Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Catherine Yang and Version 1 by Sewbert Rodrigues Jati.
Snakebite envenomation (SBE)-induced immunity refers to individuals who have been previously bitten by a snake and developed a protective immune response against subsequent envenomations. The notion stems from observations of individuals, including in the indigenous population, who present only mild signs and symptoms after surviving multiple SBEs. Indeed, these observations have engendered scientific interest and prompted inquiries into the potential development of a protective immunity from exposure to snake toxins.
- snakebite immunity
- Yanomami
- antivenom
- snakebite resistance
- immune response
1. Introduction
In Brazil, approximately 30,000 snakebites are reported each year [1], presenting high morbidity and mortality rates within the Brazilian Amazon region [2]. Snake venoms are composed of a protein cocktail of high complexity and diversity [3], triggering a variety of biochemical and toxicological effects on victims, which influences several clinical manifestations [4] ranging from mild to severe outcomes, including hospitalization for long periods, surgical procedures, and follow-up for rehabilitation [5]. Indeed, snakebite after-effects depend on several factors, including the species, region of the bite, quantity of venom injected, and influence of ecology and evolution in driving inter- and intra-specific venom variations, in addition to the health condition of the victim [6,7][6][7].
Antivenom (i.e., horse-derived polyvalent antibodies) has been the primary and single specific snakebite treatment for more than a century. Although lifesaving, antivenoms still have therapeutic limitations [8,9][8][9], presenting limited efficacy against some effects of envenoming, such as local tissue damage [4,10][4][10].
Based on that, vaccines targeting snakebites were raised as a possibility to induce protection to those at risk for death by snakebite [11]. Knowing that many snake venom-derived toxins are immunogenic, different experimental approaches and studies have demonstrated that vaccine-elicited antibodies can neutralize venoms and protect from envenomation injury [12,13,14,15][12][13][14][15]. In humans, the ability of venom to induce neutralizing antibodies has also been demonstrated [16]. This article will review evidence of human antibodies targeting snake venoms and inducing protection for the victims, aiming to understand the memory immune response.
2. Snakebites in the Yanomami Indigenous Community of the Brazilian Amazon
The Yanomamis are a hunter–horticulturist indigenous population from the interfluvial tropical forest of the western Guiana massif, who inhabit the borders between Venezuela (upper Orinoco and Cassiquiare) and Brazil (upper Rio Branco, left bank of Rio Negro). They constitute a cultural and linguistic set composed of four territorially adjacent subgroups that speak mutually intelligible languages: the Yanomami (approximately 56% of the ethnic group), the Yanomam (25%), the Sanumá (14%), and the Ninam (5%) [17].
On the Brazil side, the Yanomami Indigenous Land (YIL) was demarcated in 1992, occupying 96,560 km2 in the west of the state of Roraima and north of the state of Amazonas (Figure 1), where around 21,600 indigenous people live in 260 communities [18].
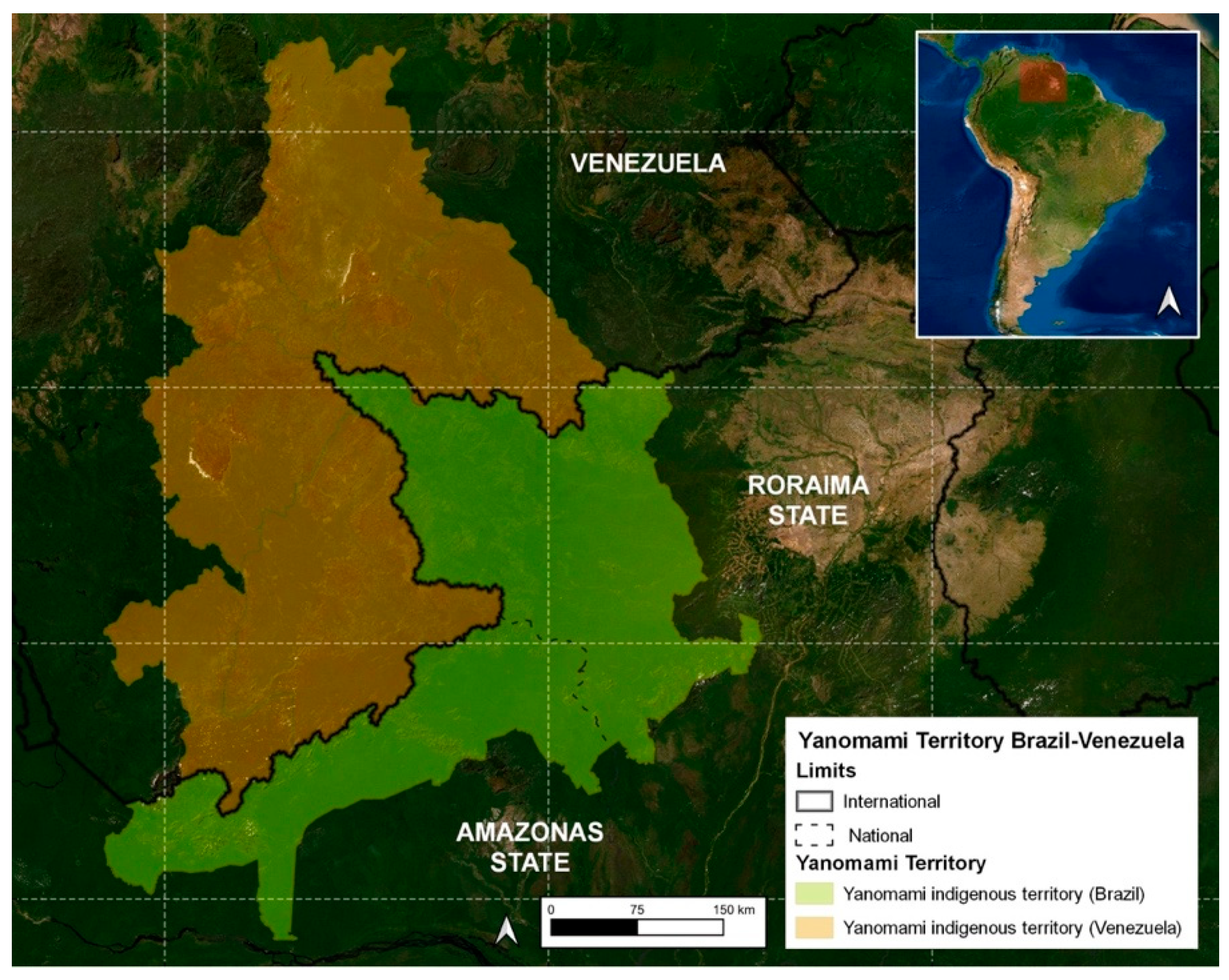
Figure 1.
