1. NGF and Autoimmune Diseases
Table 12 shows the role of NGF in autoimmune diseases. Indeed, evidence indicates that NGF may play a role in the pathogenesis of autoimmune diseases. Studies have revealed elevated levels of NGF in the blood and tissues of individuals affected by autoimmune diseases (thyroiditis, rheumatoid arthritis, and multiple sclerosis). Furthermore, NGF has been shown to modulate immune cell activity and has significant involvement in inflammatory conditions, wherein an increase in NGF, induced by inflammation or stress, might stimulate immune cells and other biological mediators during immunologic insults
[1][15].
Table 12.
NGF roles in autoimmune diseases.
| Disease |
Clinical Manifestations |
Role of NGF |
Ref. |
| Autoimmune thyroiditis |
Hyperthyroidism (e.g., Graves’ Disease) and hypothyroidism (e.g., Hashimoto Thyroiditis) with a variety of associated symptoms such as humoral psychotic symptoms, intolerance to cold/hot temperature, weight changes, difficulty in concentration, and eye disorders. |
-
Role in T cell population homeostasis regulation.
-
Increased levels of NGF and NGF autoantibodies in the blood and tissues of Autoimmune thyroiditis patients.
-
NGF may contribute to inflammation and tissue damage stimulating pro-inflammatory cytokines production and activating mast cells.
-
Anti-inflammatory treatments, able to reduce NGF levels in tears, are able to increase tear film stability and production and decrease eye congestive symptoms.
|
[2]122[3][4][,1235,124],125[6],126[7][,127] |
| Chronic arthritis |
Chronic inflammation and damage to joints and surrounding tissues, chronic pain and reduced quality of life, asthenia, psychological and social symptoms |
-
NGF overexpression in synovial fluid, serum, cerebrospinal fluid, and tissue specimens.
-
NGF concentrations are correlated with the extent of inflammation and clinical disease activity.
-
Rapid activation of NF-kB and MAP kinases regulates the bioavailability of aggrecanase and of NGF causing pain.
-
Decreased TrkA expression in immune cells of arthritis patients may contribute to chronic inflammation development and maintenance by preventing NGF regulatory feed-back mechanisms.
-
An active proNGF/p75NTR axis promotes chronic synovial inflammation.
-
Antibodies directed against NGF (NGF-Abs) have been successfully tested for the treatment of chronic pain in both animals and humans with some concerns about side effects.
|
[8][9][10][11][12][13][14][15][16[19][13,47][,12817][,12918,130,131,132,133,134,135],136,137] |
| Multiple sclerosis |
Periods of relative well-being alternate with episodes of symptom deterioration with gradual worsening over time. Tingling, numbness, pain, burning, itching, reduced sense of touch, loss of strength or dexterity in a limb, vision disorders. |
-
Increased cerebrospinal fluid and cerebral NGF levels.
-
Enhanced expression of NGF receptors in multiple sclerosis lesions.
-
NGF seems to produce anti-inflammatory effects so the induction of NGF probably represents an adaptive response against immune-mediated neuroinflammation.
-
The release of this neurotrophic factor by brain mast cells could be a key element.
-
Autocrine and paracrine factor in TrkA-expressing reactive and neoplastic glial cells.
-
p75NTR plays an important role in leukocyte-endothelial cell interactions and in the maintenance of Purkinje cells survival as well as their upregulation of sodium channel Na(v)1.8.
-
In animal studies altered NGF levels represent one of the early manifestations of these demyelinating diseases.
-
Higher levels of NGF correlate with disease phase, duration, age of patients, cognitive performance and disease progression.
-
Potential therapeutic role as NGF showed neuroprotective activity and immunomodulatory effects.
-
NGF may be useful as a marker of successful treatment.
|
[20][21]89[22][23][24][25][26][27][28][29][30][31][32][33][34][35][36][37][38][39][40][41][42][43],90[,9144,92][,9345][86,,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113] |
| Chronic granulomatous disease |
Recurrent infections, multiorgan granulomatous lesions, abscesses, lymphadenitis, hypergammaglobulinemia, anemia. |
-
The NOX2 deficiency in animal models is linked to a reduced expression of NGF and a decreased generation of mature neurons.
