Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Rita Xu and Version 1 by George Geladakis.
Environmental pollution is a growing threat to natural ecosystems and one of the world’s most pressing concerns. The increasing worldwide use of pharmaceuticals has elevated their status as significant emerging contaminants. Pharmaceuticals enter aquatic environments through multiple pathways related to anthropogenic activity.
- aquatic pollution
- biodiversity
- biomonitoring
- ecotoxicology
1. Introduction
Human activities such as industrialization, urbanization, and economic development contribute synergistically to increased environmental pollution in aquatic habitats, such as rivers, lakes, and marine environments [1]. Chemical pollutants, such as heavy metals and industrial and pharmaceutical chemicals, can disrupt the balance of essential nutrients and oxygen levels, impair water quality, and make toxic or unsuitable conditions for aquatic life [2]. Water pollution can also harm biodiversity and disrupt photosynthesis in aquatic plants, significantly impacting ecosystems relying on these plants [3]. Both terrestrial and aquatic plants can absorb pollutants from water (as their main nutrient source) and transfer them through the food chain to animals and humans [4]. Pharmaceutical drugs and their metabolites contribute to water pollution by entering water bodies, disrupting the normal biological processes of aquatic organisms, and leading to the development of drug-resistant strains of bacteria [5]. In aquatic environments, including surface water, urban wastewater, wastewater treatment plants, groundwater, drinking water, and even seawater, the concentration range of predominant individual pharmaceutical compounds is typically observed to be between nanograms per liter (ng/L) to micrograms per liter (μg/L). Nevertheless, effluent from treatment plants that receive waste from pharmaceutical manufacturing facilities has been documented to contain concentrations as high as several milligrams per liter (mg/L) [6]. Furthermore, the distribution of pharmaceuticals in aquatic environments is geographically specific and contingent upon drug use patterns [7].
In recent decades, a major concern has arisen due to the increasing use of pharmaceutical products and their detrimental effects on the environment, wildlife, and humans [8]. Hignite and Azarnoff were the pioneering authors who initially documented the existence of pharmaceutical compounds in both wastewater and natural water during the late 1970s [9]. Since then, ourthe comprehension of pharmaceuticals’ origins, fate, and ecotoxicity has advanced [10,11,12][10][11][12]. Pharmaceuticals are chemicals for diagnosing, preventing, and treating humans and animals [13]. They are vital to modern human and veterinary medicine, and their use is rising worldwide because of population increase, aging demographics, economic expansion, and the rising demand for animal protein in intensified food production [8,14][8][14]. Pharmaceuticals are one of the few chemical groups explicitly designed to act on living organisms. Pharmaceutical active chemicals (PhACs) are the biologically active components of pharmaceutical medications. These PhACs may be natural or synthetic chemical compounds typically found in therapeutic and veterinary medicines.
Over the past twenty years, the negative effects of pharmaceutical products on the environment, wildlife, and humans have been recognized as a serious problem that must be addressed globally [8,15,16,17,18,19][8][15][16][17][18][19]. Slowly degradable or non-degradable PhACs pose a unique risk when they enter, remain, or disperse in the environment and are thus considered environmentally persistent pharmaceutical pollutants (EPPPs). The extensive consumption of numerous pharmaceutical products results in their subsequent release into the environment, making them serious emerging contaminants. [5,12,20][5][12][20]. Multiple mechanisms and pathways aid PhACs and their metabolites enter aquatic environments such as seas, rivers, and aquaculture facilities [21,22,23,24][21][22][23][24]. These pathways include the excessive use of pharmaceutical products like antibiotics, β-blockers, psychoactive substances, endocrine disruptors, analgesics, anticancer drugs, and non-steroidal anti-inflammatory drugs (NSAIDs), as well as processes such as oxidation, photolysis, wastewater treatment plants, pharmaceutical manufacturing, and improper medication disposal [6,19,25,26,27][6][19][25][26][27]. Additively, microplastics can also carry pharmaceutical elements and metabolites, increasing environmental exposure [28]. Due to their massive global consumption and the inability of organisms to completely metabolize drugs [29[29][30][31],30,31], pharmaceutical residues in aquatic environments and their long-term toxic effects on living organisms are becoming more of a concern [21,26,30,32,33][21][26][30][32][33].
According to the existing literature, antibiotics are the most frequently identified pharmaceuticals in aquatic environments, followed by non-steroidal anti-inflammatory drugs (NSAIDs) and psychotropic substances [34]. Antibiotics are chemical compounds that can eradicate or impede the proliferation of pathogens. Consequently, they have been extensively employed in the management, regulation, and prevention of infectious diseases in humans, animals, and plants [35,36][35][36]. Multiple antibiotics have been documented to exhibit high levels of toxicity towards various aquatic organisms, as indicated by toxicity unit values above 100 for acute toxicity and 1000 for chronic toxicity. Erythromycin exhibited the highest level of toxicity among the antibiotics, as indicated by its elevated acute and chronic toxicity unit values [37,38][37][38].
Analgesics and NSAIDs are PhACs that are extensively utilized on a global scale [39]. These substances are commonly prescribed for analgesic purposes in human medical treatment. However, they are also frequently available for purchase without a prescription, commonly referred to as “over-the-counter” medications. Certain NSAIDs may not elicit immediate physiological responses but instead exert long-term effects on specific organisms. As an example, Cleuvers [40] reported that naproxen exhibited an EC50 (half maximal effective concentration) value of 174 mg/L and a NOEC (no-observed-effect concentration) value of 0.15 mg/L for Daphnia magna. Based on studies conducted by Martins et al. [41] and Załeska-Radziwiłl et al. [42], ciprofloxacin exhibited an EC50 value of 65.3 mg/L for Daphnia magna, while the NOEC value was 0.156 mg/L.
Psychiatric medications are pharmacological agents that possess psychoactive properties, influencing the internal neurochemical processes of the brain and the central nervous system. Therefore, these pharmaceuticals manage mental and neurological disorders [43,44][43][44]. In aquatic environments, the most frequently identified psychiatric pharmaceuticals include antidepressants, anxiolytics, and antiepileptic drugs (AEDs). According to Duarte et al. [45], the administration of fluoxetine, a psychiatric medication, resulted in significant DNA damage in meagre (Argyrosomus regius) when exposed to a concentration of 3 μg/L, as compared to the control group. Additionally, Aguirre-Martínez et al. [46] emphasized the significant DNA damage caused by carbamazepine, a psychiatric medication, to Corbicula fluminea. Notably, even at the lowest dose examined (0.1 μg/L) and after an exposure period of 21 days, carbamazepine had a considerable impact on DNA integrity.
Pharmaceuticals exhibit significant diversity in their physicochemical qualities, resulting in a wide range of biological variances. The water solubility, hydrophobicity, volatility, and other similar properties of substances can significantly influence their actions and ultimate destiny within aquatic ecosystems. The fate of pharmaceuticals is influenced by various factors, including dissociation constants (pKa), solid–water distribution coefficients (Kd), organic carbon-based sorption coefficients (log Koc), and octanol-water partition coefficients (Kow). These factors play a role in determining the extent of sorption, partitioning, hydrolysis, photodegradation, and biodegradation processes [47,48,49][47][48][49]. Furthermore, it should be noted that numerous pharmaceuticals possess acidic and/or basic functional groups, hence allowing for the existence of anionic, cationic, neutral, or zwitterionic forms under varying pH values [50]. The variability of these factors is contingent upon the pKa and Kow values of the molecule, as stated by Patel et al. [6]. The significance of chirality in relation to the environmental destiny of pharmaceuticals is noteworthy, because approximately 50% of pharmaceutical products are marketed and distributed as individual enantiomers [51]. Enantioselective reactions involve the subjection of a certain enantiomer to distinct biotransformations compared to its enantiomeric counterpart [52].