Map of the Yanomami indigenous land.
Hence, the Yanomami people’s way of life, deeply intertwined with the Urihi (Forest-Earth, as it is referred to in Yanomami language), renders them particularly susceptible to snake-related accidents. Their homes (called Xapono in the Yanomami language) lack walls that would provide insulation from the surrounding forest. Additionally, their swidden agriculture is carried out in dense forest, and they frequently visit collection sites to gather roots and other plant materials essential to their daily lives. Moreover, their hunting and fishing grounds, crucial for sustenance, expose them to further potential encounters with snakes.
Since the Yanomamis are a closed indigenous community, there is no road to access the YIL, resulting in communities with difficult access (remote), which are marked by frequent snakebite envenomings due to the typical humid tropical Amazon Forest environment. Although highly prevalent, snakebites in YIL are commonly treated in situ, with the victim’s care mainly performed by local healers and health professionals working in the area [19]. In cases of severe envenomings with no improvement following local therapy, the indigenous victims can be transferred by air to main hospitals in cities [20,21][20][21]. Although snakebites are considered one of the main diseases in the YIL, there is a lack of studies in the region compared with other regions in the Amazon such as the state of Pará [22,23,24,25][22][23][24][25] and the Central Amazon [26,27][26][27].
As the Yanomamis live in places with difficult access, different causes have been shown to be responsible for the negligence of snakebite care, including the delay in therapy and difficulty in removing patients from the isolated areas, resulting in a higher lethality rate and sequelae than in other parts of Brazil [22].
In addition, recent studies [18,28,29][18][28][29] corroborate the fact that the Yanomamis have a mythological relationship with snakes, which can be considered as an “evil” for their population. Thus, they believe that being attacked by a snake may be a kind of punishment by the gods for some act performed. As a result, many Yanomamis do not seek medical assistance following snakebites, which, consequently, may result in the worsening of the envenomation. On the other hand, many Yanomamis that do not seek any care following a snakebite based on punishment beliefs testify that they had recovered from the envenoming without any kind of treatment, raising the possibility of a memory immune response. Nevertheless, the memory response targeting snakebites in the Yanomamis has not yet been studied or demonstrated. Nevertheless, it is undeniable that coexistence between the Yanomamis and snakes has persisted for several generations of this ethnic group.
Detailed clinical-epidemiological data obtained from the Yanomami and Ye’kuana Indigenous Special Health District (DSEI-YY) from 2017 to 2022, conducted in accordance with the Declaration of Helsinki (protocol approved by the Research Ethic Committee of the Federal University of Roraima under number CAAE 53970721.4.0000.5016), performed by the research group and presented for the first time here, demonstrated that:
- (i)
-
In the YIL, men are mainly affected by snakebites at an average of 61.3%, which is statistically different in comparison to women (paired t-test, p = 0.034). However, compared with other studies [30,31][30][31], the percentage of Yanomami women (38.7%) who were affected by snakebites is 4.85 times higher than the national average (8%), probably due to the women-related activities in the community, as they also work in the field and harvest firewood, food, and medicinal products in the forest.
- (ii)
-
Most snakebites occur in the age group of 20 to 39 years old (35.2%), followed by 15 to 19 (17.6%), 10 to 14 (16.9%), 40 to 59 (16.0%), 5 to 9 (10.4%), 60 to 79 years (2.1%), up to 4 years old (1.3% of cases), and over 80 years old (0.5% of cases), similarly to the results obtained by the pioneering Vital Brazil studies [30] and others [31,32,33][31][32][33].
- (iii)
-
Regarding seasonality, the month with the highest snakebite incidence was May (mean of 19.8) and the one with the lowest incidence was December (mean of 9.5) (Figure 2). This is a different result compared to a previous study that marked July as the month with the highest occurrence of snakebites and October as the one with the lowest occurrence.
