Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Peter Tang and Version 1 by Reinald Pamplona.
Non-enzymatic modification of aminophospholipids by lipid peroxidation-derived aldehydes and reducing sugars through carbonyl-amine reactions are thought to contribute to the age-related deterioration of cellular membranes and to the pathogenesis of diabetic complications. Much evidence demonstrates the modification of aminophospholipids by glycation, glycoxidation and lipoxidation reactions. Therefore, a number of early and advanced Maillard reaction-lipid products have been detected and quantified in different biological membranes. These modifications may be accumulated during aging and diabetes, introducing changes in cell membrane physico-chemical and biological properties.
- advanced glycation endproducts
- advanced lipoxidation endproducts
- age-associated diseases
- aging
- cell membrane
- carbonyl compounds
- lipid peroxidation
- Maillard reaction products
- oxidative stress
- phosphatidylethanolamine
1. Introduction
Life demands membranes. Biological membranes are dynamic structures that generally consist of amphipathic molecules bilayers held together by non-covalent bonds [1,2][1][2]. In eukaryotic cells, phospholipids are the predominant membrane lipids and are, from a topographic point of view, asymmetrically distributed across the bilayer [3–5][3][4][5]. Phospholipids consist of a hydrophilic head group with attached hydrophobic acyl chains. The variation in head groups and aliphatic chains allows the existence of more than 1000 different phospholipid species in any eukaryotic cell [6,7][6][7]. Phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylserine (PS), phosphatidylinositol (PI) and cardiolipin (CL), as well as sphingomyelin (SM) and glycosphingolipids (GS) are the major phospholipid classes [1,2][1][2]. In most eukaryotic membranes, PC and PE represent together around 60%–85% of the phospholipid fraction, while the fraction of other phospholipids depends on the cell membrane and even on animal species [1,2,8,9][1][2][8][9]. Phospholipids play multiple roles. They constitute a permeability barrier, modulate the functional properties of membrane-associated activities, provide a matrix for the assembly and function of a wide variety of catalytic processes, and act as donors during the synthesis of macromolecules. The wide range of processes in which phospholipids are specifically involved explains the need for diversity in phospholipid structures and fatty acid composition [6,10][6][10]. This diversity requires complex metabolic and regulatory pathways [1,2][1][2]. Therefore, for example, eukaryotic cells invest around 5% of their genes to synthesize all of these lipids [9].
The various phospholipid classes that comprise cell membranes are distributed over both leaflets of the bilayer in a non-random fashion [3–5][3][4][5]. This is especially evident for the aminophospholipids, PS and PE, which preferentially reside in the plasma membranes’ inner leaflet [11,12][11][12]. Where does lipid asymmetry originate, and how is it maintained and regulated? What is the functional role of aminophospholipid asymmetry, and what are the consequences of the breakdown of regulatory processes that result in the exposure of aminophospholipids in the cells’ outer leaflet? Although these key questions mainly remain unanswered, recent studies have established that cells have developed a number of mechanisms to deal with this issue. Targeting phospholipids to specific membrane sites is essential for maintaining critical signal transduction cascades, cell shape, hemostasis, and homeostasis [12]. In particular, aminophospholipids have been implicated in a diverse array of processes ranging from cell proliferation to cell death, from catabolism to inflammation [12]. In this scenario, asymmetry is maintained by active ATP-dependent processes, suggesting that is critical to normal cell function. Specifically, aminophospholipid asymmetry is controlled by one or more specific mechanisms, which involve selective interactions between lipids and cytoskeletal proteins and an aminophospholipid-specific active transport system [11,12][11][12].
The acyl chains are composed of either saturated, monounsaturated or polyunsaturated hydrocarbon chains that normally vary from 14 to 22 carbons in length [13]. In eukaryotic cells from vertebrate species, the average chain length of a biological membrane is strictly maintained by around 18 carbon atoms, and the relative distribution between saturated and unsaturated fatty acids follows the ratio 40:60 [14]. Polyunsaturated fatty acids (PUFAs) are essential components of cellular membranes in higher eukaryotes that strongly affect their fluidity, flexibility and selective permeability. Additionally, PUFAs affect many cellular and physiological processes in animals, including modulation of ion channels and carriers, activities of membrane-associated enzymes, and regulation of gene expression, among others [13].