To evaluate the environmental hazards associated with pharmaceuticals, it is imperative to consider many factors, such as the quantities in which they are used, their physicochemical characteristics, and their potential for ecotoxicity. The necessity for conducting risk assessment analysis arises from several factors, including the high solubility of the substance in water, its ability to persist in the environment, its tendency to accumulate in organisms, and its potential to induce toxicity and carcinogenicity. Indeed, this endeavor has a significant level of difficulty. Low concentrations of pharmaceutical environmental residues can potentially cause acute and chronic impacts on microorganisms, flora, and fauna. The observed effects encompass a spectrum of metabolic alterations and disruptions in hormonal equilibrium. Organisms other than the specified target species may experience adverse effects. Although present in tiny amounts, below the established threshold, certain pharmaceutical substances have the potential to inflict serious adverse effects due to the intricate interactions exhibited by diverse pharmaceutical mixes within the environment [6].
For the quantification of PhACs in water or soil sediments, various analytical biochemical methods have been utilized, including liquid chromatography-mass spectrometry (LC-MS), gas chromatography-MS (GC-MS), solid-phase extraction (SPE), hydrophilic interaction liquid chromatography (HILIC), and high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) [53,54][53][54]. Nevertheless, in recent decades, technological advances in molecular biotechnology have improved the measurement and monitoring of pharmaceutical compounds’ ecotoxicological effects on water quality by applying and validating new biological indicators, such as bioassays and biomarkers [8,55,56,57,58,59,60,61,62][8][55][56][57][58][59][60][61][62].
The impact of human activities on different ecosystems is widely recognized, resulting in significant changes that include species extinction and biodiversity alterations. Cardinale et al. [63] highlighted that these changes can negatively affect ecosystem functioning. Hence, there is a demand for non-invasive assessments of biodiversity. According to Shim et al. [64], all living organisms release genetic material into the environment via various means, such as feces, urine, gametes, and epidermal cells, leaving detectable remnants of their DNA. In this context, biotechnological techniques based on next-generation sequencing (NGS), such as environmental DNA (eDNA) metabarcoding, can serve as a powerful bioindicator for detecting and evaluating the impacts of various pollutants, such as pharmaceutical compounds, on the diversity and composition of bacterial communities and other microorganisms such as microalgae (phytoplankton), protista, and metazoa [65,66,67,68,69][65][66][67][68][69]. Such techniques may also prove helpful for estimating the composition of animal and plant communities, including the genetic diversity of these species and their response to disease outbreaks resulting from changes in pathogen fitness and genotype–environment interactions due to the presence of specific PhACs [70]. The technique of eDNA metabarcoding entails an in-depth, thorough analysis of DNA sequences derived from environmental samples within a particular ecosystem [62,71,72,73,74][62][71][72][73][74]. The novel concept of eDNA metabarcoding, which offers to bypass many of the problems of thorough conventional research, is gaining traction as an effective and powerful approach to measuring biodiversity, albeit with pros and cons.
2. Pharmaceuticals and Pollution: Routes and Pathways
Many PhACs and byproducts exist in rivers, lakes, and groundwater [26]. Due to their widespread use, persistence, and bioaccumulation potential, several classes of PhACs have been identified as hazards to human and environmental health. They primarily infiltrate waterways through wastewater treatment plants, improper drug disposal, and human and animal waste (Figure 1).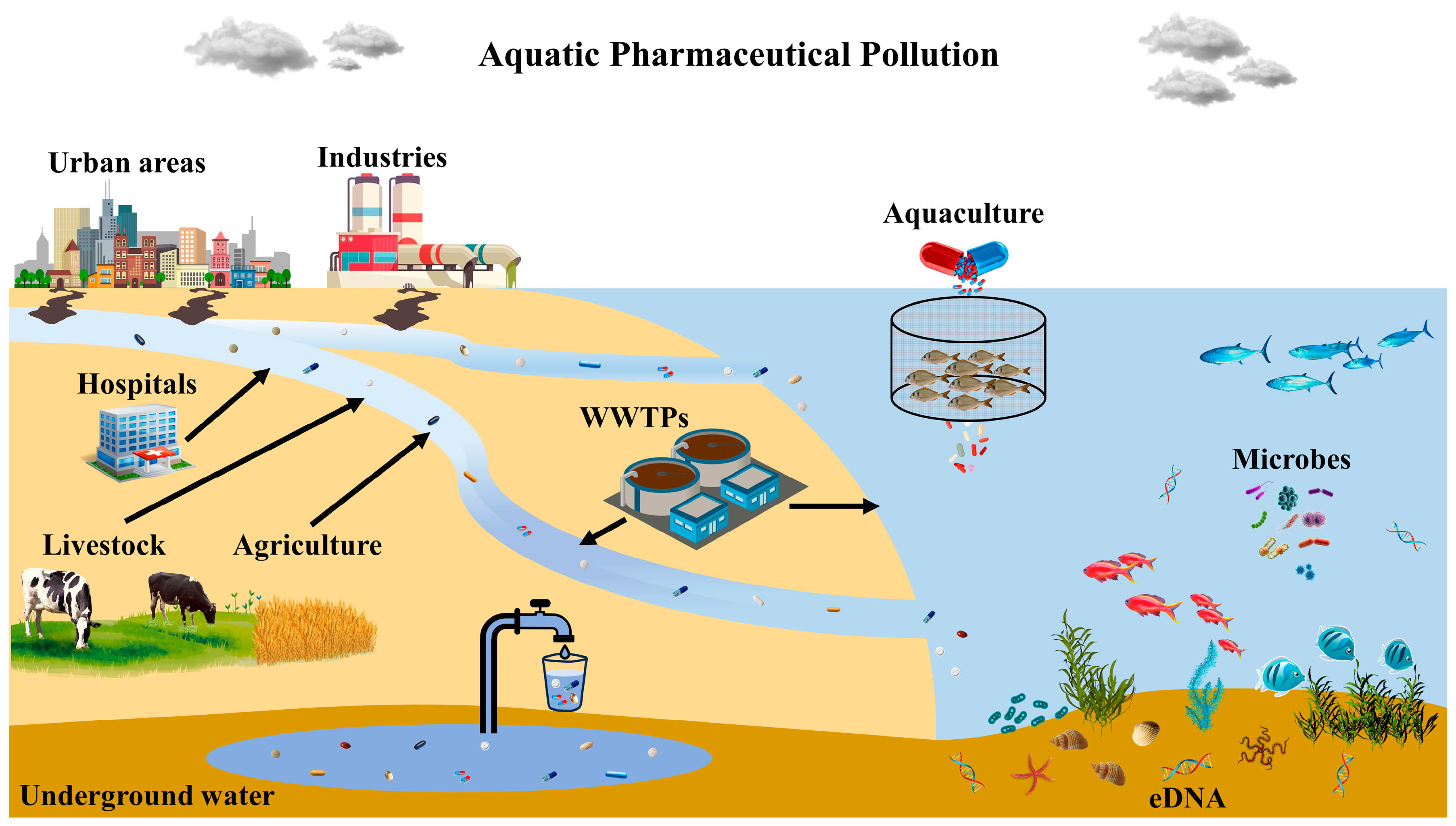
Figure 1. Main pathways of pharmaceutical aquatic pollution (WWTPs: wastewater treatment plants).