-
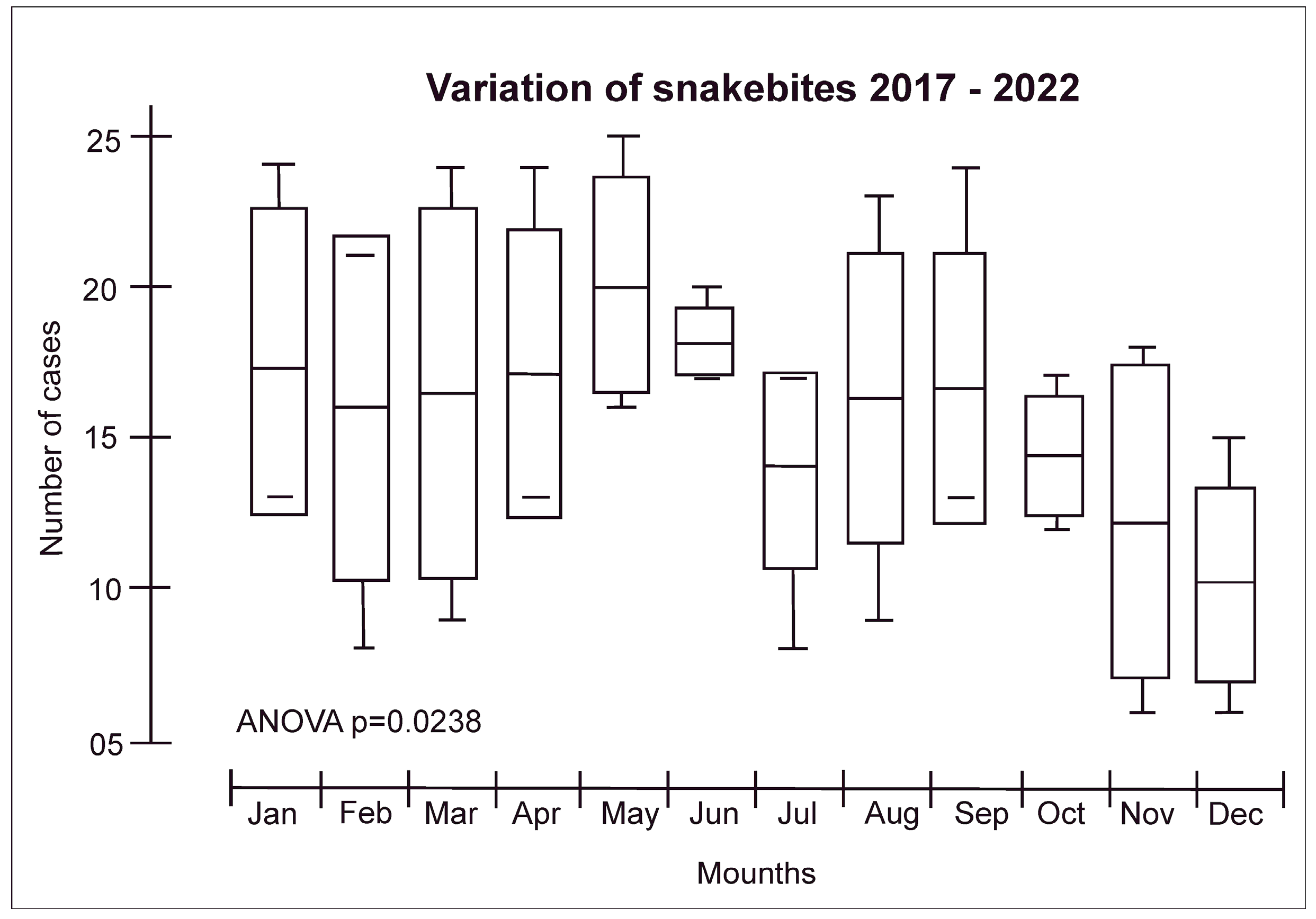 Figure 2. Snakebite distribution according to seasonality in the Yanomami Indigenous Land. The seasonality in Roraima is represented by a rainy period (from April to September) and a dry period (from October to March).
Figure 2. Snakebite distribution according to seasonality in the Yanomami Indigenous Land. The seasonality in Roraima is represented by a rainy period (from April to September) and a dry period (from October to March). - (iv)
-
The highest number of snakebites were reported in the municipality of Alto Alegre in the Roraima States/Brazil (50.5%), which was expected since the region includes the Serra dos Surucucus, located in the westernmost portion of this municipality, in the border region with Venezuela, known to be inhabited by snake-rich fauna [31].
-
The Yanomami people are particularly noteworthy as they have had recent contact with external societies. The Fundação Nacional dos Povos Indígenas (FUNAI) defines “recent contact” indigenous groups as those who maintain both permanent and/or temporary connections with segments of the national society. Regardless of the duration of contact, these groups exhibit distinct characteristics in their relationship with the national society, displaying selectivity and autonomy in their incorporation of goods and services. As a result, they preserve their unique social structures and collective dynamics, and exercise a high degree of autonomy in defining their interactions with the state and national society [34].