2. Membrane Unsaturation and Lipid Peroxidation
As a principle, chemical reactions in living cells are under strict enzyme control and are tightly regulated by the metabolic program. One of the attractors involved in biomolecular evolution is the minimizing of unnecessary side reactions. Nevertheless, uncontrolled and potentially deleterious reactions occur, even under physiological conditions. Oxidative damage is a broad term used to cover the attack upon biological molecules by free radicals—chemical species with one unpaired electron. Free radicals attack/damage all cellular constituents [14]. In this context, the susceptibility of membrane phospholipids to oxidative damage is related to two inherent traits, the physico-chemical properties of the membrane bilayer and the chemical reactivity of the fatty acids composing the membrane [15]. The first property is related to the fact that oxygen and free radicals are more soluble in the fluid lipid bilayer than in the aqueous solution. Thus, membranes contain an interior organic phase, in which oxygen may tend to concentrate. Therefore, these differences in solubility are important when considering the availability of oxygen/free radicals for chemical reactions inside living systems: Organic regions may contain more free radicals than aqueous regions [16,17][16][17] and, consequently, membrane lipids become primary targets of oxidative damage. The second property is related to the fact that PUFA residues of phospholipids are extremely sensitive to oxidation. Every membrane phospholipid contains an unsaturated fatty acid residue esterified to the 2-hydroxyl group of its glycerol moiety. Many of these are polyunsaturated and the presence of a methylene group between two double bonds renders the fatty acid sensitive to free radical-induced damage, their sensitivity to oxidation increasing exponentially as a function of the number of double bonds per fatty acid molecule [18,19][18][19]. Consequently, the high concentration of PUFAs in phospholipids not only makes them prime targets for reaction with oxidizing agents but also enables them to participate in long free radical chain reactions. Lipid peroxidation generates hydroperoxides as well as endoperoxides, which undergo fragmentation to produce a broad range of reactive intermediates called reactive carbonyl species (RCS) with three to nine carbons in length (see Figure 1), the most reactive being α,β-unsaturated aldehydes (4-hydroxy-trans-2-nonenal (HNE) and acrolein), di-aldehydes (malondialdehyde (MDA) and glyoxal), and keto-aldehydes (4-oxo-trans-2-nonenal (ONE) and isoketals) [20,21][20][21]. 2-Hydroxyheptanal (2-HH) is another major aldehydic product of lipid peroxidation of PUFAn-6, while 4-hydroxyhexenal (4-HHE) is generated in a lower yield. Additionally, a number of other short chain aldehydes are produced during lipid peroxidation through poorly understood mechanisms. These carbonyl compounds, ubiquitously generated in biological systems, have unique properties contrasted with free radicals [14]. For instance, compared with free radicals, reactive aldehydes have a much longer half-life (i.e., minutes to hours instead of microseconds to nanoseconds for most free radicals). Further, the non-charged structure of aldehydes allows them to migrate with relative ease through hydrophobic membranes and hydrophilic cytosolic media, thereby extending the migration distance far from the production site. Based on these features alone, these carbonyl compounds can be more destructive than free radicals and may have far-reaching damaging effects on target sites within or outside membranes.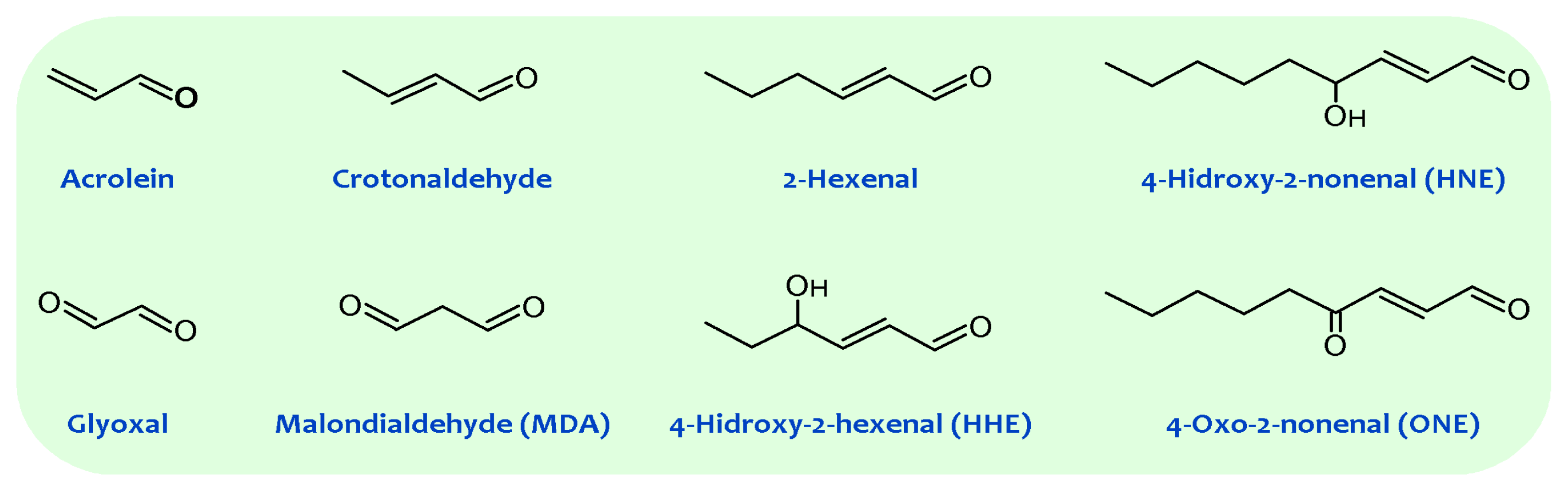
Figure 1.
General structures of principal reactive carbonyl species detected in biological systems.