Table 1. Overview of the primary sources and associated environmental effects and health risks of PhACs.
| Pharmaceuticals | Sources | Effects | References |
|---|---|---|---|
| Antibiotics | Contamination of water bodies from human and veterinary medicine wastes. |
|
[81,82,83,84,85,86,87,88,89,90,91][80][81][82][83][84][85][86][87][88][89][90] |
| Hormones and endocrine-disrupting chemicals (EDCs) | Enter the aquatic environment through agricultural and livestock manure, excretion (e.g., urine and feces), improper disposal. |
|
[92,93,94,95,][9496,][9597,][9698,][9799,100][91][92][93][98][99] |
| Analgesics and nonsteroidal anti-inflammatory drugs (NSAIDs) | Main pathways to the aquatic environment: human excretion, the inappropriate disposal of unused medications, wastewater discharges from pharmaceutical and healthcare facilities. |
|
[27,101,,105][27102,][100103,][101][102]104[103][104] |
| Psychotropic and antiepileptic drugs | Enter water bodies through human excretion, and wastewater systems via sewage or septic tanks |
|
[43,44,106,107,108][43][44][105][106][107] |
| β-blockers | Infiltrate the aquatic environment through human excretion, and wastewater systems via sewage or septic tanks |
|
[109,110,111,112][108][109][110][111] |
| Chemotherapy and anticancer drugs | Introduction to the aquatic environment via human excretion and the incorrect disposal of unused medicines |
|
[113,114,115][112][113][114] |
2.1. Antibiotics
Antibiotics are frequently employed in human as well as animal medicine for the purpose of treating bacterial infections. Penicillins, cephalosporins, lincosamides, macrolides, tetracyclines, sulfonamides, and quinolones are among the most frequently utilized classes of antibiotics in human medicine [89][88]. After ingestion, humans and animals frequently excrete antibiotics. Antibiotics and their constituents can also be released from untreated or inadequately treated effluents if conventional wastewater treatment methods are ineffective at removing them [35,36][35][36]. The excessive utilization of agricultural practices, such as farming and raising livestock, may be another source that contributes to the release of antibiotics into the surrounding environment. Due to their vast utilization, the discharge of antibiotic-containing effluent into rivers, lakes, or other water bodies contributes significantly to pharmaceutical pollution [81,84,87,88,91,116][80][83][86][87][90][115]. Despite utilizing advanced treatment methods like activated carbon adsorption, ozone treatment, or other advanced methods, completely eradicating antibiotic residues from enriched wastes may not be achievable. Furthermore, the persistence of antibiotics in the environment can be attributed to their resistance to degradation [86][85]. Hence, antibiotics in the environment can potentially contribute to developing and spreading antibiotic resistance in microorganisms, posing significant challenges in treating infections. It can also exert selective pressure by disturbing the equilibrium of microbial communities in water bodies, thereby affecting nutrient cycling and the overall ecosystem functioning. This disturbance contributes to the emergence and dissemination of antibiotic-resistant bacteria (ARBs) and antibiotic resistance genes (ARGs) [82,83][81][82].2.2. Hormones and Endocrine-Disrupting Chemicals
Endocrine-disrupting chemicals (EDCs) are naturally occurring or artificially produced compounds that interfere with the normal functioning of hormones in the body. Hormones such as estrogen are integral endocrine system components [117][116]. According to the United States Environmental Protection Agency (EPA), an EDC is an exogenous substance that possesses the capacity to interfere with the synthesis, secretion, transport, metabolism, receptor binding, or clearance of endogenous hormones, thereby inducing modifications in the endocrine and homeostatic systems [118,119][117][118]. EDCs are commonly found in a variety of everyday products, such as human and animal medications (e.g., diethylstilbestrol), cosmetics (e.g., triclosan), food and beverage packaging (e.g., perfluorochemicals, bisphenol A, phthalates), toys (e.g., lead and cadmium), industrial solvents or oils and their by-products (e.g., dioxins and polychlorinated biphenyls), and pesticides (e.g., dichlorodiphenyltrichloroethane and chlorpyrifos) [120,121,122][119][120][121]. EDCs can be classified into four distinct groups based on their source: industrial (e.g., dioxins, polychlorinated biphenyls, and alkylphenols), agricultural (including pesticides, insecticides, herbicides, phytoestrogens, and fungicides), residential (such as phthalates, polybrominated biphenyls, and bisphenol A), and pharmaceutical (including birth control pills, hormone replacement therapy, and parabens) [118,123,124][117][122][123]. Pharmaceutical EDCs can enter the environment through excretion and improper disposal [92,96,98][91][95][97]. Since hormones regulate human and animal physiological processes, their release into the environment through agricultural and livestock manure runoff can contribute to environmental pollution and ultimately disrupt the endocrine systems of aquatic organisms, resulting in reproductive and developmental abnormalities, altered sex ratios, and stunted growth and development [93,94,95,97,99,100][92][93][94][96][98][99]. Estrogenic hormones, such as estradiol and ethinyl estradiol (i.e., a synthetic estrogen present in contraceptive medications), are of special concern. The presence of these hormones has been linked to the occurrence of feminization effects in fish populations. Exposure to these hormones can induce the development of intersex traits, characterized by both male and female characteristics within a single individual, as well as the disturbance of normal reproductive processes. Fish feminization can have significant implications for population dynamics and reproductive success. It can sometimes lead to population declines or the extinction of endangered species [125,126][124][125]. Although the direct impact of hormone-contaminated water on human health is not yet fully understood, scientists continue to investigate the potential risks since it is believed that chronic exposure to low levels of hormone contaminants in potable water or consuming contaminated aquatic organisms may subtly affect human endocrine systems [127][126].2.3. Analgesics and Nonsteroidal Anti-Inflammatory Drugs
Analgesics and nonsteroidal anti-inflammatory drugs (NSAIDs) are commonly employed to manage pain and inflammation. The detection of analgesics and NSAIDs in both ground and surface water, such as lakes and rivers, has become prevalent due to their extensive utilization. Minute amounts of NSAIDs have been identified in various environmental matrices such as soil, wastewater, surface water, groundwater, sediments, snow, and drinking water [39,104][39][103]. Despite negligible detectable environmental concentrations, NSAIDs have long-lasting ecotoxic impacts on the biotic components of ecosystems [103,104][102][103]. According to Feng et al. [128][127], daily NSAID consumption exceeds 30 million doses and is rising swiftly. Due to their stability and resistance to degradation, these compounds can persist in the environment and accumulate over time. NSAIDs can enter the environment through various routes, but human excretion is the most common. The inappropriate disposal of unused medications further contributes to NSAID pollution [129][128]. Additionally, pharmaceutical manufacturing and healthcare facility effluent discharges can release NSAIDs into the environment. NSAIDs can influence organisms’ behavior, reproduction, growth, and development. For instance, NSAIDs such as ibuprofen and diclofenac have been associated with impaired reproduction and aberrant development in fish. In addition, repeated exposure to NSAIDs may cause bioaccumulation in aquatic organisms. Bioaccumulation can occur through the food chain, leading to elevated levels of NSAIDs in predators that consume contaminated prey. NSAIDs can influence the microbial communities of aquatic ecosystems by inhibiting the development and activity of beneficial bacteria, resulting in population imbalances among microorganisms and disruption of essential ecological processes [101,102][100][101].2.4. Psychotropic and Antiepileptic Drugs
Psychotropic medications and AEDs are commonly employed in managing mental health disorders, such as anxiety and depression, addiction, seizures and convulsions, and chronic pain management. Researchers have discovered traces of psychotropics and AEDs in aquatic environments, implying their widespread presence. Psychotropic and AED drugs infiltrate the environment primarily through human excretion following their use as medications. The active compounds of psychotropic medications are metabolized within the body, and the residues are excreted through urine and feces. Also, these drugs can infiltrate wastewater systems via sewage or septic tanks [43,44,108][43][44][107]. Studies have demonstrated that exposure to psychotropic medications can induce alterations in the behavior, reproductive patterns, and physiological functions of fish, invertebrates, and other aquatic organisms [106[105][106],107], contributing to further disrupting the natural ecological processes and aquatic ecosystem populations.2.5. β-Blockers
β-blockers are a class of pharmaceutical drugs (competitive antagonists) that inhibit the activity of adrenergic β-receptors in the sympathetic nervous system. The broad range of pathologies for which β-blockers are prescribed has resulted in an annual consumption increase of more than treble [130,131][129][130]. Although β-blockers have significant therapeutic value, they can potentially contribute to pharmaceutical pollution and have environmental effects. The increased consumption of β-blockers has led to increased tracing in the environment, and their presence has been detected in several bodies of water [132,133][131][132]. The administration of β-blockers has the potential to exert adverse effects on fish, as evidenced by their ability to induce alterations in pulse rate and other cardiovascular-related physiological processes. They have been found to have various impacts on aquatic organisms, such as causing disruption in testosterone levels, reducing fertility and reproduction rates, and inducing abnormal behavior [109,110,111][108][109][110]. β-blockers can alter the activities and functions of microorganisms involved in decomposing organic matter during treatment within wastewater treatment facilities [112][111].2.6. Chemotherapy and Anticancer Drugs
Chemotherapy and anticancer medications may pose risks as pharmaceutical pollutants when discharged into the environment [115][114]. Human excretion is the primary source after administering these medications to cancer patients. However, the incorrect disposal of unused medicines can contribute to chemotherapy drug pollution. Typically, conventional wastewater purification processes are ineffective at removing chemotherapy drugs. Thus, these drugs can enter the environment through treated effluents [114,134][113][133]. Certain chemotherapy drugs are designed to be exceedingly robust and resistant to degradation to exert their therapeutic effects on the human body. This stability will also allow them to endure for extended periods in the environment and, consequently, may accumulate over time, resulting in long-term exposure in particular regions [114,135][113][134]. Chemotherapeutic medications may adversely affect aquatic organisms and other non-target species [113][112]. They can affect the growth, development, and reproduction of organisms exposed to them by interfering with normal cell division and DNA replication. Several investigations have demonstrated toxic effects at environmental concentrations on fish, invertebrates, and other aquatic organisms [114][113]. Additionally, chemotherapy drugs can disrupt the growth and activity of soil and water microbial communities in the environment, leading to population imbalances and disturbances in ecological processes.References
- Goel, P.K. Water Pollution: Causes, Effects and Control; New Age International: New Delhi, India, 2006.
