Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Jessie Wu and Version 3 by Jessie Wu.
Thermal ablation is increasingly being utilized for the management of solid parenchymal tumors, such as hepatocellular cancer, renal tumors, thyroid nodules, and pulmonary tumors. However, its application in the management of pancreas lesions was delayed due to fears of causing iatrogenic thermal injury to the surrounding organs. The initial success of radiofrequency ablation (RFA) in inoperable pancreatic cancers led to its application in pancreatic neuroendocrine tumors and pancreatic cystic neoplasms (PCLs).
- microwave ablation
- thermal ablation
- pancreatic cancer
- neuroendocrine tumors
- pancreatic cystic lesions
- pancreas tumors
1. Introduction
The microwave delivery system consists of a generator, a microwave antenna needle, and a flexible coaxial cable. In Figure 1, the endoscopic ultrasound (EUS)-guided microwave ablation probe is shown with the power generator and EUS-guided Microblate™ Fine Needle (Creo Medical, Chepstow, UK) as an example. The specific power and frequency output of the generator vary according to the manufacturer. The microwave antenna is 15 to 30 cm in length with a 14 to 20-gauge needle. The generator and antenna are connected using a flexible coaxial cable, which may be internally cooled [1].
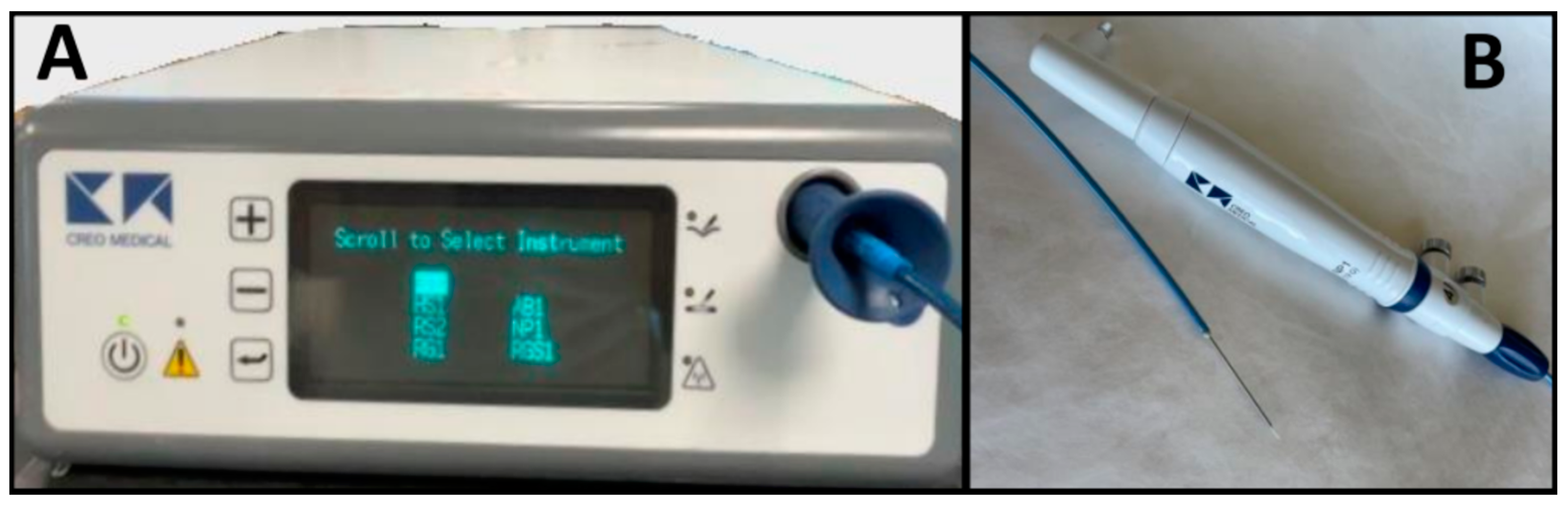
Figure 1. Microblate™ Fine Needle (Creo Medical, Chepstow, UK): microwave delivery system with generator (A) and microwave antenna needle and coaxial cable (B).