3. Non-Enzymatic Modification of Cellular Components: The Maillard Reaction-Derived Molecular Damage
Carbonyl compounds react with nucleophilic groups in macromolecules like proteins and DNA, resulting in their chemical, non-enzymatic, and irreversible modification and finally in the formation of a variety of adducts and cross-links collectively named Advanced Lipoxidation Endproducts (ALEs) [22,23][22][23]. Thus, by reacting with nucleophilic sites in proteins (belonging basically to Cys, Lys, Arg, and His residues), carbonyl compounds generate ALE adducts such as MDA-Lys, HNE-Lys, FDP-Lys, and S-carboxymethyl-cysteine; and the cross-links glyoxal-lysine dimmer, and lysine-MDA-lysine, among several others. The accumulation of MDA adducts on proteins is also involved in the formation of lipofuscin. Thus, lipofuscin becomes a nondegradable intralysosomal fluorescent pigment formed through lipoxidative reactions [24]. In addition to proteins, lipid peroxidation-derived endproducts can also react with the exocyclic amino groups of deoxyguanosine, deoxyadenosine, and deoxycytosine to form various alkylated products [25]. Guanine is, however, the most commonly modified DNA base because of its high nucleophilicity. Some common enals that cause DNA damage, analogously to proteins, are MDA, HNE, and acrolein, among others. Thus, the most common adducts arising from enals are exocyclic adducts such as etheno adducts, and MDA-deoxyguanosine (M1dG). In addition to lipid peroxidation derived carbonyl compounds, reducing sugars and carbonyl compounds derived from carbohydrate oxidation can also react with the primary amino groups of macromolecules such as proteins and DNA, following the principles of the carbonyl-amine reaction (also named Maillard reaction) [22]. The early Schiff base and Amadori adducts (glycation reaction), which form subsequently, slowly undergo a succession of intramolecular rearrangements, dehydration, and oxidation-reduction reactions to produce the terminal products termed advanced glycation end products (AGEs), which are often chemically irreversible, thus persisting throughout the life of the affected macromolecule [26,27][26][27]. More important, a major spin-off of studies on glycation during the 1980s was the recognition that oxidative reactions, and by inference, oxidative stress, catalyzed the chemical modification of proteins and DNA by Maillard reactions in vivo [28]. In this scenario, it is likely that the amino group of aminophospholipids will also react with carbonyl compounds and initiates some of the reactions occurring in proteins and DNA, expanding the biological effects of the carbonyl-amine or Maillard reaction (see Figure 2).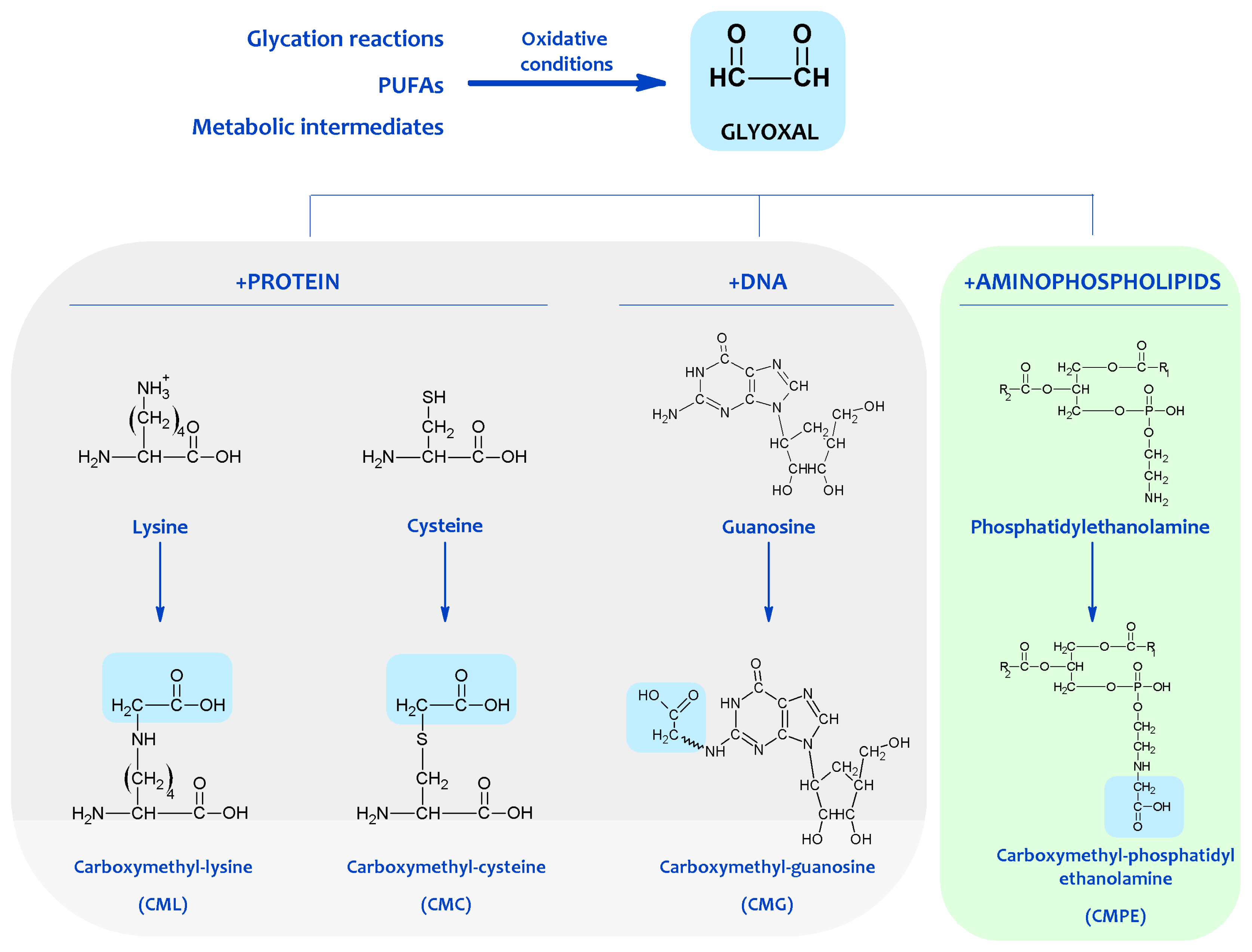
Figure 2. Protein, DNA and aminophospholipid damage resulting from carbonyl products of lipid peroxidation. Shown are examples of molecular adducts (Advanced Lipoxidation Endproducts, ALEs) generated by the reactive carbonyl compound glyoxal.