- Gautam, P.K.; Gautam, R.K.; Banerjee, S.; Chattopadhyaya, M.C.; Pandey, J.D. Heavy Metals in the Environment: Fate, Transport, Toxicity and Remediation Technologies. In Heavy Metals: Sources, Toxicity and Remediation Techniques; Pathania, S., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2016; ISBN 9781634847407.
- Obinnaa, I.B.; Ebere, E.C. A Review: Water Pollution by Heavy Metal and Organic Pollutants: Brief Review of Sources, Effects and Progress on Remediation with Aquatic Plants. Anal. Methods Environ. Chem. J. 2019, 2, 5–38.
- Tongesayi, T.; Fedick, P.; Lechner, L.; Brock, C.; Le Beau, A.; Bray, C. Daily Bioaccessible Levels of Selected Essential but Toxic Heavy Metals from the Consumption of Non-Dietary Food Sources. Food Chem. Toxicol. 2013, 62, 142–147.
- Świacka, K.; Maculewicz, J.; Kowalska, D.; Grace, M.R. Do Pharmaceuticals Affect Microbial Communities in Aquatic Environments? A Review. Front. Environ. Sci. 2023, 10, 1093920.
- Patel, M.; Kumar, R.; Kishor, K.; Mlsna, T.; Pittman, C.U.; Mohan, D. Pharmaceuticals of Emerging Concern in Aquatic Systems: Chemistry, Occurrence, Effects, and Removal Methods. Chem. Rev. 2019, 119, 3510–3673.
- Carlsson, C.; Johansson, A.K.; Alvan, G.; Bergman, K.; Kühler, T. Are Pharmaceuticals Potent Environmental Pollutants? Part I: Environmental Risk Assessments of Selected Active Pharmaceutical Ingredients. Sci. Total Environ. 2006, 364, 67–87.
- Kock, A.; Glanville, H.C.; Law, A.C.; Stanton, T.; Carter, L.J.; Taylor, J.C. Emerging Challenges of the Impacts of Pharmaceuticals on Aquatic Ecosystems: A Diatom Perspective. Sci. Total Environ. 2023, 878, 162939.
- Hignite, C.; Azarnoff, D.L. Drugs and Drug Metabolites as Environmental Contaminants: Chlorophenoxyisobutyrate and Salicyclic Acid in Sewage Water Effluent. Life Sci. 1977, 20, 337–342.
- Cardoso, O.; Porcher, J.M.; Sanchez, W. Factory-Discharged Pharmaceuticals Could Be a Relevant Source of Aquatic Environment Contamination: Review of Evidence and Need for Knowledge. Chemosphere 2014, 115, 20–30.
- Pozzebon, E.A.; Seifert, L. Emerging Environmental Health Risks Associated with the Land Application of Biosolids: A Scoping Review. Environ. Health 2023, 22, 57.
- Nguyen, M.K.; Lin, C.; Nguyen, H.L.; Hung, N.T.Q.; La, D.D.; Nguyen, X.H.; Chang, S.W.; Chung, W.J.; Nguyen, D.D. Occurrence, Fate, and Potential Risk of Pharmaceutical Pollutants in Agriculture: Challenges and Environmentally Friendly Solutions. Sci. Total Environ. 2023, 899, 165323.
- aus der Beek, T.; Weber, F.A.; Bergmann, A.; Hickmann, S.; Ebert, I.; Hein, A.; Küster, A. Pharmaceuticals in the Environment-Global Occurrences and Perspectives. Environ. Toxicol. Chem. 2016, 35, 823–835.
- Kovalakova, P.; Cizmas, L.; McDonald, T.J.; Marsalek, B.; Feng, M.; Sharma, V.K. Occurrence and Toxicity of Antibiotics in the Aquatic Environment: A Review. Chemosphere 2020, 251, 126351.
- Valdez-Carrillo, M.; Abrell, L.; Ramírez-Hernández, J.; Reyes-López, J.A.; Carreón-Diazconti, C. Pharmaceuticals as Emerging Contaminants in the Aquatic Environment of Latin America: A Review. Environ. Sci. Pollut. Res. 2020, 27, 44863–44891.
- Hughes, S.R.; Kay, P.; Brown, L.E. Global Synthesis and Critical Evaluation of Pharmaceutical Data Sets Collected from River Systems. Environ. Sci. Technol. 2013, 47, 661–677.
- Kolpin, D.W.; Furlong, E.T.; Meyer, M.T.; Thurman, E.M.; Zaugg, S.D.; Barber, L.B.; Buxton, H.T. Pharmaceuticals, Hormones, and Other Organic Wastewater Contaminants in U.S. Streams, 1999-2000: A National Reconnaissance. Environ. Sci. Technol. 2002, 36, 1202–1211.
- Fekadu, S.; Alemayehu, E.; Dewil, R.; Van der Bruggen, B. Pharmaceuticals in Freshwater Aquatic Environments: A Comparison of the African and European Challenge. Sci. Total Environ. 2019, 654, 324–337.
- Hussain, A.; Ashique, S.; Zaheen Hassan, M.; Afzal, O.; Asiri, Y.I.; Kumar, P.; Dua, K.; Webster, T.J.; Altamimi, A.S.A.; Altamimi, M.A. Pharmaceutical Contaminants in Aquatic Systems, Conventional and Green Strategies, Recent Updates, Challenges and Policies, and Potential Outcomes. J. Mol. Liq. 2023, 389, 122905.
- Mahapatra, I.; Clark, J.R.A.; Dobson, P.J.; Owen, R.; Lynch, I.; Lead, J.R. Expert Perspectives on Potential Environmental Risks from Nanomedicines and Adequacy of the Current Guideline on Environmental Risk Assessment. Environ. Sci. Nano 2018, 5, 1873–1889.
- Wilkinson, J.L.; Boxall, A.B.A.; Kolpin, D.W.; Leung, K.M.Y.; Lai, R.W.S.; Galbán-Malagón, C.; Adell, A.D.; Mondon, J.; Metian, M.; Marchant, R.A.; et al. Pharmaceutical Pollution of the World’s Rivers. Proc. Natl. Acad. Sci. USA 2022, 119, e2113947119.