3. The Immunological Response Targeting Snake Venoms
After a snakebite and the subsequent introduction of venom, the immune system is initiated in accordance with a well-established pattern, leading to the activation of both innate and adaptive immune responses. It is worth emphasizing that this immune mechanism is applicable to any other external or extracellular antigen (Figure 3) [35]. Briefly, antigen-presenting cells (APCs) are recruited to the bite site, where they recognize and process venom-derived toxins into small peptides. These peptides are then presented on the cell membrane by the major histocompatibility complex (MHC). Stimulated by the cytokines produced during the establishment of the innate immune response, the APCs migrate to the regional lymph nodes, where naive T CD8+ and T CD4+ cells recognize the antigen-MHC complex. Upon lymphocyte activation, they differentiate into effector lymphocytes. Simultaneously, naive B cells located in the lymph nodes recognize the toxin-derived components of the venom, known as antigenic epitopes. The B cells present it again on the cell membrane bound to MHC class II molecules. The subsequent interaction between the antigen-MHC class II complex on the B cell membrane and the activated helper T cell generates a second activating signal within the B cell. This signal triggers their proliferation and subsequent differentiation into plasma cells and memory cells leading to the production of specific antibodies targeting venom-derived toxins [36,37][36][37]. Memory B cells are predominantly localized in secondary lymphoid organs, such as lymph nodes and spleen. The role of memory B cells is to provide a rapid and enhanced immune response upon re-exposure to a previously encountered antigen. They are responsible for the long-term maintenance of immunological memory. Short-lived plasma cells are predominantly localized in the medullary cords of lymph nodes and the red pulp of the spleen and play a crucial role in the early immune response by producing high levels of IgM and IgG antibodies, which are important for the initial control of infections. On the other hand, long-lived plasma cells primarily reside in the bone marrow. They are responsible for maintaining a sustained production of IgG antibodies over a prolonged period [37].
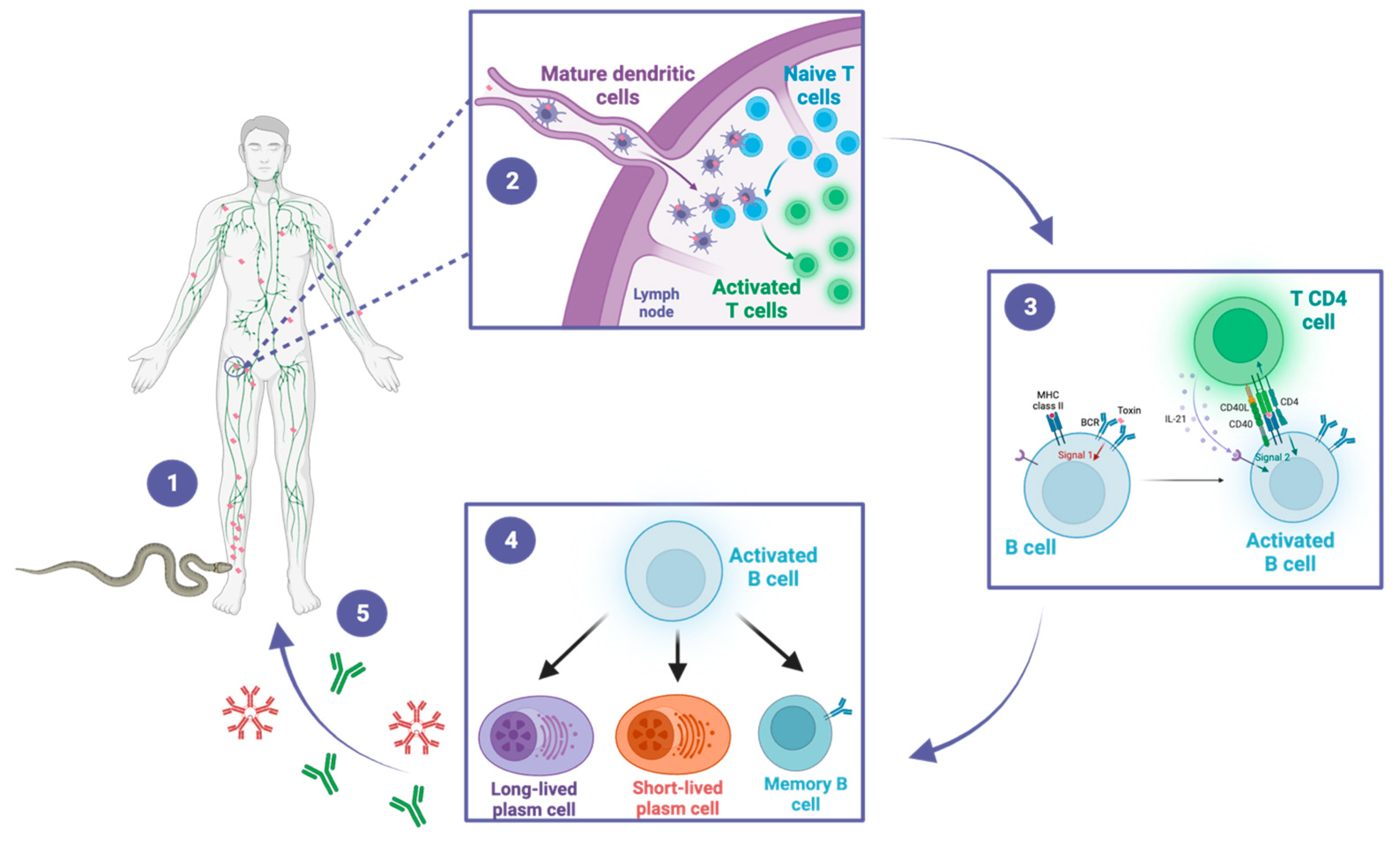
Figure 3. A snakebite inducing antivenom antibodies. This figure illustrates the sequential steps of the immune response to snakebite, highlighting the crucial roles of dendritic cells, T cells, and B cells in the generation of protective antibodies. (1) The snake bites the victim and inoculates the venom, composed of a cocktail of toxins. The toxins are distributed locally and systemically throughout the body. Dendritic cells recognize and process the venom-derived toxins and migrate to the draining lymph nodes. (2) In the lymph nodes, dendritic cells present toxin-derived peptides to naïve CD4+ T cells, leading to their activation. (3) Activated CD4+ T cells in turn activate B cells through a T-dependent pathway. (4) Activated B cells differentiate into memory B cells, as well as into short-lived or long-lived plasma cells. (5) Short-lived plasma cells generate early IgM and IgG antibodies, while long-lived plasma cells sustain the production of IgG antibodies for long-term immunity. These antibodies could contribute to the protective immunity of snakebite victims during subsequent exposures.