There are multiple ways to access the pancreatic lesion for microwave ablation, including the percutaneous, laparotomy, and endoscopic ultrasound (EUS) approaches [2][3][4][5][6]. The choice of approach depends on the operator’s preference/expertise and patient characteristics. The patient’s hemodynamics should be closely monitored throughout the procedure. The postoperative course and length of hospitalization depend on the complexity of the procedure.
The general principles of performing microwave ablation of pancreatic lesions involve the following: (a) Advancing the microwave ablation probe into the lesion until its tip is situated in the center of the lesion [2][7]. When used surgically during laparotomy, a cold, wet gauze can be placed over surrounding tissue to avoid thermal damage. (b) Applying short bursts of microwave energy ranging from 60 s to 10 min at various power wattages ranging from 20 W to 60 W. The output time, power, and frequency are usually recommended by the manufacturer to allow for optimal thermal necrosis of the tissue. Multiple bursts of energy can be applied. (c) Constant monitoring of the ablation zone, which is performed using ultrasound or computed tomography imaging [3][4][8][9][10].
There is a paucity of standardized guidelines for microwave ablation due to its emergence as a novel ablative technique for pancreatic lesions. While microwave ablation is commonly employed for hepatic tumors, a comparative study utilizing two different frequencies (915 MHz vs. 2.45 GHz) demonstrated no differences in ablation outcomes. Nevertheless, variations in parameters such as total ablation time per application, ablation time per lesion, and applied energy were observed. Further research involving microwave ablation for pancreas lesions is imperative to establish the requisite technical specifics and optimal procedural details [11].
2. Radiofrequency Ablation
Numerous studies have evaluated the use of RFA in the management of pancreatic lesions [12]. RFA generates heat through a high-frequency alternative current in the range of 400–500 kHz delivered via a needle electrode to the target tissue, causing coagulative necrosis and apoptosis [13][14]. Similar to the microwave delivery system, the RFA system also consists of a generator and a needle electrode. The RFA system also consists of a grounding pad. Only one RFA system is FDA-approved in the United States: STARmed EUSRA RF (STARmed, Seoul, Republic of Korea). The device consists of an 18- or 19-gauge RFA needle, which is connected to the generator. The needle electrode is 140 cm in length and is completely covered except for the distal segment (5–20 mm in length), which delivers energy through its conical tip. This needle electrode is internally cooled with cold saline to prevent charring on the electrode surface. RFA is susceptible to the heat sink effect, where the heat is absorbed and dissipated by the bloodstream from adjacent structures, thereby reducing the ablative effect [15]. Depending on the target lesion, RFA can be applied via a percutaneous, EUS-guided, or surgical approach.
3. Photodynamic Therapy
Photodynamic therapy is a novel technique with limited clinical usage. Initial studies have reported the technical feasibility and safety of the technique in patients with inoperable pancreatic cancer [16]. During the procedure, a photosensitizer drug is administered intravenously, and multiple animal studies have shown that these photosensitizing drugs preferentially accumulate in malignant pancreatic tissue [17]. Subsequently, the target tissue is exposed to a predetermined wavelength of light that activates the photosensitized drug and causes localized necrosis. The one published study on pancreatic cancer utilized a chlorin e6 derivative (Photolon; Belmedpreparaty, Minsk, Belarus) as the photosensitizing drug [16]. Using an EUS-guided approach, they advanced a flexible laser-light catheter preloaded on a 19-gauge needle into the pancreatic tail to activate the drug and cause necrosis. Future studies should further investigate these photosensitizing drugs, the depth of penetration for various wavelengths of light, and other technical aspects of the procedure.
4. High-Intensity Focused Ultrasound
High-intensity focused ultrasound causes thermal coagulative necrosis in target tissue using a focused ultrasound beam [18]. The high-intensity focused ultrasound system consists of three main parts: I. a special transducer that bundles ultrasound waves into a beam and focuses it at the target tissue; II. a generator; and III. an imaging modality (magnetic resonance imaging or ultrasound) that targets and monitors waves in real time. Depending on the manufacturer of the system, the emitted frequency and penetrable tissue depth can differ. During the procedure, very short bursts of ultrasound energy are delivered repeatedly while the target tissue is constantly monitored via imaging modalities [19]. One benefit of a high-intensity focused ultrasound method is that it is non-invasive and does not require any needles. Many studies have reported the safety and technical feasibility of high-intensity focused ultrasound on pancreatic cancer [20]. However, more studies are needed to evaluate the use of high-intensity focused ultrasound on pancreatic neuroendocrine tumors and PCLs.