- Feckler, A.; Wolfram, J.; Schulz, R.; Bundschuh, M. Reducing Pollution to Levels Not Harming Biodiversity and Ecosystem Functions: A Perspective on the Post-2020 Global Biodiversity Framework. Curr. Opin. Environ. Sci. Health 2023, 35, 100495.
- Fatimazahra, S.; Latifa, M.; Laila, S.; Monsif, K. Review of Hospital Effluents: Special Emphasis on Characterization, Impact, and Treatment of Pollutants and Antibiotic Resistance. Environ. Monit. Assess. 2023, 195, 393.
- Fernandes, J.P.; Almeida, C.M.R.; Salgado, M.A.; Carvalho, M.F.; Mucha, A.P. Pharmaceutical Compounds in Aquatic Environments— Occurrence, Fate and Bioremediation Prospective. Toxics 2021, 9, 257.
- Salimi, M.; Esrafili, A.; Gholami, M.; Jonidi Jafari, A.; Rezaei Kalantary, R.; Farzadkia, M.; Kermani, M.; Sobhi, H.R. Contaminants of Emerging Concern: A Review of New Approach in AOP Technologies. Environ. Monit. Assess. 2017, 189, 414.
- Ortúzar, M.; Esterhuizen, M.; Olicón-Hernández, D.R.; González-López, J.; Aranda, E. Pharmaceutical Pollution in Aquatic Environments: A Concise Review of Environmental Impacts and Bioremediation Systems. Front. Microbiol. 2022, 13, 869332.
- Lin, J.Y.; Zhang, Y.; Bian, Y.; Zhang, Y.X.; Du, R.Z.; Li, M.; Tan, Y.; Feng, X.S. Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) in the Environment: Recent Updates on the Occurrence, Fate, Hazards and Removal Technologies. Sci. Total Environ. 2023, 904, 166897.
- Santos, L.H.M.L.M.; Rodríguez-Mozaz, S.; Barceló, D. Microplastics as Vectors of Pharmaceuticals in Aquatic Organisms—An Overview of Their Environmental Implications. Case Stud. Chem. Environ. Eng. 2021, 3, 100079.
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial Resistance: A Global Multifaceted Phenomenon. Pathog. Glob. Health 2015, 109, 309–318.
- Świacka, K.; Maculewicz, J.; Kowalska, D.; Caban, M.; Smolarz, K.; Świeżak, J. Presence of Pharmaceuticals and Their Metabolites in Wild-Living Aquatic Organisms—Current State of Knowledge. J. Hazard. Mater. 2022, 424, 127350.
- Rios-Miguel, A.B.; van Bergen, T.J.H.M.; Zillien, C.; Ragas, A.M.J.; van Zelm, R.; Jetten, M.S.M.; Hendriks, A.J.; Welte, C.U. Predicting and Improving the Microbial Removal of Organic Micropollutants during Wastewater Treatment: A Review. Chemosphere 2023, 333, 138908.
- Pinheiro, M.; Martins, I.; Raimundo, J.; Caetano, M.; Neuparth, T.; Santos, M.M. Stressors of Emerging Concern in Deep-Sea Environments: Microplastics, Pharmaceuticals, Personal Care Products and Deep-Sea Mining. Sci. Total Environ. 2023, 876, 162557.
- Bethke, K.; Kropidłowska, K.; Stepnowski, P.; Caban, M. Review of Warming and Acidification Effects to the Ecotoxicity of Pharmaceuticals on Aquatic Organisms in the Era of Climate Change. Sci. Total Environ. 2023, 877, 162829.
- Hejna, M.; Kapuścińska, D.; Aksmann, A. Pharmaceuticals in the Aquatic Environment: A Review on Eco-Toxicology and the Remediation Potential of Algae. Int. J. Environ. Res. Public Health 2022, 19, 7717.
- Carvalho, I.T.; Santos, L. Antibiotics in the Aquatic Environments: A Review of the European Scenario. Environ. Int. 2016, 94, 736–757.
- Nannou, C.; Ofrydopoulou, A.; Evgenidou, E.; Heath, D.; Heath, E.; Lambropoulou, D. Antiviral Drugs in Aquatic Environment and Wastewater Treatment Plants: A Review on Occurrence, Fate, Removal and Ecotoxicity. Sci. Total Environ. 2020, 699, 134322.
- Eguchi, K.; Nagase, H.; Ozawa, M.; Endoh, Y.S.; Goto, K.; Hirata, K.; Miyamoto, K.; Yoshimura, H. Evaluation of Antimicrobial Agents for Veterinary Use in the Ecotoxicity Test Using Microalgae. Chemosphere 2004, 57, 1733–1738.
- González-Pleiter, M.; Gonzalo, S.; Rodea-Palomares, I.; Leganés, F.; Rosal, R.; Boltes, K.; Marco, E.; Fernández-Piñas, F. Toxicity of Five Antibiotics and Their Mixtures towards Photosynthetic Aquatic Organisms: Implications for Environmental Risk Assessment. Water Res. 2013, 47, 2050–2064.
- Świacka, K.; Michnowska, A.; Maculewicz, J.; Caban, M.; Smolarz, K. Toxic Effects of NSAIDs in Non-Target Species: A Review from the Perspective of the Aquatic Environment. Environ. Pollut. 2021, 273, 115891.
- Cleuvers, M. Mixture Toxicity of the Anti-Inflammatory Drugs Diclofenac, Ibuprofen, Naproxen, and Acetylsalicylic Acid. Ecotoxicol. Environ. Saf. 2004, 59, 309–315.
- Martins, N.; Pereira, R.; Abrantes, N.; Pereira, J.; Gonçalves, F.; Marques, C.R. Ecotoxicological Effects of Ciprofloxacin on Freshwater Species: Data Integration and Derivation of Toxicity Thresholds for Risk Assessment. Ecotoxicology 2012, 21, 1167–1176.
- Załęska-Radziwiłł, M.; Łebkowska, M.; Affek, K.; Zarzeczna, A. Environmental Risk Assessment of Selected Pharmaceuticals Present in Surface Waters in Relation to Animals. Arch. Environ. Prot. 2011, 37, 31–42.
- Hartwig, C.; Muth-Köhne, E.; Düring, R.A. Screening for Ecotoxicological Effects of Antiepileptic Drugs in Biologically Treated Waste Water Originating from an Epilepsy Ward by Danio Rerio Embryos. Environ. Sci. Eur. 2013, 25, 29.
- Argaluza, J.; Domingo-Echaburu, S.; Orive, G.; Medrano, J.; Hernandez, R.; Lertxundi, U. Environmental Pollution with Psychiatric Drugs. World J. Psychiatry 2021, 11, 791–804.
- Duarte, I.A.; Reis-Santos, P.; Novais, S.C.; Rato, L.D.; Lemos, M.F.L.; Freitas, A.; Pouca, A.S.V.; Barbosa, J.; Cabral, H.N.; Fonseca, V.F. Depressed, Hypertense and Sore: Long-Term Effects of Fluoxetine, Propranolol and Diclofenac Exposure in a Top Predator Fish. Sci. Total Environ. 2020, 712, 136564.
- Aguirre-Martínez, G.V.; Del Valls, T.A.; Martín-Díaz, M.L. Identification of Biomarkers Responsive to Chronic Exposure to Pharmaceuticals in Target Tissues of Carcinus Maenas. Mar. Environ. Res. 2013, 87–88, 1–11.
- Yamamoto, H.; Nakamura, Y.; Moriguchi, S.; Nakamura, Y.; Honda, Y.; Tamura, I.; Hirata, Y.; Hayashi, A.; Sekizawa, J. Persistence and Partitioning of Eight Selected Pharmaceuticals in the Aquatic Environment: Laboratory Photolysis, Biodegradation, and Sorption Experiments. Water Res. 2009, 43, 351–362.