To date, the only strategy available for assessing the memory response to snakebites is by measuring the humoral response through the quantification of venom-specific antibodies in serum. This approach involves determining the levels of venom-specific IgM and IgG antibodies using techniques such as enzyme-linked immunosorbent assay (ELISA). By measuring the antibody titers in the serum, valuable information can be obtained regarding the presence and magnitude of the immune memory generated after a snakebite. However, assessing other aspects of the memory response, such as the presence and functionality of memory cells (e.g., effector memory cells) and the dynamics of long-lived and short-lived plasma cells, poses challenges due to technical limitations and the complexity of accessing these cell populations in living human subjects [38].
5. Vaccine Approaches for Populations Living in Areas of Snakebite Risk
The optimal preventive measure against snakebites is to avoid encounters with snakes. Nevertheless, accidental encounters still occur in most situations, leading to envenomation and necessitating the best approach for overcoming it, which is treatment with antivenom. In 1877, Sewall conducted the first investigation on a prophylaxis for snakebite envenomation, subcutaneously inoculating rattlesnake venom into pigeons repeatedly. The observed signs and symptoms in the animals ranged from paw paralysis to death, occurring 3 to 20 h after injection. However, the study demonstrated that when animals received repeated venom injections they evolved to a “resistance” to the venom’s harmful effects, recovering quickly from the venom’s effects, even when exposed to lethal or higher doses, although the resistance lasted no longer than five months, as observed in one of the tested animals [57][39]. Later, Wallis and Wallis (2005) described the vaccination of dogs against rattlesnake venoms, showing that the dogs’ produced antibodies were able to bind and neutralize the venom effects of Crotalus atrox in vitro and in vivo [12]. Based on this information, a vaccine specifically designed for C. atrox has been developed and is currently available through Red Rock Biologics (http://redrockbiologics.com, accessed on 28 August 2023) for both dogs and horses. However, a study conducted by Leonard, Bresee, and Cruikshank (2014) suggests that there was no discernible difference in terms of morbidity and mortality rates between dogs that were vaccinated and those that were not [58][40]. Another study reports the vaccination of cattle with Bothrops asper venom, showing that the systemic effects that occur during moderate envenoming were prevented, and coagulopathies were delayed, in relation to non-vaccinated animals; however, the infusion of antivenom was still required [59][41]. Active immunization was tested not only with venoms, but also with isolated toxins derived from venoms. A study using α-cobratoxin from Naja kaouthia observed that the animals that were immunized with the toxin and later challenged with it survived, avoiding lethality [11]. Similarly, mice immunization with α-bungarotoxin peptides from Bungarus multicinctus led to a protective effect, even using high doses of the toxin [60][42]. These studies are not restricted to snake venoms, but also are conducted on toxins from other animal venoms. Costa et al. (2020) produced monoclonal antibodies able to recognize metalloprotease of Loxosceles spp. spider venom, through the immunization of mice with a recombinant multiepitopic protein derived from loxoscelic toxin (rMEPLox), and observed that the monoclonal antibodies produced were also able to neutralize the fibrinogenolytic activity of L. intermedia spider venom [61][43]. Moreover, Cerni et al. (2023) reported that the immunization of mice with Ts5, a toxin from Tityus serrulatus, was able to trigger the production of antibodies capable of inhibiting the pain caused during the scorpion envenomation [62][44]. In the context of human immunization against snake venoms, significant observations have been made. Haast and Winer (1955) conducted a study on a man who deliberately self-immunized using Elapidae venoms and managed to survive a snakebite incident. The individual described his symptoms from the time of the bite until his arrival at the hospital. Surprisingly, by the third day following the accident, he had fully recovered and was able to resume work the next day [63][45]. Another case study presented by Watt, Parrish, and Pollard (1956) involved a herpetologist who, over a period of 12 years, was bitten by ten venomous snakes and, remarkably, survived each encounter [64][46]. Building upon this line of research, Parrish and Pollard (1959) examined North American pit viper bites in 14 patients who had experienced multiple bites. Contrary to expectations, the study found that these repeated bites did not induce permanent immunity. In fact, some cases resulted in hypersensitivity reactions, which could potentially lead to fatal outcomes [65][47]. Flowers (1963) documented the immunization process of an individual who received 17 injections of Naja naja venom over a period of five months. After the immunization period, the individual’s antibodies were used in neutralization assays in mice, where 1 mL of his serum was able to neutralize 0.244 mg of venom [66][48]. Interestingly, despite having received 17 doses of venom, the individual continued to receive monthly injections of 