5. Cryothermal Ablation
Similar to other thermal ablative techniques, cryothermal ablation relies on the generation of heat and subsequent irreversible cellular damage and coagulative necrosis. However, in addition to this thermal injury, cryothermal ablation induces in situ freezing, vascular injury, and apoptosis by application of a cryogenic gas such as carbon dioxide [21]. The HybridTherm Probe (Erbe Elektromedizin GmbH, Tübingen, Germany) consists of a bipolar RFA device paired with an internal carbon dioxide cooling system used under EUS guidance and has undergone limited clinical investigation [22][23]. Most recently, the system was applied to patients with locally advanced pancreatic adenocarcinoma in conjunction with standard chemotherapy versus chemotherapy alone; however, meaningful conclusions about survival could not be drawn because the study was underpowered [23]. Cryothermal ablation is thought to overcome some of the existing limitations of standard RFA; however, additional investigation is needed.
References
- Seki, T.; Wakabayashi, M.; Nakagawa, T.; Itho, T.; Shiro, T.; Kunieda, K.; Sato, M.; Uchiyama, S.; Inoue, K. Ultrasonically guided percutaneous microwave coagulation therapy for small hepatocellular carcinoma. Cancer 1994, 74, 817–825.
- Carrafiello, G.; Ierardi, A.M.; Fontana, F.; Petrillo, M.; Floridi, C.; Lucchina, N.; Cuffari, S.; Dionigi, G.; Rotondo, A.; Fugazzola, C. Microwave Ablation of Pancreatic Head Cancer: Safety and Efficacy. J. Vasc. Interv. Radiol. 2013, 24, 1513–1520.
- Ierardi, A.M.; Biondetti, P.; Coppola, A.; Fumarola, E.M.; Biasina, A.M.; Alessio Angileri, S.; Carrafiello, G. Percutaneous microwave thermosphere ablation of pancreatic tumours. Gland. Surg. 2018, 7, 59–66.
- D’Amico, F.; Finotti, M.; Di Renzo, C.; Pasquale, A.; Bertacco, A.; Caturegli, G.; Gondolesi, G.E.; Cillo, U. Microwave Thermal Ablation in an Unusual Case of Malignant and Locally Advanced Rare Tumor of Pancreas in ASA IV Old Male Patient and Literature Review. Case Rep. Gastrointest. Med. 2018, 2018, 6064912.
- Lygidakis, N.J.; Sharma, S.K.; Papastratis, P.; Zivanovic, V.; Kefalourous, H.; Koshariya, M.; Lintzeris, I.; Porfiris, T.; Koutsiouroumba, D. Microwave ablation in locally advanced pancreatic carcinoma—A new look. Hepato-gastroenterology 2007, 54, 1305–1310.
- Robles-Medranda, C.; Arevalo-Mora, M.; Oleas, R.; Alcivar-Vasquez, J.; Del Valle, R. Novel EUS-guided microwave ablation of an unresectable pancreatic neuroendocrine tumor. VideoGIE 2022, 7, 74–76.
- Carrafiello, G.; Ierardi, A.M.; Piacentino, F.; Lucchina, N.; Dionigi, G.; Cuffari, S.; Fugazzola, C. Microwave Ablation with Percutaneous Approach for the Treatment of Pancreatic Adenocarcinoma. CardioVascular Interv. Radiol. 2012, 35, 439–442.
- Chen, O.T.; Dojki, F.K.; Weber, S.M.; Hinshaw, J.L. Percutaneous Microwave Ablation of an Insulinoma in a Patient with Refractory Symptomatic Hypoglycemia. J. Gastrointest. Surg. 2015, 19, 1378–1381.
- Murtha, T.D.; Cornman-Homonoff, J.; Ayyagari, R.; Zhang, X.; Salem, R.R. A Novel Treatment for Metastatic Serous Cystadenocarcinoma Using a Microwave Ablation: Case Report and Review of the Literature. Pancreas 2021, 50, 434–440.
- Jayawickreme, K.P.; Muthukuda, D.T.; Kariyawasam, C.; Piyarisi, L.; Abeywickrama, B.A. A rare case of insulinoma presenting with deep vein thrombosis, successfully treated with minimally invasive procedures including microwave ablation. Endocrinol. Diabetes Metab. Case Rep. 2022, 2022, 21–0127.