- Pal, A.; Gin, K.Y.H.; Lin, A.Y.C.; Reinhard, M. Impacts of Emerging Organic Contaminants on Freshwater Resources: Review of Recent Occurrences, Sources, Fate and Effects. Sci. Total Environ. 2010, 408, 6062–6069.
- Lapworth, D.J.; Baran, N.; Stuart, M.E.; Ward, R.S. Emerging Organic Contaminants in Groundwater: A Review of Sources, Fate and Occurrence. Environ. Pollut. 2012, 163, 287–303.
- Kummerer, K. Pharmaceuticals in the Environment. Annu. Rev. Environ. Resour. 2010, 35, 57–75.
- Kasprzyk-Hordern, B. Pharmacologically Active Compounds in the Environment and Their Chirality. Chem. Soc. Rev. 2010, 39, 4466–4503.
- Kasprzyk-Hordern, B.; Baker, D.R. Estimation of Community-Wide Drugs Use via Stereoselective Profiling of Sewage. Sci. Total Environ. 2012, 423, 142–150.
- Miller, T.H.; Bury, N.R.; Owen, S.F.; MacRae, J.I.; Barron, L.P. A Review of the Pharmaceutical Exposome in Aquatic Fauna. Environ. Pollut. 2018, 239, 129–146.
- Wilkinson, J.L.; Boxall, A.B.A.; Kolpin, D.W. A Novel Method to Characterise Levels of Pharmaceutical Pollution in Large-Scale Aquatic Monitoring Campaigns. Appl. Sci. 2019, 9, 1368.
- Kontana, A.; Papadimitriou, C.A.; Samaras, P.; Zdragas, A.; Yiangou, M. Bioassays and Biomarkers for Ecotoxicological Assessment of Reclaimed Municipal Wastewater. Water Sci. Technol. 2008, 57, 947–953.
- Maranho, L.A.; Baena-Nogueras, R.M.; Lara-Martín, P.A.; DelValls, T.A.; Martín-Díaz, M.L. Bioavailability, Oxidative Stress, Neurotoxicity and Genotoxicity of Pharmaceuticals Bound to Marine Sediments. The Use of the Polychaete Hediste Diversicolor as Bioindicator Species. Environ. Res. 2014, 134, 353–365.
- Mercado, S.A.S.; Galvis, D.G.V. Paracetamol Ecotoxicological Bioassay Using the Bioindicators Lens Culinaris Med. and Pisum Sativum L. Environ. Sci. Pollut. Res. 2023, 30, 61965–61976.
- Rodrigues, S.; Pinto, I.; Martins, F.; Formigo, N.; Antunes, S.C. Can Biochemical Endpoints Improve the Sensitivity of the Biomonitoring Strategy Using Bioassays with Standard Species, for Water Quality Evaluation? Ecotoxicol. Environ. Saf. 2021, 215, 112151.
- Gavrilescu, M.; Demnerová, K.; Aamand, J.; Agathos, S.; Fava, F. Emerging Pollutants in the Environment: Present and Future Challenges in Biomonitoring, Ecological Risks and Bioremediation. New Biotechnol. 2015, 32, 147–156.
- Chauhan, B.; Dodamani, S.; Malik, S.; Almalki, W.H.; Haque, S.; Sayyed, R.Z. Microbial Approaches for Pharmaceutical Wastewater Recycling and Management for Sustainable Development: A Multicomponent Approach. Environ. Res. 2023, 237, 116983.
- Mishra, S.; Singh, A.K.; Cheng, L.; Hussain, A.; Maiti, A. Occurrence of Antibiotics in Wastewater: Potential Ecological Risk and Removal through Anaerobic–Aerobic Systems. Environ. Res. 2023, 226, 115678.
- Lin, Y.; Zhong, W.; Zhang, X.; Zhou, X.; He, L.; Lv, J.; Zhao, Z. Environmental DNA Metabarcoding Revealed the Impacts of Anthropogenic Activities on Phytoplankton Diversity in Dianchi Lake and Its Three Inflow Rivers. Ecol. Evol. 2023, 13, e10088.
- Cardinale, B.J.; Duffy, J.E.; Gonzalez, A.; Hooper, D.U.; Perrings, C.; Venail, P.; Narwani, A.; MacE, G.M.; Tilman, D.; Wardle, D.A.; et al. Biodiversity Loss and Its Impact on Humanity. Nature 2012, 486, 59–67.
- Shim, K.Y.; Shin, H.; Yeo, I.C.; Kim, K.R.; Kwak, I.S.; Jeong, C.B. Environmental DNA Surveillance of Biocontamination in a Drinking Water Treatment Plant. J. Hazard. Mater. 2023, 456, 131656.
- Bernhard, A.E.; Field, K.G. Identification of Nonpoint Sources of Fecal Pollution in Coastal Waters by Using Host-Specific 16S Ribosomal DNA Genetic Markers from Fecal Anaerobes. Appl. Environ. Microbiol. 2000, 66, 1587–1594.
- Li, F.; Peng, Y.; Fang, W.; Altermatt, F.; Xie, Y.; Yang, J.; Zhang, X. Application of Environmental DNA Metabarcoding for Predicting Anthropogenic Pollution in Rivers. Environ. Sci. Technol. 2018, 52, 11708–11719.
- Wang, S.; Zhang, P.; Zhang, D.; Chang, J. Evaluation and Comparison of the Benthic and Microbial Indices of Biotic Integrity for Urban Lakes Based on Environmental DNA and Its Management Implications. J. Environ. Manag. 2023, 341, 118026.
- Zan, R.; Blackburn, A.; Plaimart, J.; Acharya, K.; Walsh, C.; Stirling, R.; Kilsby, C.G.; Werner, D. Environmental DNA Clarifies Impacts of Combined Sewer Overflows on the Bacteriology of an Urban River and Resulting Risks to Public Health. Sci. Total Environ. 2023, 889, 164282.
- Zhang, L.; Yang, J.; Zhang, Y.; Shi, J.; Yu, H.; Zhang, X. eDNAeDNA Biomonitoring Revealed the Ecological Effects of Water Diversion Projects between Yangtze River and Tai Lake. Water Res. 2022, 210, 117994.
- Aulsebrook, L.C.; Wong, B.B.M.; Hall, M.D. Can Pharmaceutical Pollution Alter the Spread of Infectious Disease? A Case Study Using Fluoxetine. Philos. Trans. R. Soc. B Biol. Sci. 2023, 378, 20220010.
- Barnes, M.A.; Turner, C.R.; Jerde, C.L.; Renshaw, M.A.; Chadderton, W.L.; Lodge, D.M. Environmental Conditions Influence eDNA Persistence in Aquatic Systems. Environ. Sci. Technol. 2014, 48, 1819–1827.
- Greco, M.; Lejzerowicz, F.; Reo, E.; Caruso, A.; Maccotta, A.; Coccioni, R.; Pawlowski, J.; Frontalini, F. Environmental RNA Outperforms eDNA Metabarcoding in Assessing Impact of Marine Pollution: A Chromium-Spiked Mesocosm Test. Chemosphere 2022, 298, 134239.
- Jerde, C.L.; Olds, B.P.; Shogren, A.J.; Andruszkiewicz, E.A.; Mahon, A.R.; Bolster, D.; Tank, J.L. Influence of Stream Bottom Substrate on Retention and Transport of Vertebrate Environmental DNA. Environ. Sci. Technol. 2016, 50, 8770–8779.
- Suarez-Menendez, M.; Planes, S.; Garcia-Vazquez, E.; Ardura, A. Early Alert of Biological Risk in a Coastal Lagoon Through eDNA Metabarcoding. Front. Ecol. Evol. 2020, 8, 9.
