Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Susana P. Pereira and Version 2 by Peter Tang.
Chronic diseases represent one of the major causes of death worldwide. It has been suggested that pregnancy-related conditions, such as gestational diabetes mellitus (GDM), maternal obesity (MO), and intra-uterine growth restriction (IUGR) induce an adverse intrauterine environment, increasing the offspring’s predisposition to chronic diseases later in life. There are several CD risks factors an individual can manage. Recent research suggested that the intrauterine environment, which is modulated by maternal behaviors and disease, severely influences the offspring’s CD development risk.
- maternal antioxidant supplementation
- disease prevention
- chronic diseases
- developmental programming
- metabolic dysfunction
- oxidative stress
1. Introduction
Non-communicable chronic diseases (CD) are considered to be one of the major threats to global health and include cardiovascular disease, diabetes, obesity, cancer, chronic respiratory diseases, and chronic liver disease [1]. It is estimated that, in 2016, non-communicable CD contributed to two-thirds of mortality worldwide [2]. There are several CD risks factors an individual can manage, including high blood pressure, tobacco smoking, high body mass index, physical inactivity, and constant consumption of poor diets [1]. In addition, unmanageable risk factors can also contribute to CD development, such as age, sex, and genetic background [3]. On top of that, recent research has suggested that the intrauterine environment, which is modulated by maternal behaviors and disease, including gestational diabetes mellitus (GDM) [4][5][4,5], maternal obesity (MO) [5][6][7][8][5,6,7,8], and IUGR [9], severely influences the offspring’s CD development risk.
Gestational diabetes mellitus (GDM) is defined as hyperglycemic and glucose intolerance states that are detected, for the first time, either in the second or third trimester of pregnancy [10]. It is well established that GDM is the most prevalent pregnancy complication, affecting 13.9% of pregnancies [4][11][4,11]. Identified risk factors for GDM development encompass maternal age and obesity, with obese pregnant women presenting a 2.4-fold higher risk of developing GDM [12]. Furthermore, MO itself represents a highly prevalent pregnancy complication [5]. It is estimated that approximately 50% of pregnancies occur in overweight or obese women [6]. Both GDM and MO contribute to an increased risk of inducing a fetoplacental environment resembling prolonged hypoxia, along with other factors, such as maternal smoking, vascular dysfunction, and maternal nutrient reduction [13]. These conditions can potentiate suboptimal fetal development and growth, a condition referred to as intra-uterine growth restriction (IUGR). Indeed, neonatal complications associated with GDM, MO, and IUGR include increased perinatal mortality and morbidity, deviations in birthweight, and preterm birth [14][15][16][14,15,16]. These pregnancy-associated disorders induce structural, functional, and metabolic adaptations across several organs as early as the fetal stage. In this context, it has been pointed out that oxidative stress and mitochondrial dysfunction may be pivotal mechanisms of developmental programming in pregnancy-related disorders [7]. Therefore, these mechanisms can be strategic targets to modulate the programming of non-communicable CD in offspring during pregnancy.
2. Developmental Programming of Chronic Diseases by Pregnancy-Associated Disorders
2.1. (Patho)physiologic Role of Reactive Oxygen Species in Fetal Development
Proper fetal development hinges on an interplay of several critical factors, among which a consistent and uninterrupted provision of nutrients and oxygen plays a pivotal role [17]. Oxygen levels exhibit a fine orchestration throughout gestation according to the specific requirements of the developing fetus [18]. In the early stages, oxygen is maintained at lower levels, particularly up until the 12th week following conception [19]. This intentionally lowered oxygen levels stimulate angiogenesis, promoting the formation of new blood vessels, which is a vital process for sustaining early development [19]. Around the 16th week of gestation, a significant shift occurs, with intrauterine oxygen levels increasing significantly and then remaining stable until birth [19]. Even in this carefully regulated environment, a small fraction of the oxygen required for oxidative metabolism undergoes incomplete reduction, giving rise to reactive oxygen species (ROS) [19]. ROS production predominantly occurs via the escape of electrons from the mitochondrial electron transport chain (ETC), with complex-I and -III being notable contributors, resulting in the formation of the superoxide (•O2−) radical. Notwithstanding, other sources of ROS production can be considered, such as dihydroorotate dehydrogenase (DHODH), among others. It is important to state that ROS have a biphasic effect [20]. On the one hand, at moderate levels, ROS are key players in pregnancy physiology, acting as signaling molecules in developmental processes including placental growth [21], embryo development, and implantation [22], and are involved in the replication, differentiation, and maturation of cells and organs. However, on the flip side, excessive ROS levels, when not counterbalanced by the antioxidant capacity, can usher in oxidative stress [5]. This state of oxidative stress can inflict severe damage, compromising the structural integrity of cells and organelles’ membranes and hindering proper protein function. Furthermore, it poses a significant risk to fetal development and the intricate process of proper organogenesis. In sum, the orchestration of oxygen levels and the balance of ROS levels within the maternal–fetal interface are pivotal to ensuring the proper progression of fetal development.2.2. The Impact of Pregnancy-Associated Disorders on Offspring’s Organs Oxidative Stress and Mitochondrial Function