5 mg of venom. However, during the study, approximately two weeks after the 24th venom injection, an accidental snakebite occurred. The snake responsible for the bite was identified as N. naja. Despite being admitted to the hospital 15 min after the bite, the individual did not receive antivenom therapy as he had already received venom doses for his immunization. Notably, he presented only mild symptoms of envenomation, and experienced only pain, swelling, and necrosis in the affected finger [67][49]. These human studies, involving repeated venom injections, have provided valuable insights that allow us to conclude that individuals can develop immunity to venom, rendering them protected against future bites [68][50]. However, it could be argued that the largest and most noteworthy study was the exceptional immunization campaign of the Amami and Okinawa Islands (Japan) performed over a period of three years (1957–1960), involving over 40,000 volunteers, aiming to immunize against Trimeresurus flavoviridis venom. This pioneering study evaluated the effectiveness of the immunization protocol in preventing local lesions caused by the venom. Meticulous data collection and analysis revealed that the immunization successfully prevented the occurrence of local lesions in vaccinated individuals exposed to the venom, highlighting the success of large-scale efforts in addressing the devastating impacts of snakebite envenomation and providing valuable insights for future research and the development of effective immunization strategies [69][51]. Despite the partial effectiveness observed in these human studies, it is important to highlight the cost-effectiveness of vaccines. In Brazil, snakebite care costs USD 78.15 per patient daily, including the price of the antivenom and the indirect care [70][52]. On the other hand, in sub-Saharan Africa the average cost of an antivenom vial is approximately USD 124 [71][53], which, although expensive, is relatively more affordable compared to vaccine production in low-income countries [72][54]. Thus, while previous studies have explored vaccination strategies, it is essential to acknowledge that these approaches have many limitations for effectively mitigating the risks associated with snakebites. The incidence of snakebites is comparatively lower than that of other epidemic diseases. Moreover, developing snakebite-specific vaccination strategies for various venomous snake species can be cost-prohibitive and may lack cost-effectiveness. Furthermore, snakebites can result from numerous snake species, each with distinct venom compositions, which should reveal the financial constraints associated with the development of multiple snake-specific vaccines and the challenges in providing widespread coverage for a relatively rare event like a snakebite.References
- Ministério da Saúde Acidente Por Animais Peçonhentos—Notificações Registradas No Sistema de Informação de Agravos de Notificação—Brasil. Available online: http://tabnet.datasus.gov.br/cgi/tabcgi.exe?sinannet/cnv/animaisbr.def (accessed on 28 February 2023).
- Sampaio, V.S.; Gomes, A.A.; Silva, I.M.; Sachett, J.; Ferreira, L.C.L.; Oliveira, S.; Sabidò, M.; Chalkidis, H.; Barbosa Guerra, M.G.V.; Salinas, J.L.; et al. Low Health System Performance, Indigenous Status and Antivenom Underdosage Correlate with Spider Envenoming Severity in the Remote Brazilian Amazon. PLoS ONE 2016, 11, e0156386.
- Calvete, J.J. Proteomic Tools against the Neglected Pathology of Snake Bite Envenoming. Expert. Rev. Proteom. 2011, 8, 739–758.
- Gutiérrez, J.M.; Calvete, J.J.; Habib, A.G.; Harrison, R.A.; Williams, D.J.; Warrell, D.A. Snakebite Envenoming. Nat. Rev. Dis. Primers 2017, 3, 17063.
- Burki, T. Resolution on Snakebite Envenoming Adopted at the WHA. Lancet 2018, 391, 2311.
- Gold, B.S.; Dart, R.C.; Barish, R.A. Bites of Venomous Snakes. N. Engl. J. Med. 2002, 347, 347–356.
- Kaur, N.; Iyer, A.; Sunagar, K. Evolution Bites—Timeworn Inefficacious Snakebite Therapy in the Era of Recombinant Vaccines. Indian. Pediatr. 2021, 58, 219–223.
- Harrison, R.A.; Cook, D.A.; Renjifo, C.; Casewell, N.R.; Currier, R.B.; Wagstaff, S.C. Research Strategies to Improve Snakebite Treatment: Challenges and Progress. J. Proteom. 2011, 74, 1768–1780.
- Harrison, R.; Gutiérrez, J. Priority Actions and Progress to Substantially and Sustainably Reduce the Mortality, Morbidity and Socioeconomic Burden of Tropical Snakebite. Toxins 2016, 8, 351.
- Laustsen, A.H.; María Gutiérrez, J.; Knudsen, C.; Johansen, K.H.; Bermúdez-Méndez, E.; Cerni, F.A.; Jürgensen, J.A.; Ledsgaard, L.; Martos-Esteban, A.; Øhlenschlæger, M.; et al. Pros and Cons of Different Therapeutic Antibody Formats for Recombinant Antivenom Development. Toxicon 2018, 146, 151–175.
- Pergolizzi, R.G.; Dragos, R.; Ropper, A.E.; Menez, A.; Crystal, R.G. Protective Immunity against α-Cobratoxin Following a Single Administration of a Genetic Vaccine Encoding a Non-Toxic Cobratoxin Variant. Hum. Gene Ther. 2005, 16, 292–298.
- Wallis, D.; Wallis, J. Rattlesnake Vaccine to Prevent Envenomation Toxicity in Dogs. In Proceedings of the Western Veterinary Conference, Las Vegas, NV, USA, 1 January 2005; Volume SIS1.
- Ayala, M.S.; Arevalo, B.; Vanegas, V. Design of a Therapeutic Vaccine against Snake Venom Disintegrins, 2015.
- Freitas, T.; Diniz, C.; Frézard, F. The Use of Liposomes as Snake Venom Vehicles. Application in Protective Immunization. J. Toxicol. Toxin Rev. 1998, 17, 441–466.