4. Chemical Modification of Aminophospholipids by Carbonyl-Amine Reactions
Recent reports indicate that, like proteins and DNA, aminophospholipids are also targets of Maillard-type reactions. In early studies, the formation of products resulting from the reaction between aminophospholipids and lipoperoxidation-derived aldehydes such as MDA and HNE were described, but the exact structure of the products was not established. In these works, it was established that the amount of free amino groups significantly decreased during oxidation of phospholipids, developing a brownish-yellow color attributed to a Maillard-type reaction [29]. It was also reported that free amino groups of PE disappeared during oxidation in proportion to the oxygen absorbed [30]. Probably, both findings can be attributed to the reaction of the PE amino group with carbonyls, mainly MDA produced by lipid oxidation, leading to Schiff base formation as assessed by fluorescence. So, during peroxidation, PE and PS formed fluorescent chromophores with maximum emission ranging from 440 to 490 nm and maximum excitation between 360 and 400 nm [31,32][31][32]. Fluorescence development was related to (i) formation of thiobarbituric acid reactive substances (TBARS), especially MDA; (ii) reaction time; (iii) availability of reactive amino groups on the aminophospholipids, and (iv) antioxidant (alpha-tocopherol) content in an inverse fashion. These chromophores showed similarities to those formed in model membranes [33] or in rat liver mitochondrial and microsomal fractions peroxidized in vitro [31,32][31][32]. Furthermore, lipid extracts isolated from lipofuscin [34] and from tissues of lipid peroxidation-experimental models such as old, vitamin E deficient animals or animals stressed with highly unsaturated lipid diets, showed similar fluorescence properties [31,32][31][32]. Using thin-layer chromatography (TLC), non-enzymatic modification of aminophospholipids by lipoperoxidation derived products were also detected in red blood cells (RBC) [35–42][35][36][37][38][39][40][41][42] and in eye lens membranes [43,44][43][44]. These modifications corresponded to a Schiff’s base adduct formed by cross-linking the PE and PS amino with MDA aldehyde groups, based on the following evidence: (i) A new lipid spot appeared between PS and PE; (ii) its intensity was proportional to MDA concentration both in vivo and in vitro; (iii) in selective staining procedures, it was phosphorus positive and ninhydrin negative; (iv) when this compound was exposed to acid vapors and then developed in a second direction, the “adduct” was resolved into two equimolar spots of PS and PE which were ninhydrin positive; (v) other non-amino phospholipids were ineffective in the formation of this compound; (vi) its fluorescence characteristics were compatible with a Schiff base conjugate formed between MDA and aminophospholipids; and finally, (vii) added antioxidants, blocking MDA formation, avoided its appearance. Lipid peroxidation leads to the formation of other aldehydes, such as HNE, able to react with aminophospholipids. Accordingly, the