-
NGF may play a key role in the development of effective therapeutic genetic modification strategies.
|
[46][47][48][49][50][51][46,138,139,140,141,142] |
| Systemic lupus erythematosus |
Fever, fatigue, butterfly rash on the face, erythematous lesions in areas exposed to the sun, hair loss, purple-red lesions of the hard and nasal palate, cutaneous vasculitis, and multiorgan involvement. |
-
Increased B cells and serum NGF levels.
-
Major role in the inflammatory phase being closely correlated with disease activity.
-
Higher NGF concentrations related to subcortical atrophy.
-
NGF may have a prognostic value.
-
p75NTR is increased on CD16+ and CD56+ leucocytes of affected patients.
|
[52][53],144[54],145[55],146[56],147[57],148[58][59][60][143,149,150,151] |
| Mastocytosis |
Itching, dyspnea, urticaria, dizziness, sense of fainting, multi-organ dysfunctions. |
-
Mast cells are involved in neuroimmune interactions related to tissue inflammation.
-
NGF promotes mast cell differentiation and survival while mast cells produce NGF and other neurotrophins.
-
Elevated serum levels of NGF are related to mast cells load.
-
Increased expression of modified Trk receptors on mast cells may contribute to the pathophysiology of mastocytosis in paracrine and autocrine loops.
|
[61][62][63][64][2,152,153,154] |
Autoimmune thyroid diseases (AITDs) affect approximately 5% of the population and are the most prevalent organ-specific autoimmune conditions
[65][66][155,156]. These diseases are more common in women, with a prevalence of 5–15%, compared to men with a prevalence of 1–5%. The two most frequent AITDs are Graves’ Disease (GD) and Hashimoto Thyroiditis (HT), which are the major causes of hyperthyroidism and hypothyroidism, respectively. Their pathologic features involve reactive T-cells infiltration (predominant in HT) and B cells (predominant in GD), leading to follicular destruction, gradual atrophy, and fibrosis
[67][157].
The etiology of AITDs is multifactorial and involves various factors, including genes like
HLA and
CTLA4, as well as smoking, stress, alcohol, and iodine consumption. The onset of injury occurs when autoantibodies and/or sensitized T-cells respond against thyroid cells, causing an inflammatory reaction and cell lysis
[68][69][70][158,159,160]. About 20% of patients with AITDs also have other organ-specific or systemic autoimmune disorders. In the immune system, death receptors from the TNF/NGF receptor superfamily play a crucial role in regulating the adaptive immune response
[3][123]. During the adaptive immune response to antigens, after the peak of the immune response, most activated antigen-specific T cells are eliminated to maintain T cell population homeostasis. This elimination occurs either by death caused by cytokine withdrawal or by activation-induced cell death, through death receptor engagement.
Numerous studies have reported increased levels of NGF and NGF autoantibodies in the blood and tissues of individuals with AITDs
[2][4][5][122,124,125]. Additionally, NGF is believed to contribute to inflammation and tissue damage associated with these diseases. It has been found that NGF stimulates the production of pro-inflammatory cytokines and may activate mast cells, which release inflammatory mediators, further contributing to the inflammatory status and tissue damage observed in AITDs. Studies on AITDs-associated ophthalmopathy have emphasized the importance of NGF in the neuroprotection of orbital tissues. This suggests that anti-inflammatory treatments aimed at reducing NGF levels in tears could enhance tear film stability and production while reducing congestive symptoms
[6][7][126,127].
3. Chronic Arthritis
NGF is overexpressed in numerous inflammatory and degenerative rheumatic diseases
[10][128]. Its presence can be detected in synovial fluid, serum, cerebrospinal fluid, and tissue specimens, with NGF concentrations often correlating with the degree of inflammation and/or clinical activities in various circumstances.
Chronic arthritis is a significant contributor to joint and surrounding tissue inflammation and damage, resulting in chronic pain and reduced quality of life
[71][48]. The upstream mechanism that activates mechanoflammation in chronic arthritis remains unidentified. However, it leads to the rapid activation of NFkB and inflammatory mitogen-activated protein (MAP) kinases, controlling aggrecanase bioavailability and NGF regulation, which in turn causes pain
[11][129]. Numerous studies have demonstrated altered levels of NGF and its receptors in the sera and tissues of patients with chronic arthritis
[5][12][13][125,130,131]. The reduced expression of TrkA in the immune cells of arthritis patients may hinder the activation of regulatory feedback mechanisms by NGF, thereby contributing to the development and maintenance of chronic inflammation
[8][13].