- Barra Caracciolo, A.; Topp, E.; Grenni, P. Pharmaceuticals in the Environment: Biodegradation and Effects on Natural Microbial Communities. A Review. J. Pharm. Biomed. Anal. 2015, 106, 25–36.
- Arnold, K.E.; Brown, A.R.; Brown, A.R.; Ankley, G.T.; Sumpter, J.P. Medicating the Environment: Assessing Risks of Pharmaceuticals to Wildlife and Ecosystems. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130569.
- Grenni, P.; Ancona, V.; Barra Caracciolo, A. Ecological Effects of Antibiotics on Natural Ecosystems: A Review. Microchem. J. 2018, 136, 25–39.
- King, K.C.; Hall, M.D.; Wolinska, J. Infectious Disease Ecology and Evolution in a Changing World. Philos. Trans. R. Soc. B Biol. Sci. 2023, 378, 20220002.
- Caban, M.; Stepnowski, P. How to Decrease Pharmaceuticals in the Environment? A Review. Environ. Chem. Lett. 2021, 19, 3115–3138.
- Bilal, M.; Mehmood, S.; Rasheed, T.; Iqbal, H.M.N. Antibiotics Traces in the Aquatic Environment: Persistence and Adverse Environmental Impact. Curr. Opin. Environ. Sci. Health 2020, 13, 68–74.
- Bondarczuk, K.; Piotrowska-Seget, Z. Microbial Diversity and Antibiotic Resistance in a Final Effluent-Receiving Lake. Sci. Total Environ. 2019, 650, 2951–2961.
- Lai, C.C.; Chen, S.Y.; Ko, W.C.; Hsueh, P.R. Increased Antimicrobial Resistance during the COVID-19 Pandemic. Int. J. Antimicrob. Agents 2021, 57, 106324.
- López-Serna, R.; Jurado, A.; Vázquez-Suñé, E.; Carrera, J.; Petrović, M.; Barceló, D. Occurrence of 95 Pharmaceuticals and Transformation Products in Urban Groundwaters Underlying the Metropolis of Barcelona, Spain. Environ. Pollut. 2013, 174, 305–315.
- Michaelis, C.; Grohmann, E. Horizontal Gene Transfer of Antibiotic Resistance Genes in Biofilms. Antibiotics 2023, 12, 328.
- Mukhtar, A.; Manzoor, M.; Gul, I.; Zafar, R.; Jamil, H.I.; Niazi, A.K.; Ali, M.A.; Park, T.J.; Arshad, M. Phytotoxicity of Different Antibiotics to Rice and Stress Alleviation upon Application of Organic Amendments. Chemosphere 2020, 258, 127353.
- Peng, X.; Yu, Y.; Tang, C.; Tan, J.; Huang, Q.; Wang, Z. Occurrence of Steroid Estrogens, Endocrine-Disrupting Phenols, and Acid Pharmaceutical Residues in Urban Riverine Water of the Pearl River Delta, South China. Sci. Total Environ. 2008, 397, 158–166.
- Peng, X.; Zhang, K.; Tang, C.; Huang, Q.; Yu, Y.; Cui, J. Distribution Pattern, Behavior, and Fate of Antibacterials in Urban Aquatic Environments in South China. J. Environ. Monit. 2011, 13, 446–454.
- Samal, K.; Mahapatra, S.; Hibzur Ali, M. Pharmaceutical Wastewater as Emerging Contaminants (EC): Treatment Technologies, Impact on Environment and Human Health. Energy Nexus 2022, 6, 100076.
- Serwecińska, L. Antimicrobials and Antibiotic-Resistant Bacteria: A Risk to the Environment and to Public Health. Water 2020, 12, 3313.
- Valcárcel, Y.; González Alonso, S.; Rodríguez-Gil, J.L.; Gil, A.; Catalá, M. Detection of Pharmaceutically Active Compounds in the Rivers and Tap Water of the Madrid Region (Spain) and Potential Ecotoxicological Risk. Chemosphere 2011, 84, 1336–1348.
- Andrade-Eiroa, A.; Canle, M.; Leroy-Cancellieri, V.; Cerdà, V. Solid-Phase Extraction of Organic Compounds: A Critical Review (Part I). Trends Anal. Chem. 2016, 80, 641–654.
- Braun, J.M. Early-Life Exposure to EDCs: Role in Childhood Obesity and Neurodevelopment. Nat. Rev. Endocrinol. 2017, 13, 161–173.
- Forte, M.; Di Lorenzo, M.; Carrizzo, A.; Valiante, S.; Vecchione, C.; Laforgia, V.; De Falco, M. Nonylphenol Effects on Human Prostate Non Tumorigenic Cells. Toxicology 2016, 357–358, 21–32.
- Forte, M.; Di Lorenzo, M.; Iachetta, G.; Mita, D.G.; Laforgia, V.; De Falco, M. Nonylphenol Acts on Prostate Adenocarcinoma Cells via Estrogen Molecular Pathways. Ecotoxicol. Environ. Saf. 2019, 180, 412–419.
- Gröger, T.M.; Käfer, U.; Zimmermann, R. Gas Chromatography in Combination with Fast High-Resolution Time-of-Flight Mass Spectrometry: Technical Overview and Perspectives for Data Visualization. Trends Anal. Chem. 2020, 122, 115677.
- Heindel, J.J.; Newbold, R.; Schug, T.T. Endocrine Disruptors and Obesity. Nat. Rev. Endocrinol. 2015, 11, 653–661.
- Li, C.; Wei, Y.; Zhang, S.; Tan, W. Advanced Methods to Analyze Steroid Estrogens in Environmental Samples. Environ. Chem. Lett. 2020, 18, 543–559.
- Marotta, V.; Russo, G.; Gambardella, C.; Grasso, M.; La Sala, D.; Chiofalo, M.G.; D’Anna, R.; Puzziello, A.; Docimo, G.; Masone, S.; et al. Human Exposure to Bisphenol AF and Diethylhexylphthalate Increases Susceptibility to Develop Differentiated Thyroid Cancer in Patients with Thyroid Nodules. Chemosphere 2019, 218, 885–894.
- Nadal, A.; Quesada, I.; Tudurí, E.; Nogueiras, R.; Alonso-Magdalena, P. Endocrine-Disrupting Chemicals and the Regulation of Energy Balance. Nat. Rev. Endocrinol. 2017, 13, 536–546.
- Izadi, P.; Izadi, P.; Salem, R.; Papry, S.A.; Magdouli, S.; Pulicharla, R.; Brar, S.K. Non-Steroidal Anti-Inflammatory Drugs in the Environment: Where Were We and How Far We Have Come? Environ. Pollut. 2020, 267, 115370.
- Jan-Roblero, J.; Cruz-Maya, J.A. Ibuprofen: Toxicology and Biodegradation of an Emerging Contaminant. Molecules 2023, 28, 2097.
- Parolini, M. Toxicity of the Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) Acetylsalicylic Acid, Paracetamol, Diclofenac, Ibuprofen and Naproxen towards Freshwater Invertebrates: A Review. Sci. Total Environ. 2020, 740, 140043.
- Tyumina, E.A.; Bazhutin, G.A.; Cartagena Gómez, A.d.P.; Ivshina, I.B. Nonsteroidal Anti-Inflammatory Drugs as Emerging Contaminants. Microbiology 2020, 89, 148–163.
- Huynh, N.C.; Nguyen, T.T.T.; Nguyen, D.T.C.; Tran, T. Van Occurrence, Toxicity, Impact and Removal of Selected Non-Steroidal Anti-Inflammatory Drugs (NSAIDs): A Review. Sci. Total Environ. 2023, 898, 165317.
- Gao, S.; Yang, F. Behavioral Changes and Neurochemical Responses in Chinese Rare Minnow Exposed to Four Psychoactive Substances. Sci. Total Environ. 2022, 808, 152100.