formation of fluorescent chromolipids was detected when HNE was incubated with aminophospholipids, microsomes and mitochondrial fractions [45,46][45][46]. Spectral characteristics of these chromolipids showed excitation maxima at 350–360 nm and emission at 430 nm, with the fluorescence intensity being linearly related to the number of HNE molecules reacting with aminophospholipids [45]. More recently, HNE potential capacity to react with aminophospholipids has been reported. Thus, by using TLC-high performance liquid chromatography (HPLC)-liquid chromatography (LC)-mass spectrometry (MS) techniques, it has been found that the main resulting compounds were a Michael adduct plus a minor Schiff base adduct, partly cyclized as a pyrrole derivative via a loss of water, with PE being more reactive than PS [46]. Much evidence demonstrates the in vitro and in vivo occurrence of the Schiff base, Amadori and AGEs-lipid products resulting from the Maillard reaction (see also Figure 3). The Schiff base formation between glucose and aminophospholipids was documented in experimental models and in human RBC membranes, plasma, and low-density lipoproteins (LDL) [53,77–79][47][48][49][50]. The existence of glycated aminophospholipids in its Schiff base form was confirmed by using HPLC LC-electrospray ionization (ESI)-MS. Reduction with NaBH3CN, shifting the retention time and increasing the detected mass of glycated lipids by two units, confirmed the identity of the major analyte as a Schiff base. Surprisingly, only the diacyl species became glycated and neither the alkylacyl nor the alkenylacyl were modified; furthermore, in contrast with in vitro experiments, PS glycation was not detected.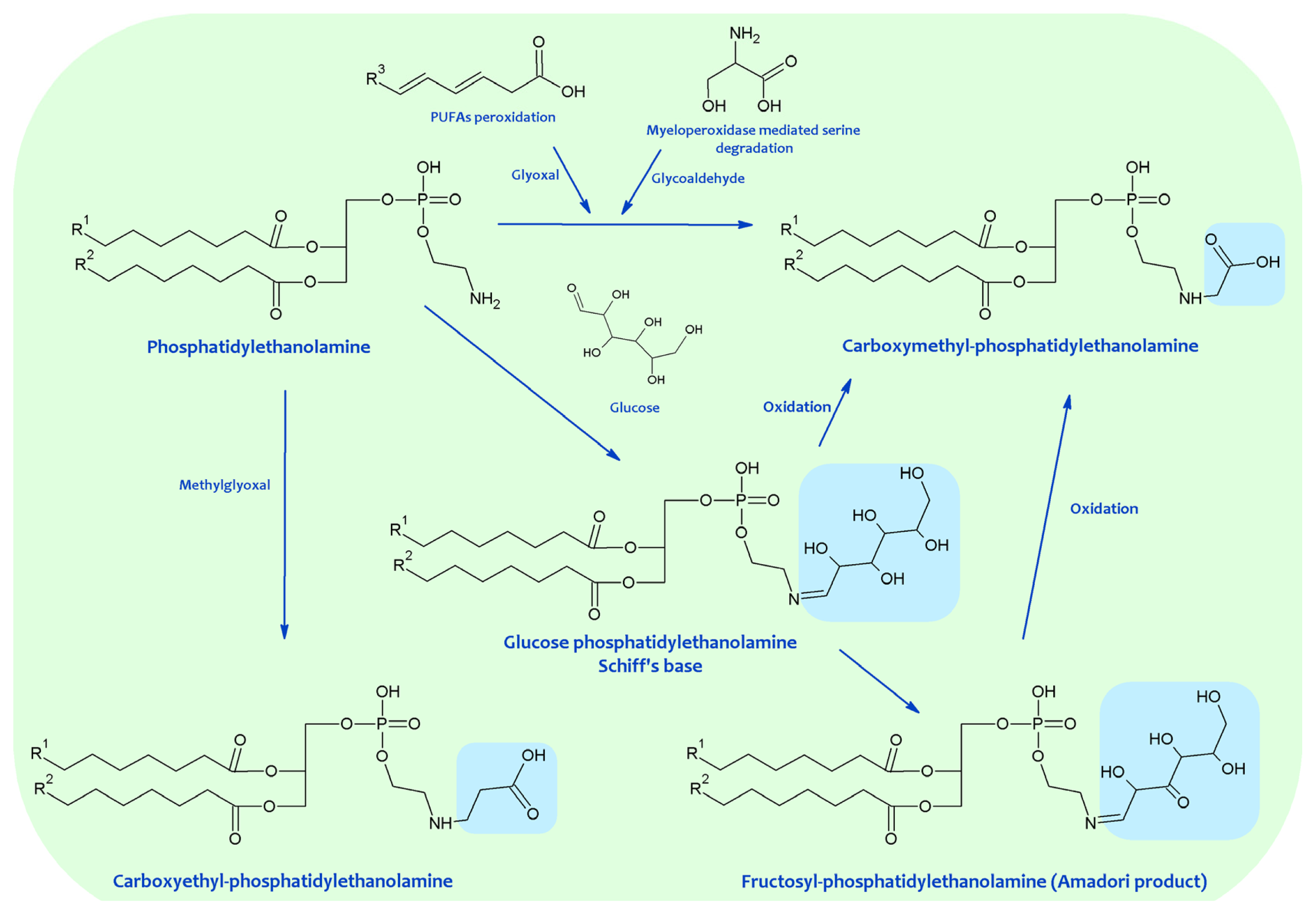
Figure 3.
Advanced glycation end products (AGEs)-lipid products resulting from the Maillard reaction.
References
- Yeagle, P. The Membranes of Cells; Academic Press: San Diego, CA, USA, 1993; pp. 1–349.
- Vance, D.E.; Vance, J.E. Biochemistry of Lipids, Lipoproteins and Membranes; Elsevier Science BV: Amsterdam, The Netherlands, 1996; pp. 1–553.
- Vereb, G.; Szollosi, J.; Matko, J.; Nagy, P.; Farkas, T.; Vigh, L.; Matyus, L.; Waldmann, T.A.; Damjanovich, S. Dynamic, yet structured: The cell membrane three decades after the Singer-Nicolson model. Proc. Natl. Acad. Sci. USA 2003, 100, 8053–8058.
- Ikeda, M.; Kihara, A.; Igarashi, Y. Lipid asymmetry of the eukaryotic plasma membrana: Functions and related enzymes. Biol. Pharm. Bull 2006, 29, 1542–1546.
- Lenoir, G; Williamson, P.; Holthuis, J.C.M. On the origin of lipid asymmetry: The flip side of ion transport. Curr. Opin. Chem. Biol. 2007, 11, 654–661.
- Dowhan, W. Molecular basis for membrane phospholipid diversity: Why are there so many lipids? Ann. Rev. Biochem 1997, 66, 199–232.
- Van Meer, G. Cellular lipidomics. EMBO J 2005, 24, 3159–3165.
- Portero-Otín, M.; Bellmunt, M.J.; Ruiz, M.C.; Barja, G.; Pamplona, R. Correlation of fatty acid unsaturation of the major liver mitochondrial phospholipid classes in mammals to their maximum life span potential. Lipids 2001, 36, 491–498.