Additionally, evidence indicates a role for the p75NTR receptor and its preferential ligand proNGF in potentiating inflammatory responses in synovial mononuclear cells of patients affected by chronic arthritis
[9][47]. This suggests that an active proNGF/p75NTR axis may promote pro-inflammatory responses in synovial fibroblasts, further contributing to chronic synovial inflammation. Consequently, p75NTR inhibitors could represent a potential novel therapeutic approach for chronic arthritis. Despite the availability of various non-pharmacologic and pharmacologic treatment options, chronic pain continues to be a significant global burden, affecting approximately 30% of the adult population. Therefore, the development of new analgesics with novel mechanisms of action is of utmost importance. Antibodies targeting NGF (NGF-Abs) have been developed for treating chronic pain conditions such as osteoarthritis and chronic low-back pain, as NGF contributes to peripheral and central sensitization through the activation of TrkA and stimulation of local neuronal sprouting
[14][15][132,133].
These NGF-Abs have demonstrated significant pain relief and functional improvement in both animal models and clinical patients affected by knee and/or hip osteoarthritis
[16][17][134,135]. However, their efficacy in non-specific lower back pain has yielded mixed results. Unfortunately, studies have raised safety concerns regarding NGF-Abs, as they may potentially cause or worsen peripheral neuropathies and lead to rapid joint destruction necessitating joint replacement surgery
[18][19][136,137]. The underlying causes of these side effects have been widely debated, and their pathophysiology remains poorly understood, limiting the practical use of these compounds. Nevertheless, most subjects have shown acceptable tolerability to NGF-Abs, with low rates of discontinuation reported in clinical trials to date
[72][73][161,162]. Interestingly, research has demonstrated that pretreatment with NGF-Abs reduces or prevents arthritis induced by carrageenan, indicating a functional role of NGF in this type of peripheral inflammation
[74][163].
4. Multiple Sclerosis
Multiple sclerosis is a chronic, predominantly immune-mediated, disease of the central nervous system (CNS) and one of the most common reasons of neurological disability in young adults, characterized by inflammation, demyelination and axonal loss leading to loss of vision in an eye and loss of power or sensibility in an arm or leg
[75][165]. The onset usually begins in young adulthood (between 20 and 40 years of age), and it is more common in women (the female to male ratio is 3 to 1) especially in Europe and North America
[76][77][166,167]. The etiology is complex and mostly unclear, amongst the environmental factors evidence supports an increased risk in patients with Epstein–Barr virus infection, cigarette smoking, low levels of vitamin D, and an increased BMI during adolescence
[78][168].
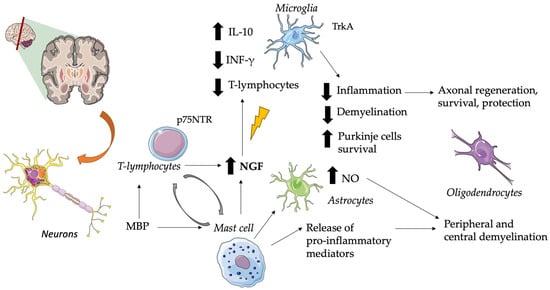
In particular, multiple sclerosis is an autoimmune demyelinating disease that produces brain plaques containing mast cells and areas of demyelination demarcated by T-lymphocytes and monocytes in cellular infiltrates
[61][79][2,169]. NGF seems to produce anti-inflammatory effects by upregulating the production of interleukin 10 by glial cells, T cells infiltrating the CNS, and downregulating the production of interferon-gamma
[80][170]. The major suspected immunogen in multiple sclerosis is the myelin basic protein (MBP) which stimulates mast cell secretion of pro-inflammatory mediators, capable of causing peripheral and central demyelination, and of cytokines that can induce astrocyte production of neurotoxic amounts of nitric oxide (NO)
[81][171]. See
Figure 12 for further information.