- Gupta, I.; Clauder-Münster, S.; Klaus, B.; Järvelin, A.I.; Aiyar, R.S.; Benes, V.; Wilkening, S.; Huber, W.; Pelechano, V.; Steinmetz, L.M. Alternative Polyadenylation Diversifies Post-Transcriptional Regulation by Selective RNA-Protein Interactions. Mol. Syst. Biol. 2014, 10, 719.
- Mousel, D.; Bastian, D.; Firk, J.; Palmowski, L.; Pinnekamp, J. Removal of Pharmaceuticals from Wastewater of Health Care Facilities. Sci. Total Environ. 2021, 751, 141310.
- Huggett, D.B.; Brooks, B.W.; Peterson, B.; Foran, C.M.; Schlenk, D. Toxicity of Select Beta Adrenergic Receptor-Blocking Pharmaceuticals (B-Blockers) on Aquatic Organisms. Arch. Environ. Contam. Toxicol. 2002, 43, 229–235.
- Maszkowska, J.; Stolte, S.; Kumirska, J.; Łukaszewicz, P.; Mioduszewska, K.; Puckowski, A.; Caban, M.; Wagil, M.; Stepnowski, P.; Białk-Bielińska, A. Beta-Blockers in the Environment: Part II. Ecotoxicity Study. Sci. Total Environ. 2014, 493, 1122–1126.
- De Oliveira, L.L.D.; Antunes, S.C.; Gonçalves, F.; Rocha, O.; Nunes, B. Acute and Chronic Ecotoxicological Effects of Four Pharmaceuticals Drugs on Cladoceran Daphnia Magna. Drug Chem. Toxicol. 2016, 39, 13–21.
- Rutere, C.; Posselt, M.; Ho, A.; Horn, M.A. Biodegradation of Metoprolol in Oxic and Anoxic Hyporheic Zone Sediments: Unexpected Effects on Microbial Communities. Appl. Microbiol. Biotechnol. 2021, 105, 6103–6115.
- Li, D.; Chen, H.; Liu, H.; Schlenk, D.; Mu, J.; Lacorte, S.; Ying, G.G.; Xie, L. Anticancer Drugs in the Aquatic Ecosystem: Environmental Occurrence, Ecotoxicological Effect and Risk Assessment. Environ. Int. 2021, 153, 106543.
- Nassour, C.; Barton, S.J.; Nabhani-Gebara, S.; Saab, Y.; Barker, J. Occurrence of Anticancer Drugs in the Aquatic Environment: A Systematic Review. Environ. Sci. Pollut. Res. 2020, 27, 1339–1347.
- Rowney, N.C.; Johnson, A.C.; Williams, R.J. Cytotoxic Drugs in Drinking Water: A Prediction and Risk Assessment Exercise for the Thames Catchment in the United Kingdom. Environ. Toxicol. Chem. 2009, 28, 2733–2743.
- Kümmerer, K. Antibiotics in the Aquatic Environment—A Review—Part I. Chemosphere 2009, 75, 417–434.
- Gore, A.C.; Chappell, V.A.; Fenton, S.E.; Flaws, J.A.; Nadal, A.; Prins, G.S.; Toppari, J.; Zoeller, R.T. Executive Summary to EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr. Rev. 2015, 36, 593–602.
- Kabir, E.R.; Rahman, M.S.; Rahman, I. A Review on Endocrine Disruptors and Their Possible Impacts on Human Health. Environ. Toxicol. Pharmacol. 2015, 40, 241–258.
- Kiyama, R.; Wada-Kiyama, Y. Estrogenic Endocrine Disruptors: Molecular Mechanisms of Action. Environ. Int. 2015, 83, 11–40.
- Gore, A.C.; Crews, D.; Doan, L.L.; Merrill, M.L.; Patisaul, H.; Zota, A. Introduction to Endocrine Disrupting Chemicals (EDCs): A Guide for Public Interest Organizations and Policymakers; IPEN: Göteborg, Sweden, 2014; pp. 21–22.
- Tijani, J.O.; Fatoba, O.O.; Babajide, O.O.; Petrik, L.F. Pharmaceuticals, Endocrine Disruptors, Personal Care Products, Nanomaterials and Perfluorinated Pollutants: A Review. Environ. Chem. Lett. 2016, 14, 27–49.
- Huang, S.; Qi, Z.; Ma, S.; Li, G.; Long, C.; Yu, Y. A Critical Review on Human Internal Exposure of Phthalate Metabolites and the Associated Health Risks. Environ. Pollut. 2021, 279, 116941.
- De Coster, S.; Van Larebeke, N. Endocrine-Disrupting Chemicals: Associated Disorders and Mechanisms of Action. J. Environ. Public Health 2012, 2012, 713696.
- Monneret, C. What Is an Endocrine Disruptor? Comptes Rendus. Biol. 2017, 340, 403–405.
- Akhbarizadeh, R.; Russo, G.; Rossi, S.; Golianova, K.; Moore, F.; Guida, M.; De Falco, M.; Grumetto, L. Emerging Endocrine Disruptors in Two Edible Fish from the Persian Gulf: Occurrence, Congener Profile, and Human Health Risk Assessment. Mar. Pollut. Bull. 2021, 166, 112241.
- Vieira, W.T.; de Farias, M.B.; Spaolonzi, M.P.; da Silva, M.G.C.; Vieira, M.G.A. Removal of Endocrine Disruptors in Waters by Adsorption, Membrane Filtration and Biodegradation. A Review. Environ. Chem. Lett. 2020, 18, 1113–1143.
- Gonsioroski, A.; Mourikes, V.E.; Flaws, J.A. Endocrine Disruptors in Water and Their Effects on the Reproductive System. Int. J. Mol. Sci. 2020, 21, 1929.
- Feng, L.; van Hullebusch, E.D.; Rodrigo, M.A.; Esposito, G.; Oturan, M.A. Removal of Residual Anti-Inflammatory and Analgesic Pharmaceuticals from Aqueous Systems by Electrochemical Advanced Oxidation Processes. A Review. Chem. Eng. J. 2013, 228, 944–964.
- Azmi Hassali, M.; Shakeel, S. Unused and Expired Medications Disposal Practices among the General Public in Selangor, Malaysia. Pharmacy 2020, 8, 196.
- Vieno, N.; Tuhkanen, T.; Kronberg, L. Elimination of Pharmaceuticals in Sewage Treatment Plants in Finland. Water Res. 2007, 41, 1001–1012.
- Xu, J.; Sun, H.; Zhang, Y.; Alder, A.C. Occurrence and Enantiomer Profiles of β-Blockers in Wastewater and a Receiving Water Body and Adjacent Soil in Tianjin, China. Sci. Total Environ. 2019, 650, 1122–1130.
- Godoy, A.A.; Kummrow, F.; Pamplin, P.A.Z. Occurrence, Ecotoxicological Effects and Risk Assessment of Antihypertensive Pharmaceutical Residues in the Aquatic Environment—A Review. Chemosphere 2015, 138, 281–291.
- Maszkowska, J.; Stolte, S.; Kumirska, J.; Łukaszewicz, P.; Mioduszewska, K.; Puckowski, A.; Caban, M.; Wagil, M.; Stepnowski, P.; Białk-Bielińska, A. Beta-Blockers in the Environment: Part I. Mobility and Hydrolysis Study. Sci. Total Environ. 2014, 493, 1112–1121.
- Heath, E.; Filipič, M.; Kosjek, T.; Isidori, M. Fate and Effects of the Residues of Anticancer Drugs in the Environment. Environ. Sci. Pollut. Res. 2016, 23, 14687–14691.
- Wormington, A.M.; De María, M.; Kurita, H.G.; Bisesi, J.H.; Denslow, N.D.; Martyniuk, C.J. Antineoplastic Agents: Environmental Prevalence and Adverse Outcomes in Aquatic Organisms. Environ. Toxicol. Chem. 2020, 39, 967–985.
More