- Van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane lipids: Where they are and how they behave. Nat. Rev. Mol. Cell Biol 2008, 9, 112–124.
- Hermansson, M.; Hokynar, K.; Somerharju, P. Mechanisms of glycerophospholipid homeostasis in mammalian cells. Prog. Lipid Res 2011, 50, 240–257.
- Schroit, A.J.; Zwaal, R.F.A. Transbilayer movement of phospholipids in red cell and platelet membranes. Biochim. Biophys. Acta 1991, 1071, 313–329.
- Balasubramanian, K.; Schroit, A.J. Aminophospholipid asymmetry: A matter of lifeand death. Ann. Rev. Physiol 2003, 65, 701–734.
- Wallis, J.G.; Watts, J.L.; Browse, J. Polyunsaturated fatty acid synthesis: What will they think of next? Trends Biochem. Sci 2002, 27, 467–473.
- Pamplona, R. Membrane phospholipids, lipoxidative damage and molecular integrity: A causal role in aging and longevity. Biochim. Biophys. Acta 2008, 1777, 1249–1262.
- Hulbert, A.J.; Pamplona, R.; Buffenstein, R.; Buttemer, W.A. Life and death: Metabolic rate, membrane composition, and life span of animals. Physiol. Rev 2007, 87, 1175–1213.
- Moller, M.; Botti, H.; Batthyany, C.; Rubbo, H.; Radi, R.; Denicola, A. Direct measurement of nitric oxide and oxygen partitioning into liposomes and low density lipoprotein. J. Biol. Chem 2005, 280, 8850–8854.
- Gamliel, A.; Afri, M.; Frimer, A.A. Determining radical penetration of lipid bilayers with new lipophilic spin traps. Free Radical Biol. Med 2008, 44, 1394–1405.
- Holman, R.T. Autoxidation of Fats and Related Substances. In Progress in Chemistry of Fats and Other Lipids; Holman, R.T., Lundberg, W.O., Malkin, T., Eds.; Pergamon Press: London, UK, 1954; pp. 51–98.
- Bielski, B.H.; Arudi, R.L.; Sutherland, M.W. A study of the reactivity of HO2/O2− with unsaturated fatty acids. J. Biol. Chem 1983, 258, 4759–4761.
- Esterbauer, H.; Schaur, R.J.; Zollner, H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radical Biol. Med 1991, 11, 81–128.
- Aldini, G.; Dalle-Donne, I.; Facino, R.M.; Milzani, A.; Carini, M. Intervention strategies to inhibit protein carbonylation by lipoxidation-derived reactive carbonyls. Med. Res. Rev 2007, 27, 817–868.
- Thorpe, S.R.; Baynes, J.W. Maillard reaction products in tissue proteins: New products and new perspectives. Amino Acids 2003, 25, 275–281.
- Pamplona, R. Advanced lipoxidation end-products. Chem.-Biol. Interact 2011, 192, 14–20.
- Terman, A.; Brunk, U.T. Lipofuscin. Int. J. Biochem. Cell Biol 2004, 36, 1400–1404.
- West, J.D.; Marnett, L.J. Endogenous reactive intermediates as modulators of cell signalling and cell death. Chem. Res. Toxicol 2006, 19, 173–194.
- Baynes, J.W.; Monnier, V.M. The Maillard Reaction in Aging, Diabetes and Nutrition; Liss: New York, NY, USA, 1989.
- Portero-Otin, M.; Pamplona, R. Is Endogenous Oxidative Protein Damage Envolved in the Aging Process? In Protein Oxidation and Disease; Pietzsch, J., Ed.; Research Signpost: Kerala, India, 2006; pp. 91–142.
- Baynes, J.W. The Role of Oxidation in the Maillard Reaction in vivo. In The Maillard Reaction: Consequences for the Chemical and Life Sciences; Ika, R., Ed.; John Wiley & Sons: Chichester, UK, 1996; pp. 55–72.
- Corliss, G.A.; Dugan, L.R. Phospholipid oxidation in emulsions. Lipids 1970, 5, 846–853.
- Popjak, G.; LeBreton, E. Biochemical Problems of Lipids; Interscience Publishers Inc.: New York, NY, USA, 1956; p. 81.
- Dillard, C.J.; Tappel, A.L. Fluorescent products from reaction of peroxidizing polyunsaturated fatty acids with phosphatidyl ethanolamine and phenylalanine. Lipids 1973, 8, 183–189.
- Bidlack, W.R.; Tappel, A.L. Fluorescent products of phospholipids during lipid peroxidation. Lipids 1973, 8, 203–207.
- Shimasaki, H.; Ueta, N.; Mowri, H.-O.; Inoue, K. Formation of age pigment-like fluorescent substances during peroxidation of lipids in model membranes. Biochim. Biophys. Acta 1984, 792, 123–129.
- Tsuchida, M.; Miura, T.; Aibara, K. Lipofucsin and lipofucsin-like substances. Chem. Phys. Lipids 1987, 44, 297–325.
- Jain, S.K.; Hochstein, P. Polymerization of membrane components in aging red blood cells. Biochem. Biophys. Res. Commun 1980, 92, 247–254.
- Jain, S.K.; Yip, R.; Hoesch, R.M.; Pramanik, A.K.; Dallman, P.R.; Shohet, S.B. Evidence of peroxidative damage to the erythrocyte membrane in iron deficiency. Am. J. Clin. Nutr 1983, 37, 26–30.