Figure 12. Role of NGF in Multiple Sclerosis. Brain plaques characterized by mast cells and demyelination zones containing T-lymphocytes and monocytes are typical of this disease. In this context, NGF exerts anti-inflammatory effects by downregulating the production of IFN-γ, reducing T-lymphocyte infiltration, and upregulating the production of IL-10 by glial cells. This suggests a role as an adaptive response against immune-mediated neuroinflammation. T-cell specific antigens (i.e., MBP) stimulate T-cell and mast cell NGF secretion, but also release pro-inflammatory mediators and astrocyte production of neurotoxic amounts of NO. When p75NTR is expressed, it plays a crucial role in leukocyte-endothelial cell interactions and in maintaining the survival of Purkinje cells. Altered NGF levels are associated with the loss of its neuroprotective role in axonal and oligodendrocyte regulation. CNS, central nervous system; INF-γ, interferon-gamma; IL, interleukin; MBP, myelin basic protein; NO, nitric oxide; NGF, nerve growth factor; p75NTR, p75 neurotrophin receptor; Trk, tyrosine kinase receptor. The pictures were obtained by Servier Medical Art. Servier Medical Art by Servier is licensed under a Creative Commons Attribution 3.0 Unported License (
https://creativecommons.org/licenses/by/3.0/ accessed on 5 November 2023).
Early release of mast cell mediators may influence a delayed T-cell response, whereas T-cell products can cause mast cell activation
[82][172]. Since both lymphocytes and monocytes respond to NGF, the release of this neurotrophic factor by brain mast cells could be a key element in such a cycle. It has been suggested that the reactivation of CNS autoimmune T cells by locally presented antigens to which they are specific (e.g., MBP) can lead to enhanced secretion of NTs
[83][173]. So, the induction of NGF probably represents an adaptive response against immune-mediated neuroinflammation
[84][85][174,175]. There are various reports of increased cerebrospinal fluid and cerebral NGF levels in patients with multiple sclerosis
[86][87][88][89][90][176,177,178,179,180]. Furthermore, enhanced expression of NGF receptors has also been demonstrated in multiple sclerosis lesions
[91][92][181,182].
These receptors play important and different roles in multiple sclerosis, for example, NGF acts as an autocrine or paracrine factor in TrkA-expressing reactive and neoplastic glial cells, while p75NTR plays an important role in leukocyte-endothelial cell interactions and in the maintenance of Purkinje cell survival
[93][94][183,184]. As studies have highlighted, in addition to demyelination and axonal degeneration, dysregulated ion channel expression also contributes to the pathophysiology of multiple sclerosis; moreover, it has been suggested that NGF acts via p75 to contribute to the upregulation of sodium channel Na(v)1.8 in Purkinje cells
[95][185].
In animal studies altered NGF levels represent one of the early manifestations of these demyelinating diseases
[96][186]. Interestingly, a recent article suggested a correlation between higher cerebrospinal fluid levels of iodothyronines, nerve growth factor and multiple sclerosis
[97][187]. This is in line with the evidence suggesting that thyroid hormones activate oligodendrocyte precursors (OPs) and increase myelin-forming protein and NGF content
[98][188]. During the acute phase of the disease, there is an increase of BDNF, TNF-alpha and IFN-gamma synthesis and release, while significantly higher levels of NGF, GDNF, NT3 and NT4 can be found in the post-relapse phase, with the neuroprotective potential of immune cells being inversely related to disease duration and with the age of patients
[99][189]. Interestingly, cognitive performance and disease progression, especially in the case of relapsing-remitting multiple sclerosis patients, are strongly linked to NGF, which might play a neuroprotective role
[100][101][190,191].
On the other hand, the use of NTs as therapeutic agents has been suggested as a novel option for restoring and maintaining neuronal function during neurodegenerative diseases such as multiple sclerosis. NGF induces axonal regeneration, protection, survival, and differentiation of oligodendrocytes (OGs), it facilitates migration and proliferation of OPs to the sites of myelin damage
[102][192]. NGF also directly regulates key structural proteins that comprise myelin and induces the production of BDNF which is also involved in myelination
[102][192].
As NGF showed neuroprotective activity and immunomodulatory effects, it has been suggested that new therapeutic approaches for the treatment of numerous brain disorders, including multiple sclerosis should focus on NGF and NTs
[103][104][193,194]. Furthermore, autoimmune and mesenchymal stem cells may protect neuronal populations and suppress the formation of new lesions by the release of NTs, suggesting that these cells could be an alternative source for delivering NTs into the CNS
[105][195].
The NGF and NTs levels are often used as markers of successful treatment in neurodegenerative and autoimmune diseases
[106][107][196,197]. Interferon beta (INF-β) therapy, which reduces the rate of clinical relapse and the frequency of lesions in patients with multiple sclerosis, has been shown to promote NGF and NT secretion early in the course of this disease, leading to better clinical effects in those patients who presented a significant increase in NTs
[108][109][110][198,199,200].
