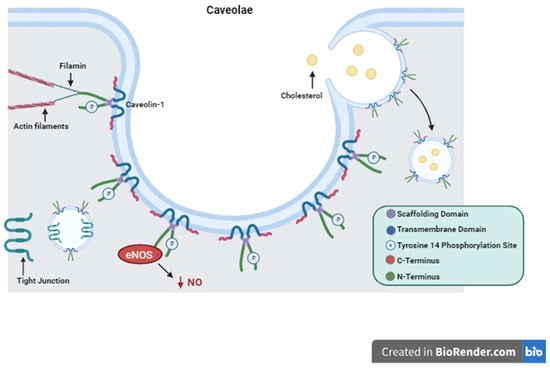
Caveolin-1 Structure and Function. Depiction of Caveolin-1 structure including its scaffolding domain, transmembrane domain, and Tyrosine 14 phosphorylation site. The structure of caveolae is represented with Caveolin-1 proteins forming the surface of the plasma membrane invagination. Important functions of Caveolin-1 are illustrated showing cytosolic interactions with actin filaments, endocytosis and cholesterol trafficking, tight junction shuttling, and intracellular signaling eNOS inactivation and decreased production of NO as an example.
Cav1 is a 21 kDa protein that is associated with the plasma membrane having two hydrophilic termini residing on the cytosolic side separated by a hydrophobic segment lying within the membrane
[78]. Here it performs its many functions with its two main functional domains: the tyrosine 14 phosphorylation site and its scaffolding domain
[78]. The scaffolding domain is responsible for interactions with other proteins that regulate intracellular signaling cascades
[78]. The Src family proteins, G proteins, protein kinase A and C, tyrosine kinase receptors (TKRs), and nitric oxide synthase (NOS) are just a few of these regulator proteins
[79][80][81][82][79,80,81,82]. Phosphorylation of Cav1 on specific amino acid residues causes upregulation or downregulation of certain processes such as cholesterol trafficking
[83][84][83,84]. Cav1 is also involved in the formation of caveolae. Caveolae, first described in 1955 while looking at gallbladder epithelium in mice, are invaginations of the cell plasma membrane that form vesicles which communicate extracellularly
[85].Interestingly, Cav1 is a protein that is palmitoylated on three cysteine residues on the C-terminus
[86][87][86,87]. This stabilizes the protein–membrane relationship and is required for certain protein–protein interactions as is the case with G proteins
[86][87][86,87]. Cav1 has been demonstrated to induce cholesterol clustering and higher degrees of membrane curvature in cholesterol-rich membranes
[86]. This is a process critical for cell signaling and the formation of caveolae
[86]. Cav1 was first thought to perform only absorptive functions via caveolae formation without any mention of its secretory or intracellular functions
[85].
3. Cellular Signaling
Cav1 serves as a key component of multiple cellular signaling pathways and both positive and negative regulatory functions have been identified
[47][48][49][50][51][52][47,48,49,50,51,52]. For example, there is evidence of Cav1 involvement in the p38 mitogen-activated protein kinase (MAPK) signaling pathway, by which decreased Cav1-mediated activation of c-Jun N-terminal kinase (JNK), nuclear factor kappa B (NF-kB), and activator protein-1 (AP-1) leads to protection against inflammatory mechanisms in the setting of sepsis
[47]. In contrast, Cav1 also interacts with toll-like receptor 4 (TLR4), a protein that activates the innate immune system, and increases endothelial cell permeability in the setting of hypoxia
[48]. When Cav1 is knocked out, the inflammatory response mediated through the TLR4 pathway is suppressed
[49]. C
4. Cell Proliferation, Differentiation, Migration and Survival
4.1. Proliferation
Cav1 plays a part in multiple mechanisms that maintain cell viability and stabilization
[1][2][3][1,2,3]. In general, Cav1 is noted to have inhibitory effects on cell proliferation as demonstrated in both mouse and human Cav1 knockout mesenchymal stem cells in which proliferation increased. There is further evidence of this phenomenon with multiple studies showing that upregulation of Cav1 has the opposite result and decreases proliferation
[1][2][1,2]. In vivo, Cav1 overexpression arrested the cell cycle in mouse embryonic fibroblasts at the G0/G1 phase causing growth and mitotic activity to come to a halt
[3].