- Jain, S.K.; Shohet, S.B. A novel phospholipid in irreversibly sickled cells: Evidence for in vivo peroxidative membrane damage in sickle cell disease. Blood 1984, 63, 362–367.
- Jain, S.K. The accumulation of malonyldialdehyde, a product of fatty acid peroxidation, can disturb aminophospholipid organization in the membrane bilayer of human erythrocytes. J. Biol. Chem 1984, 259, 3391–3394.
- Jain, S.K. In vivo externalization of phosphatidylserine and phosphatidylethanolamine in the membrane bilayer and hypercoagulability by the lipid peroxidation of erythrocytes in rats. J. Clin. Invest 1985, 76, 281–286.
- Jain, S.K. Evidence for membrane lipid peroxidation during the in vivo aging of human erythrocytes. Biochim. Biophys. Acta 1988, 937, 205–210.
- Jain, S.K.; McVie, R.; Duett, J.; Herbst, J.J. Erythrocyte membrane lipid peroxidation and glycosylated hemoglobin in diabetes. Diabetes 1989, 38, 1539–1543.
- Jain, S.K. Hyperglycemia can cause membrane lipid peroxidation and osmotic fragility in human red blood cells. J. Biol. Chem 1989, 264, 21340–21345.
- Bhuyan, K.C.; Master, R.W.; Coles, R.S.; Bhuyan, D.K. Molecular mechanisms of cataractogenesis: IV. Evidence of phospholipid.malondialdehyde adduct in human senile cataract. Mech. Ageing Dev 1986, 34, 289–296.
- Bhuyan, D.K.; Master, R.W.; Bhuyan, K.C. Crosslinking of aminophospholipids in cellular membranes of lens by oxidative stress in vitro. Biochim. Biophys. Acta 1996, 1285, 21–28.
- Esterbauer, H.; Koller, E.; Slee, R.G.; Koster, J.F. Possible involvement of the lipid-peroxidation product 4-hydroxynonenal in the formation of fluorescent chromolipids. Biochem. J 1986, 239, 405–409.
- Guichardant, M.; Taibi-Tronche, P.; Fay, L.B.; Lagarde, M. Covalent modifications of aminophospholipids by 4-hydroxynonenal. Free Radic. Biol. Med 1998, 25, 1049–1056.
- Ravandi, A.; Kuksis, A.; Marai, L.; Myher, J.J. Preparation and characterization of glucosylated aminoglycerophospholipids. Lipids 1995, 30, 885–891.
- Ravandi, A.; Kuksis, A.; Marai, L.; Myher, J.J.; Steiner, G.; Lewisa, G.; Kamido, H. Isolation and identification of glycated aminophospholipids from red cells and plasma of diabetic blood. FEBS Lett 1996, 381, 77–81.
- Ravandi, A.; Kuksis, A.; Shaikh, N.; Jackowski, G. Preparation of Schiff base adducts of phosphatidylcholine core aldehydes and aminophospholipids, amino acids, and myoglobin. Lipids 1997, 32, 989–1001.
- Ravandi, A.; Kuksis, A.; Shaikh, N.A. Glycated phosphatidylethanolamine promotes macrophage uptake of low density lipoprotein and accumulation of cholesteryl esters and triacylglycerols. J. Biol. Chem 1999, 274, 16494–16500.
- Lederer, M.O.; Dreisbusch, C.M.; Bundschuh, R.M. Amadori products from model reactions of d-glucose with phosphatidyl ethanolamine. Independent synthesis and identification of 1-deoxy-1-(2-hydroxyethylamino)-d-fructose derivatives. Carbohyd. Res 1997, 301, 111–121.
- Lertsiri, S.; Shiraishi, M.; Miyazawa, T. Identification of deoxy-D-fructosyl phosphatidyl ethanolamine as a non-enzymic glycation product of phosphatidyl ethanolamine and its occurrence in human blood plasma and red blood cells. Biosci. Biotechnol. Biochem 1998, 62, 893–901.
- Argirov, O.K.; Kerina, I.I.; Uzunova, J.I.; Argirova, M.D. Modeling of Protein and Aminophospholipid Glycation Using Low Molecular Weight Analogs. A Comparative Study. In The Maillard Reaction in Foods and Medicine; O’brien, J., Nursten, H.E., Crabbe, M.J.C., Ames, J.M., Eds.; The Royal Society of Chemistry: Cambridge, UK, 1998; pp. 245–249.
- Fountain, W.C.; Requena, J.R.; Jenkins, A.J.; Lyons, T.J.; Smyth, B.; Baynes, J.W.; Thorpe, S.R. Quantification of N-(Glucitol)ethanolamine and N-(Carboxymethyl)serine: Two products of nonenzymatic modification of aminophospholipids formed in vivo. Anal. Biochem 1999, 272, 48–55.
- Pamplona, R.; Bellmunt, M.J.; Portero-Otin, M.; Riba, D.; Prat, J. Chromatographic evidence for Amadori product formation in rat liver aminophospholipids. Life Sci 1995, 57, 873–879.