- Simo, K.A.; Tsirline, V.B.; Sindram, D.; McMillan, M.T.; Thompson, K.J.; Swan, R.Z.; McKillop, I.H.; Martinie, J.B.; Iannitti, D.A. Microwave ablation using 915-MHz and 2.45-GHz systems: What are the differences? HPB 2013, 15, 991–996.
- Karaisz, F.G.; Elkelany, O.O.; Davies, B.; Lozanski, G.; Krishna, S.G. A Review on Endoscopic Ultrasound-Guided Radiofrequency Ablation (EUS-RFA) of Pancreatic Lesions. Diagnostics 2023, 13, 536.
- Chu, K.F.; Dupuy, D.E. Thermal ablation of tumours: Biological mechanisms and advances in therapy. Nat. Rev. Cancer 2014, 14, 199–208.
- Paiella, S.; Salvia, R.; Ramera, M.; Girelli, R.; Frigerio, I.; Giardino, A.; Allegrini, V.; Bassi, C. Local ablative strategies for ductal pancreatic cancer (radiofrequency ablation, irreversible electroporation): A review. Gastroenterol. Res. Pract. 2016, 2016, 4508376.
- Wright, A.S.; Sampson, L.A.; Warner, T.F.; Mahvi, D.M.; Lee, F.T., Jr. Radiofrequency versus microwave ablation in a hepatic porcine model. Radiology 2005, 236, 132–139.
- Choi, J.H.; Oh, D.; Lee, J.H.; Park, J.H.; Kim, K.P.; Lee, S.S.; Lee, Y.J.; Lim, Y.S.; Song, T.J.; Lee, S.S.; et al. Initial human experience of endoscopic ultrasound-guided photodynamic therapy with a novel photosensitizer and a flexible laser-light catheter. Endoscopy 2015, 47, 1035–1038.
- Algorri, J.F.; Ochoa, M.; Roldán-Varona, P.; Rodríguez-Cobo, L.; López-Higuera, J.M. Photodynamic Therapy: A Compendium of Latest Review. Cancers 2021, 13, 4447.
- Zhou, Y.-F. High intensity focused ultrasound in clinical tumor ablation. World J. Clin. Oncol. 2011, 2, 8.
- Izadifar, Z.; Izadifar, Z.; Chapman, D.; Babyn, P. An introduction to high intensity focused ultrasound: Systematic review on principles, devices, and clinical applications. J. Clin. Med. 2020, 9, 460.
- Stanislavova, N.; Karamanliev, M.; Ivanov, T.; Yotsov, T.; Zhou, K.; Dimitrov, D. Is high-intensity focused ultrasound (HIFU) an option for neoadjuvant therapy for borderline resectable pancreatic cancer patients? a systematic review. Int. J. Hyperth. 2021, 38, 75–80.
- Testoni, S.G.G.; Capurso, G.; Petrone, M.C.; Barbera, M.; Linzenbold, W.; Enderle, M.; Gusmini, S.; Nicoletti, R.; Della Torre, E.; Mariani, A.; et al. Necrosis volume and Choi criteria predict the response to endoscopic ultrasonography-guided HybridTherm ablation of locally advanced pancreatic cancer. Endosc. Int. Open 2020, 8, E1511–E1519.
- Arcidiacono, P.G.; Carrara, S.; Reni, M.; Petrone, M.C.; Cappio, S.; Balzano, G.; Boemo, C.; Cereda, S.; Nicoletti, R.; Enderle, M.D.; et al. Feasibility and safety of EUS-guided cryothermal ablation in patients with locally advanced pancreatic cancer. Gastrointest. Endosc. 2012, 76, 1142–1151.
- Testoni, S.G.G.; Petrone, M.C.; Reni, M.; Rossi, G.; Barbera, M.; Nicoletti, V.; Gusmini, S.; Balzano, G.; Linzenbold, W.; Enderle, M.; et al. Efficacy of endoscopic ultrasound-guided ablation with the HybridTherm probe in locally advanced or borderline resectable pancreatic cancer: A phase II randomized controlled trial. Cancers 2021, 13, 4512.
More