4.2. Differentiation
There is evidence to suggest that Cav1 has both inhibitory and promoter effects on cellular differentiation
[50][51][50,51]. Knocking out Cav1 in mouse stem cells decreased mRNA expression of pluripotency markers while overexpression of Cav1 had the opposite effect
[4]. It is hypothesized that Cav1 activity may allow progenitor cells to be more sensitive to certain stimuli that cause ongoing differentiation
[4]. As cells reach their final phenotype, Cav1 can then function as part of a negative feedback loop to prevent ongoing growth and differentiation
[5]. This was also demonstrated with mesenchymal stem cells undergoing osteogenic differentiation as Cav1 activity enhanced the process before stabilizing the final phenotype
[6][7][6,7].
4.3. Migration
One illustration of how Cav1 influences cell polarity and migration is how the protein assists with stromal-derived factor-1 (SDF-1) interactions with its receptor, CXCR4. This plays a crucial role cell adhesion during leukocyte recruitment
[8]. Cav1 is required for mobilization of progenitor cells from mouse bone marrow
[9]. Through interactions with the cytoskeleton, Cav1 promotes cellular polarity and morphological changes that allow for cellular migration
[9]. This was further found to be regulated via Cav1 activation of Src and Rho GTPase
[10]. Grande-Garcia et al. found that Cav1-deficient mouse embryonic fibroblasts did not exhibit polarized morphology via actin rearrangements
[10]. Cells can also relocalize Cav1 depending on the type of movement they are performing
[10][11][10,11]. During three-dimensional movement, endothelial cells release Cav1 from caveolar structures in the cell rear and relocalize it to the front
[11].
4.4. Survival
Cav1 involvement in the regulation of cell survival and programmed death in response to stress or insult has important implications for tissue repair and wound healing. For example, downregulation of Cav1 expression in myogenic precursor cells is mandatory for regeneration and healing in skeletal muscle tissue
[12]. Dysregulation of these mechanisms is pertinent to certain disease states. Cav1 is an integral player in both cell survival and apoptosis. Apoptosis is cell death that can occur as a controlled part of an organism’s natural growth cycle or in response to extra or intracellular stressors. Just as essential to the cell cycle is the process of autophagy, in which Cav1 has also been shown to play a major role. Autophagy is the degradation of damaged or unnecessary cellular components through a lysosome-dependent mechanism. This process can be part of an adaptive response that promotes cell survival in the setting of starvation. There are multiple examples of Cav1 being a positive regular of autophagy and a negative regulator of apoptosis
[13][14][15][13,14,15].
4.5. Caveolin-1 Role in Cancer Cell Death and Survival
The role of Cav1 in tumor growth, metastasis, and response to treatment appears to be most closely linked with its role in regulating apoptosis and cell death mechanisms. Cav1 can act in accordance with tumor suppressor or promotor modalities depending on the cell type, tumor stage, and nature of the tumor stroma
[17][18][53][17,18,53]. In specific cancers including breast, lung, colon, and ovarian, Cav1 is expressed in low levels, which is maintained throughout the process of tumor cell proliferation and metastasis
[19][20][21][22][23][19,20,21,22,23].
5. Caveolin-1 Tissue-Specific Roles
To further understand the roles that Cav1 plays in individual tissue types, it must first be explained that Cav1 takes on multiple different forms within the cell itself in order to perform these specific functions. As a membrane protein, Cav1 forms caveolae to interact with cytoskeletal elements and signaling cascade proteins
[47][48][49][50][51][52][54][47,48,49,50,51,52,54]. In the cytoplasm, it is a soluble protein that participates in transporting and delivering lipids to the cell surface, mitochondria, endoplasmic reticulum, and other cellular compartments
[24]. Examples of cell types in which Cav1 is localized to the membrane in the form of caveolae include fibroblasts, endothelial, and polarized epithelial cells of the intestine
[24].
5.1. Vascular
Cav1 expression in vascular endothelial cells regulates the progression of atherosclerosis in large vessels
[27]. Increased autophagy in Cav1 knockout cells has shown to protect against the progression of atherosclerosis via a decrease in vascular inflammation, macrophage infiltration, and LDL transcytosis
[27]. Cav1 also inhibits endothelial nitric oxide synthase activity via direct binding of endothelial nitric oxide synthase (eNOS) and regulation of its expression
[28].