- de Melo, P.D.V.; Lima, S.d.A.; Araújo, P.; Santos, R.M.; Gonzalez, E.; Belo, A.A.; Machado-De-Ávila, R.A.; Costal-Oliveira, F.; Soccol, V.T.; Guerra-Duarte, C.; et al. Immunoprotection against Lethal Effects of Crotalus durissus Snake Venom Elicited by Synthetic Epitopes Trapped in Liposomes. Int. J. Biol. Macromol. 2020, 161, 299–307.
- Theakston, R.D.; Reid, H.A.; Larrick, J.W.; Kaplan, J.; Yost, J.A. Snake Venom Antibodies in Ecuadorian Indians. J. Trop. Med. Hyg. 1981, 84, 199–202.
- Albert, B. URIHI: Terra, Economia e Saûde Yanomami; Universidade de Brasília: Brasília, Brazil, 1991.
- Kopenawa, D. A Queda do Céu: Palavras de um Xamã Yanomami; Companhia das Letras: São Paulo, Brazil, 2015; ISBN 978-85-359-2620-0.
- Freitas, F.P.d.P.; Luna, W.F.; Bastos, L.O.d.A.; Ávila, B.T. Experiências de Médicos Brasileiros Em Seus Primeiros Meses Na Atenção Primária à Saúde Na Terra Indígena Yanomami. Interface 2021, 25, e200212.
- Cristino, J.S.; Salazar, G.M.; Machado, V.A.; Honorato, E.; Farias, A.S.; Vissoci, J.R.N.; Silva Neto, A.V.; Lacerda, M.; Wen, F.H.; Monteiro, W.M.; et al. A Painful Journey to Antivenom: The Therapeutic Itinerary of Snakebite Patients in the Brazilian Amazon (The QUALISnake Study). PLoS Negl. Trop. Dis. 2021, 15, e0009245.
- Oliveira, I.S.; Ananias, C.B.; Medeiros, J.M.; Franco, M.V.S.; Ferreira, I.G.; Cerni, F.A.; Sandri, E.A.; Monteiro, W.M.; Pucca, M.B. Medical Management after Lancehead Snakebite in North Amazon: A Case Report of Long-Term Disability. Toxins 2022, 14, 494.
- da Cunha, O.R.; Nascimento, F.P. Do Ofídios da Amazônia: As Cobras da Região Leste do Pará; Museu Emílio Goeldi: Belém, Brazil, 1993; Volume 9.
- da Frota, J.G.; dos Santos, A.P., Jr.; de Chalkidis, H.M.; Guedes, A.G. As Serpentes Da Regiao Do Baixo Rio Amazonas, Oeste Do Estado Do Pará, Brasil (Squamata). Biociências 2005, 13, 211–220.
- da Prudente, A.L.C.; Sarmento, J.F.M.; Avila-Pires, T.C.S.; Maschio, G.; Sturaro, M.J. How Much Do We Know about the Diversity of Squamata (Reptilia) in the Most Degraded Region of Amazonia? S. Am. J. Herpetol. 2018, 13, 117.
- Prudente, A.L.; Ramos, L.; Silva, T.; Sarmento, J.; Dourado, A.; Silva, F.; Almeida, P.; Santos, C.; Sousa, M. Dataset from the Snakes (Serpentes, Reptiles) Collection of the Museu Paraense Emílio Goeldi, Pará, Brazil. Biodivers. Data J. 2019, 7, e34013.
- de Fraga, R.; Stow, A.J.; Magnusson, W.E.; Lima, A.P. The Costs of Evaluating Species Densities and Composition of Snakes to Assess Development Impacts in Amazonia. PLoS ONE 2014, 9, e105453.
- Martins, M.; Oliveira, M. Natural History of Snakes in Forests of the Manaus Region, Central Amazonia, Brazil. Herpetol. Nat. Hist. 1998, 6, 78–150.
- Kawaguchi, D. Lições de Um Xamã Yanomami Para a Construção de Uma Identidade Pós-Antropocêntrica. Rev. Abordagem Gestáltica Phenomenol. Stud. 2021, 27, 328–338.
- Hata, L. A Potência Do Falso: A Cosmogonia Yanomami Na Obra de Claudia Andujar. Esferas 2021, 16, 16–31.
- Brazil, I.V. A Defesa Contra o Ofidismo; Pocai & Weiss: São Paulo, Brazil, 1911.
- Nascimento, S.P. do Aspectos epidemiológicos dos acidentes ofídicos ocorridos no Estado de Roraima, Brasil, entre 1992 e 1998. Cad. Saúde Pública 2000, 16, 271–276.
- Cunha, L.E.R. DSEI YANOMAMI e os Acidentes Ofídicos No Norte do Brasil: Do Perfil Epidemiológico à Avaliação da Termoestabilidade dos Soros Antiofídicos como Estratégia de Saúde Pública; Doutorado em Medicina Tropical, Fundação Oswaldo Cruz; Instituto Oswaldo Cruz: Rio de Janeiro, Brazil, 2020.
- Jati, S.R.; Sousa, F.; Sant’Ana, A.C.; Mello, G.T.; Ferreira, D.E.S.; Gama, H.F.; Jati, N.S. Epidemiology of Accidents by Snakes in the Yanomami Indigenous Lands, in Roraima. In VenoRaima Abstract Book; Journal of Venomous Animals Including Tropical Diseases: Boa Vista, Brazil, 2022; Volume 28, p. 100.