Pregnancy is a state of high energetic demand that is sustained mainly by fetoplacental metabolic activity [23]. Mitochondria are metabolism’s key players and one of the main cellular energy sources [24], being highly responsive organelles to energy demands. Consequently, placental mitochondrial function can be modulated [24] in response to an adverse intra-uterine environment. In pregnancy-related disorders, placental metabolic dysfunction has been extensively documented, which may translate into long-lasting consequences for the offspring in the prenatal and postnatal periods. This section aims to discuss how GDM, MO, and IUGR are related with fetoplacental dysfunction and offspring organ dysfunction via mitochondrial malfunction and oxidative stress. The characteristic hyperglycemic state during GDM may adversely impact the placental mitochondrial structure and function [25] (Figure 1). Indeed, placentas from GDM-portraying women presented swollen and disrupted mitochondria, some of which were completely damaged [26]. Since mitochondria are highly dynamic organelles, changing the number, morphology, network, and size according to cellular energy needs, mitochondrial shape and function are tightly linked [27]. For instance, GDM-derived human cytotrophoblasts present a decreased mitochondrial maximum respiratory capacity and decreased ATP production rates [28][29][28,29], highlighting GDM-induced mitochondrial bioenergetic dysfunction (Figure 1). Maternal diabetes, either pregestational type 2 diabetes or GDM, impair placental mitochondrial biogenesis (formation of new mitochondria), with decreased mitochondrial transcription factor A (TFAM) [30] and peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1-α) expression levels [28][29][28,29]. In addition to impaired placental mitochondrial biogenesis, GDM leads to altered mitochondrial dynamics, with studies in humans reporting either an increase in placental mitochondrial fusion events [28][31][28,31] or a decrease [32]. Fusion events have been suggested as essential for mitochondrial DNA (mtDNA) copy number maintenance [33] (Figure 1). Although inconsistent, mtDNA copy number alterations are reported in GDM-related studies, either in maternal serum [34][35][34,35] or placental tissue [28][30][28,30]. A potential direct relationship between the mtDNA copy number and oxidative stress has been suggested [36]. This hypothesis was raised because the placental mtDNA copy number was positively correlated with placental DNA oxidation [36] both in GDM and control pregnancies. Further studies are required to understand the mechanisms linking DNA oxidation and the mtDNA copy number to solidify this hypothesis. Despite this, placental oxidative stress has been widely reported in GDM placentas. Although ROS are generated from several sources, mitochondria are considered as one of the major ones [5]. GDM human pregnancies present placental increased biomarkers of oxidative stress, such as malondialdehyde (MDA) [37][38][37,38], reduced antioxidant defenses (decreased catalase (CAT) activity [38], and glutathione peroxidase (GPx) 1 [39]) (Figure 1). In addition to the placenta, GDM human umbilical cord mesenchymal stem cells (hUC-MSC) present increased ROS production, detected via 2′,7′-Dichlorofluorescin diacetate (DCFDA), and impaired mitochondrial bioenergetics, including a diminished basal respiration state and FCCP-induced maximum respiratory capacity [40]. Given that mesenchymal stem cells (MSC) are multipotent, they can differentiate into a wide range of cell types during fetal development, including adipocytes, cardiomyocytes, myocytes, and neurons [41]. Dysfunctional MSC may imprint dysfunctional cells and organs in the offspring that may later lead to an increased CD risk.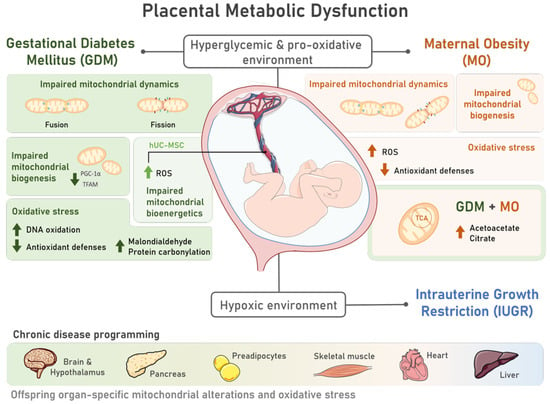
Figure 1. Placental metabolic dysfunction during pregnancies characterized by the presence of Gestational Diabetes Mellitus (GDM) and Maternal Obesity (MO) includes mitochondrial structural and functional alterations and oxidative stress, contributing to offspring chronic disease programming. The characteristic hyperglycemic and pro-oxidative intrauterine environment of pregnancies complicated by GDM and MO induces placental metabolic dysfunction via alterations in mitochondrial dynamics, with unbalanced fission and fusion events; mitochondrial biogenesis, via decreased peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) and mitochondrial transcription factor A (TFAM) levels; and impaired mitochondrial bioenergetics, which was also observed in multipotent human umbilical cord mesenchymal stem cells (hUC-MSC) of GDM pregnancies. Increased reactive oxygen species (ROS) are also common to both placenta and hUC-MSC tissues in both pregnancy disorders. Placental oxidative stress is further marked by reduced antioxidant defenses and increased DNA oxidation, protein carbonylation, and malondialdehyde levels in GDM placentas. MO during a GDM pregnancy may further contribute to mitochondrial dysfunction, as increased levels of metabolites involved in the tricarboxylic acid (TCA) cycle and ketogenesis (citrate and acetoacetate, respectively) were detected. This compromised intrauterine environment can progress into a hypoxic state, increasing the risk of Intrauterine Growth Restriction (IUGR) development. GDM, MO, and IUGR have been associated with an increased risk of offspring chronic disease. Mitochondrial alterations and oxidative stress, which are intimately involved in chronic diseases, were observed in human and animal studies in different tissues of GDM and MO offspring, such as the brain and hypothalamus, pancreas, preadipocytes, skeletal muscle, heart, and liver, highlighting the role of metabolic pregnancy disorders in disease programming.