- Lederer, M.O.; Dreisbusch, C.M.; Bundschuh, R.M. Amadori products from model reactions of d-glucose with phosphatidyl ethanolamine. Independent synthesis and identification of 1-deoxy-1-(2-hydroxyethylamino)-d-fructose derivatives. Carbohyd. Res 1997, 301, 111–121.
- Lertsiri, S.; Shiraishi, M.; Miyazawa, T. Identification of deoxy-D-fructosyl phosphatidyl ethanolamine as a non-enzymic glycation product of phosphatidyl ethanolamine and its occurrence in human blood plasma and red blood cells. Biosci. Biotechnol. Biochem 1998, 62, 893–901.
- Fountain, W.C.; Requena, J.R.; Jenkins, A.J.; Lyons, T.J.; Smyth, B.; Baynes, J.W.; Thorpe, S.R. Quantification of N-(Glucitol)ethanolamine and N-(Carboxymethyl)serine: Two products of nonenzymatic modification of aminophospholipids formed in vivo. Anal. Biochem 1999, 272, 48–55.
- Pamplona, R.; Bellmunt, M.J.; Portero-Otin, M.; Riba, D.; Prat, J. Chromatographic evidence for Amadori product formation in rat liver aminophospholipids. Life Sci 1995, 57, 873–879.
- Bucala, R.; Makita, Z.; Koschinsky, T.; Cerami, A.; Vlassara, H. Lipid advanced glycosylation: Pathway for lipid oxidation in vivo. Proc. Natl. Acad. Sci. USA 1993, 90, 6434–6438.
- Reddy, S.; Bichler, J.; Wells-Knecht, K.J.; Thorpe, S.R.; Baynes, J.W. Ne-(Carboxymethyl)lysine is a dominant advanced glycation end product (AGE) antigen in tissue proteins. Biochemistry 1995, 34, 10872–10878.
- Requena, J.R.; Ahmed, M.U.; Fountain, C.W.; Degenhardt, T.P.; Reddy, S.; Perez, C.; Lyons, T.J.; Jenkins, A.J.; Baynes, J.W.; Thorpe, S.R. Carboxymethylethanolamine, a biomarker of phospholipid modification during the Maillard reaction in vivo. J. Biol. Chem 1997, 272, 17473–17479.
- Pamplona, R.; Requena, J.R.; Portero-Otin, M.; Prat, J.; Thorpe, S.R.; Bellmunt, M.J. Carboxymethylated phosphatidylethanolamine in mitochondrial membranes of mammals. Evidence for intracellular lipid glycoxidation. Eur. J. Biochem 1998, 225, 685–689.
- Requena, J.R.; Ahmed, M.U.; Reddy, S.; Fountain, C.W.; Degenhardt, T.P.; Jenkins, A.J.; Smyth, B.; Lyons, T.J.; Thorpe, S.R. Detection of AGE-Lipids in vivo: Glycation and Carboxymethylation of Aminophospholipids in Red Cell Membranes. In The Maillard Reaction in Foods and Medicine; O’brien, J., Nursten, H.E., Crabbe, M.J.C., Ames, J.M., Eds.; The Royal Society of Chemistry: Cambridge, UK, 1998; pp. 363–368.
- Jenkins, A.; Lyons, T.J.; Smyth, B.; Requena, J.R.; Fountain, C.W.; Hermayer, K.L.; Phillips, K.D.; King, L.P.; Baynes, J.W.; Thorpe, S.R. Glycoxidation and lipoxidation products in red blood cell membranes in IDDM. Relationship to glycemic control and microvascular complications. Diabetes 1998, 47, A127.
- Fu, M.X.; Requena, J.R.; Jenkins, A.J.; Lyons, T.J.; Baynes, J.W.; Thorpe, S.R. The advanced glycation end product Ne-(Carboxymethyl)lysine is a product of both lipid peroxidation and glycoxidation reactions. J. Biol. Chem 1996, 271, 9982–9986.
- Anderson, M.M.; Requena, J.R.; Crowley, J.R.; Thorpe, S.R.; Heinecke, J.W. The myeloperoxidase system of human phagocytes generates Ne-(carboxymethyl)lysine on proteins: A mechanism for producing advanced glycation end products at sites of inflammation. J. Clin. Invest 1999, 104, 103–113.
- Wang, J.-Y.; Wang, Z.-Y.; Kouyama, T.; Shibata, T.; Ueki, T. Significance of amino groups of phosphatidylethanolamine in phospholipid peroxidation of mixed liposomes. Chem. Phys. Lipids 1994, 71, 197–203.
- Prat, J.; Bellmunt, M.J.; Portero-Otín, M.; Pamplona, R. Fluorescent Products from Aminophospholipids and Glucose. In The Maillard Reaction in Foods and Medicine; O’brien, J., Nursten, H.E., Crabbe, M.J.C., Ames, J.M., Eds.; The Royal Society of Chemistry: Cambridge, UK, 1998; p. 438.
- Al-Abed, Y.; Liebich, H.; Voelter, W.; Bucala, R. Hydroxyalkenal formation induced by advanced glycosylation of low density lipoprotein. J. Biol. Chem 1996, 271, 2892–2896.
- Ravandi, A.; Kuksis, A.; Shaikh, N.A. Glycated phosphatidylethanolamine promotes macrophage uptake of low density lipoprotein and accumulation of cholesteryl esters and triacylglycerols. J. Biol. Chem 1999, 274, 16494–16500.
More