- FUNAI Povos de Recente Contato. Available online: https://www.gov.br/funai/pt-br/atuacao/povos-indigenas/povos-indigenas-isolados-e-de-recente-contato-2/povos-de-recente-contato-1 (accessed on 21 May 2023).
- Leon, G.; Sanchez, L.; Hernandez, A.; Villalta, M.; Herrera, M.; Segura, A.; Estrada, R.; Maria Gutierrez, J. Immune Response towards Snake Venoms. Inflamm. Allergy-Drug Targets 2011, 10, 381–398.
- Ryan, R.Y.M.; Seymour, J.; Loukas, A.; Lopez, J.A.; Ikonomopoulou, M.P.; Miles, J.J. Immunological Responses to Envenomation. Front. Immunol. 2021, 12, 661082.
- Abbas, A.K.; Lichtman, A.H.; Pillai, S.; Baker, D.L.; Baker, A. Cellular and Molecular Immunology, 9th ed.; Elsevier: Philadelphia, PA, USA, 2018; ISBN 978-0-323-47978-3.
- Chen, W.; Ghobrial, R.M.; Li, X.C. The Evolving Roles of Memory Immune Cells in Transplantation. Transplantation 2015, 99, 2029–2037.
- Sewall, H. Experiments on the Preventive Inoculation of Rattlesnake Venom. J. Physiol. 1887, 8, 203–210.
- Leonard, M.; Bresee, C.; Cruikshank, A. Effects of the Canine Rattlesnake Vaccine in Moderate to Severe Cases of Canine Crotalid Envenomation. Veter. Med. Res. Rep. 2014, 5, 153–158.
- Herrera, M.; González, K.; Rodríguez, C.; Gómez, A.; Segura, Á.; Vargas, M.; Villalta, M.; Estrada, R.; León, G. Active Immunization of Cattle with a Bothropic Toxoid Does Not Abrogate Envenomation by Bothrops asper. Venom, but Increases the Likelihood of Survival. Biologicals 2017, 46, 1–5.
- Dolimbek, B.Z.; Zouhair Atassi, M. Protection against α-Bungarotoxin Poisoning by Immunization with Synthetic Toxin Peptides. Mol. Immunol. 1996, 33, 681–689.
- Costa, T.G.F.; Costal-Oliveira, F.; de Assis, T.C.S.; Lima, S.A.; Martins, C.A.; Finco, A.B.; Veiga, S.S.; Soccol, V.T.; Machado-de-Ávila, R.A.; Figueiredo, L.F.M.; et al. Engineered Antigen Containing Epitopes from Loxosceles spp. Spider Toxins Induces a Monoclonal Antibody (Lox-mAb3) against Astacin-like Metalloproteases. Int. J. Biol. Macromol. 2020, 162, 490–500.
- Cerni, F.; Oliveira, I.; Cordeiro, F.; Bordon, K.; Ferreira, I.; Monteiro, W.; Arantes, E.; Cunha, T.; Pucca, M. The Nociceptive Response Induced by Different Classes of Tityus serrulatus Neurotoxins: The Important Role of Ts5 in Venom-Induced Nociception. PLoS Neglected Trop. Dis. 2023, 17, e0011057.
- Haast, W.E.; Winer, M.L. Complete and Spontaneous Recovery from the Bite of a Blue Krait Snake (Bungarus caeruleiis). Am. J. Trop. Med. Hyg. 1955, 4, 1135–1137.
- Watt, H.F.; Parrish, H.M.; Pollard, C.B. Repeated Poisonous Snakebites in the Same Patient: An Unusual Case Report. North Carol. Med. J. 1956, 17, 174–179.
- Parrish, H.M.; Pollard, C.B. Effects of Repeated Poisonous Snakebites in Man. Am. J. Med. Sci. 1959, 237, 277–286.
- Flowers, H.H. Active immunization of a human being against cobra (Naja naja) venom. Nature 1963, 200, 1017–1018.
- Canan, E.D.; Flowers, H.H. Cobra Bite Following Immunization against Cobra Venom. JAMA 1965, 193, 625–626.
- Repeated Poisonous Snakebites in Man. Am. J. Public Health Nations Health 1959, 49, 946.
- Sawai, Y.; Kawamura, Y.; Fukuyama, T.; Okonogi, T.; Ebisawa, I. Studies on the Improvement of Treatment of Habu (Trimeresurus flavoviridis) Bites. 8. A Field Trial of Prophylactic Inoculation of the Habu Venom Toxoid. Jap. J. Expo Med. 1969, 39, 197–203.
- Magalhães, S.F.V.; Peixoto, H.M.; De Almeida Gonçalves Sachett, J.; Oliveira, S.S.; Alves, E.C.; Dos Santos Ibiapina, H.N.; Monteiro, W.M.; De Oliveira, M.R.F. Snakebite Envenomation in the Brazilian Amazon: A Cost-of-Illness Study. Trans. R. Soc. Trop. Med. Hyg. 2020, 114, 642–649.
- Brown, N.I. Consequences of Neglect: Analysis of the Sub-Saharan African Snake Antivenom Market and the Global Context. PLoS Neglected Trop. Dis. 2012, 6, e1670.
- Habib, A.G.; Lamorde, M.; Dalhat, M.M.; Habib, Z.G.; Kuznik, A. Cost-Effectiveness of Antivenoms for Snakebite Envenoming in Nigeria. PLoS Neglected Trop. Dis. 2015, 9, e3381.
More