5.2. Adipose
Adipose tissue has been described as one of the most abundant sources of caveolin proteins
[31]. Caveolins are established cholesterol binding proteins
[31][78][31,78]. Exogenous, extracellular lipids induce plasma membrane caveolins to associate with lipid droplets in adipocytes
[32][33][32,33]. This indicates a trafficking pathway by which Cav1 shuttles cholesterol and fatty acids from plasma membrane to the organelle and vice versa
[32][33][32,33]. In Cav1 null mice there is evidence of aberrant lipid metabolism, namely a lipoatrophic phenotype and hyperlipidemia, which results from reduced lipid storage within adipocytes and therefore increased levels of lipids remaining in circulation
[34]. Cav1 null mice also have resistance to diet-induced obesity
[35]. Cav1 performs key interactions with insulin receptors by affecting downstream signaling and stabilizing the receptors to the plasma membrane
[32].
5.3. Brain
There are a host of factors that influence the integrity and permeability of the blood-brain barrier (BBB). Caveolins are thought to perform vital functions in the regulation of the junctional proteins that create the barrier between vascular endothelial cells and the brain parenchyma
[38][39][40][38,39,40]. Choi et al. delineated this relationship by showing that Cav1 null mice had significant degradation of tight junction proteins and an increase in matrix me
5.4. Pneumocytes
Caveolin-1 is essential for pulmonary function and development. As previously discussed, Cav1 is involved in the proliferation and differentiation of stem cells
[1][2][3][4][5][6][7][1,2,3,4,5,6,7]. This process is also true for fetal lung development, specifically as it relates to the differentiation into type II alveolar epithelial cells, also known as pneumocyte
[43]. Type II pneumocytes have a high expression of Cav1 and structurally contain an abundance of caveolae
[44][45][44,45]. It is thought that these caveolae are partly responsible for maintaining the homeostasis of water and protein transcytosis across the epithelial membrane
[43][44][45][46][43,44,45,46]. In bronchopulmonary dysplasia, exposure of type II pneumocytes to hyperoxia showed disruption of the pulmonary epithelial barrier
[46].
5.4. Pneumocytes
Caveolin-1 is essential for pulmonary function and development. As previously discussed, Cav1 is involved in the proliferation and differentiation of stem cells
[1][2][3][4][5][6][7][1,2,3,4,5,6,7]. This process is also true for fetal lung development, specifically as it relates to the differentiation into type II alveolar epithelial cells, also known as pneumocyte
[43]. Type II pneumocytes have a high expression of Cav1 and structurally contain an abundance of caveolae
[44][45][44,45]. It is thought that these caveolae are partly responsible for maintaining the homeostasis of water and protein transcytosis across the epithelial membrane
[43][44][45][46][43,44,45,46].
6. Caveolin-1 and the Intestinal Barrier
Multiple components contribute to the maintenance of the intestinal barrier. The intestinal epithelial layer acts as a semipermeable membrane allowing the absorption of nutrients and performance of immune functions while preventing the transport of harmful substances
[55]. The mucosal layer is the first line of defense for epithelial cells coming into direct contact with potentially toxic microorganisms. IgA and antimicrobial proteins are secreted into this layer in order to neutralize these microorganisms
[56]. The layer of polarized epithelial cells that separates the intestinal lumen from the lamina propria underneath is highly selective in what is allowed to be transported across
[55]. This is in part due to regulation via the presence of junctional complexes, which consist of tight junctions, adherens junctions, and desmosomes
[57][58][57,58]. Tight junctions determine a healthy epithelial barrier. Disruption of the tight junctions by systemic stressors can lead to intestinal hyperpermeability and trigger systemic immune responses
[59]. These components of the intestinal barrier are the major points of interest when researching and describing the pathogenesis of gastrointestinal diseases.
Although Cav1 is seen in the cytosolic and intracellular compartment forms, it is most commonly thought to be associated with the cell surface
[24]. One purpose this might serve is to integrate junctional complexes into the plasma membrane. This anchors epithelial cells at the basolateral surface both to each other and to the basement membrane
(Figure 2). The location of junctional complexes within the cell and their level of expression is, in part, Cav1-dependent
[60][61][62][63][64][65][66][67][60,61,62,63,64,65,66,67]. Cav1-mediated shuttling and endocytosis of tight junctions during their relocation has been described previously
[60][61][62][63][64][65][66][67][60,61,62,63,64,65,66,67].
7. Conclusions
Caveolin-1 is a vital protein for many cellular processes and its involvement in both the positive and negative regulation of these processes has been clearly demonstrated. Its pervasiveness throughout a variety of tissue and cell types has established the protein as a point of interest for many researchers to better understand the pathophysiologic mechanisms of disease. Cav1 as a plasma membrane protein carries out regulatory functions of many intracellular signaling cascades.