.; et al. Can multi-cropping affect soil microbial stoichiometry and functional diversity, decreasing potential soil-borne pathogens? A study on European organic vegetable cropping systems. Front. Plant Sci. 2022, 13, 952910.
- Schoeny, A.; Menat, J.; Darsonval, A.; Rouault, F.; Jumel, S.; Tivoli, B. Effect of pea canopy architecture on splash dispersal of Mycosphaerella pinodes conidia. Plant Pathol. 2008, 57, 1073–1085.
- Yang, X.; TeBeest, D. Rain dispersal of Colletotrichum gloeosporioides in simulated rice field conditions. Phytopathology 1992, 82, 1219–1222.
- Yang, X.; Madden, L.; Wilson, L.; Ellis, M. Effects of surface topography and rain intensity on splash dispersal of Colletotrichum acutatum. Phytopathology 1990, 80, 1115–1120.
- Richard, B.; Bussière, F.; Langrume, C.; Rouault, F.; Jumel, S.; Faivre, R.; Tivoli, B. Effect of pea canopy architecture on microclimate and consequences on ascochyta blight infection under field conditions. Eur. J. Plant Pathol. 2013, 135, 509–524.
- Simon, S.; Lauri, P.E.; Brun, L.; Defrance, H.; Sauphanor, B. Does manipulation of fruit-tree architecture affect the development of pests and pathogens? A case study in an organic apple orchard. J. Hortic. Sci. Biotechnol. 2006, 81, 765–773.
- Vidal, T.; Boixel, A.-L.; Durand, B.; de Vallavieille-Pope, C.; Huber, L.; Saint-Jean, S. Reduction of fungal disease spread in cultivar mixtures: Impact of canopy architecture on rain-splash dispersal and on crop microclimate. Agric. For. Meteorol. 2017, 246, 154–161.
- Bent, A.F.; Mackey, D. Elicitors, effectors, and R genes: The new paradigm and a lifetime supply of questions. Annu. Rev. Phytopathol. 2007, 45, 399–436.
- Jones, J.D.; Vance, R.E.; Dangl, J.L. Intracellular innate immune surveillance devices in plants and animals. Science 2016, 354, aaf6395.
- Shao, Z.-Q.; Xue, J.-Y.; Wu, P.; Zhang, Y.-M.; Wu, Y.; Hang, Y.-Y.; Wang, B.; Chen, J.-Q. Large-Scale Analyses of Angiosperm Nucleotide-Binding Site-Leucine-Rich Repeat Genes Reveal Three Anciently Diverged Classes with Distinct Evolutionary Patterns. Plant Physiol. 2016, 170, 2095–2109.
- Shao, F.; Golstein, C.; Ade, J.; Stoutemyer, M.; Dixon, J.E.; Innes, R.W. Cleavage of Arabidopsis PBS1 by a bacterial type III effector. Science 2003, 301, 1230–1233.
- Chisholm, S.T.; Coaker, G.; Day, B.; Staskawicz, B.J. Host-Microbe Interactions: Shaping the Evolution of the Plant Immune Response. Cell 2006, 124, 803–814.
- Mur, L.A.J.; Kenton, P.; Lloyd, A.J.; Ougham, H.; Prats, E. The hypersensitive response; the centenary is upon us but how much do we know? J. Exp. Bot. 2008, 59, 501–520.
- Liu, P.-P.; Yang, Y.; Pichersky, E.; Klessig, D.F. Altering Expression of Benzoic Acid/Salicylic Acid Carboxyl Methyltransferase 1 Compromises Systemic Acquired Resistance and PAMP-Triggered Immunity in Arabidopsis. Mol. Plant-Microbe Interact. 2010, 23, 82–90.
- Tsuda, K.; Sato, M.; Stoddard, T.; Glazebrook, J.; Katagiri, F. Network properties of robust immunity in plants. PLoS Genet. 2009, 5, e1000772.
- Kaur, B.; Bhatia, D.; Mavi, G.S. Eighty years of gene-for-gene relationship and its applications in identification and utilization of R genes. J. Genet. 2021, 100, 50.
- Khajuria, Y.P.; Kaul, S.; Wani, A.A.; Dhar, M.K. Genetics of resistance in apple against Venturia inaequalis (Wint.) Cke. Tree Genet. Genomes 2018, 14, 16.
- Kou, Y.; Wang, S. Broad-spectrum and durability: Understanding of quantitative disease resistance. Curr. Opin. Plant Biol. 2010, 13, 181–185.
- Ashfield, T.; Ong, L.E.; Nobuta, K.; Schneider, C.M.; Innes, R.W. Convergent evolution of disease resistance gene specificity in two flowering plant families. Plant Cell 2004, 16, 309–318.
- Stahl, E.A.; Dwyer, G.; Mauricio, R.; Kreitman, M.; Bergelson, J. Dynamics of disease resistance polymorphism at the Rpm1 locus of Arabidopsis. Nature 1999, 400, 667–671.
- Rimbaud, L.; Papaïx, J.; Barrett, L.G.; Burdon, J.J.; Thrall, P.H. Mosaics, mixtures, rotations or pyramiding: What is the optimal strategy to deploy major gene resistance? Evol. Appl. 2018, 11, 1791–1810.
- Mundt, C.C. Durable resistance: A key to sustainable management of pathogens and pests. Infect. Genet. Evol. 2014, 27, 446–455.
- Pandolfi, V.; Neto, J.; da Silva, M.D.; Amorim, L.L.B.; Wanderley-Nogueira, A.C.; de Oliveira Silva, R.L.; Kido, E.A.; Crovella, S.; Iseppon, A.M.B. Resistance (R) Genes: Applications and Prospects for Plant Biotechnology and Breeding. Curr. Protein Pept. Sci. 2017, 18, 323–334.
- Mundt, C. Pyramiding for Resistance Durability: Theory and Practice. Phytopathology 2018, 108, 792–802.
- Perez, W.; Salas, A.; Raymundo, R.; Huamán, Z.; Nelson, R.; Bonierbale, M. Evaluation of Wild Potato Species for Resistance to Late Blight. CIP Program Rep. 1999, 2000, 49–62.
- Vyska, M.; Cunniffe, N.; Gilligan, C. Trade-off between disease resistance and crop yield: A landscape-scale mathematical modelling perspective. J. R. Soc. Interface 2016, 13, 20160451.
- Zaidi, S.S.; Mukhtar, M.S.; Mansoor, S. Genome Editing: Targeting Susceptibility Genes for Plant Disease Resistance. Trends Biotechnol. 2018, 36, 898–906.
- van Schie, C.C.; Takken, F.L. Susceptibility genes 101: How to be a good host. Annu. Rev. Phytopathol. 2014, 52, 551–581.
- Kieu, N.P.; Lenman, M.; Wang, E.S.; Petersen, B.L.; Andreasson, E. Mutations introduced in susceptibility genes through CRISPR/Cas9 genome editing confer increased late blight resistance in potatoes. Sci. Rep. 2021, 11, 4487.
- Zhou, H.; Bai, S.; Wang, N.; Sun, X.; Zhang, Y.; Zhu, J.; Dong, C. CRISPR/Cas9-Mediated Mutagenesis of MdCNGC2 in Apple Callus and VIGS-Mediated Silencing of MdCNGC2 in Fruits Improve Resistance to Botryosphaeria dothidea. Front. Plant Sci. 2020, 11, 575477.
- Yin, K.; Qiu, J.L. Genome editing for plant disease resistance: Applications and perspectives. Philos. Trans. R. Soc. B 2019, 374, 20180322.