A recent study demonstrated that six months of probiotic supplementation results in greater improvement in mental health parameters significantly increasing BDNF (but not NGF) levels and reducing the IL-6 levels
[111][201]. Other interesting articles found that moderate exercise training may alter markers of blood-brain barrier (BBB) permeability and neurotrophic factor status, especially in normal-weight persons with multiple sclerosis influencing the health-related quality of life, while overweight participants may be more resistant to these effects. However, there is still a need for more high-quality studies to clarify the impact of exercise on chronic levels of NTs and long-term health of patients
[112][113][114][115][116][117][202,203,204,205,206,207].
New evidence on the murine model of multiple sclerosis suggests that metformin-induced AMP-activated protein kinase (AMPK) pathway activation stimulates remyelination through induction of neurotrophic factors (NGF, BDNF and ciliary neurotrophic factor), downregulation of neurite outgrowth inhibitor (NogoA) and recruitment of Olig2+ precursor cells opening the way for new therapeutic strategies based on AMPK activation
[118][208].
The anti-inflammatory effects of new drugs and molecules for treating experimental autoimmune encephalomyelitis may provide further insights into the understanding of their neuroprotective activities in multiple sclerosis
[119][120][121][122][123][124][209,210,211,212,213,214]. Unfortunately, most of the evidence on these compounds has not reached the clinical level so their effectiveness on human disease is still unclear.
It has been highlighted that the importance of NTs and NGF as targets for autoimmune neuroprotection, represents a novel therapeutic approach aimed at shifting the balance between the immune and neuronal cells towards survival pathways in a variety of CNS injuries including multiple sclerosis
[125][215]. These findings are in line with animal evidence that NGF prevents demyelination, cell death, and progression of the disease in experimental allergic encephalomyelitis murine models
[126][127][216,217]. During the acute phase of the disease, the glial cells become more receptive to NGF, pointing to the glia as an important target for possible pharmacological manipulations such as exogenously administered NGF
[128][218]. In fact, new drugs have been developed that may serve as lead molecules to develop protective agents for oligodendrocyte populations and myelin (NT-like compounds) permeable to the BBB
[129][219].
5. Systemic Lupus Erythematosus
Systemic lupus erythematosus (SLE) is a rheumatic autoimmune disorder affecting multiple systems, characterized by connective tissue damage due to B-cell hyperactivity and abnormal immune regulation
[52][143]. It is more prevalent in women aged 15 to 40 years, initially manifesting as cutaneous and mucosal erythematous symptoms and photosensitivity. Subsequently, the disease can involve almost all organs and systems, including the kidneys, joints, central nervous system, serous membranes, and hematopoietic system, due to the deposition of immune complexes and complement activation. Patients with SLE have shown increased B cells and higher serum levels of both NGF and BDNF
[5][53][54][125,144,145]. Elevated concentrations of NGF and BDNF have been associated with subcortical atrophy in neuropsychiatric SLE patients
[55][146]. NGF also plays a significant role in the inflammatory phase of the disease, and studies have suggested its involvement, along with interleukin-13 (IL-13), in the pathogenesis of SLE, is closely correlated with disease activity
[56][57][58][147,148,149].
Notably, NGF levels have been found to be elevated in childhood SLE, with a correlation to disease activity, indicating its potential role in SLE pathogenesis and its usefulness as a prognostic marker for evaluating disease progression and guiding clinical management
[59][150]. Additionally, higher levels of these factors in SLE patients may be associated with epigenetic changes due to DNA hypomethylation
[130][220]. Interestingly, IL-34, strongly related to myeloid cell subsets (e.g., brain microglia), appears to be associated with disease progression, severity, and chronicity
[131][132][221,222]. Therefore, blocking NGF, IL-13, and/or IL-34 might be considered to suppress the expression of proinflammatory cytokines in the blood of SLE patients, potentially benefiting the patient’s condition.
The role of NGF and its receptors in SLE is still under investigation. The expression of the NGF high-affinity receptor (TrkA) and low-affinity receptor (p75) has been analyzed on all major leukocyte subsets of patients with SLE. When comparing SLE patients with healthy control subjects, TrkA expression was not found to be differentially expressed, while p75 expression was increased on CD16+ and CD56+ leukocytes of patients
[60][151].
6. Mastocytosis
Mastocytosis is a rare and heterogeneous disease characterized by an increased number of mast cells (MCs) in various body tissues. Two main types of mastocytosis can be distinguished based on their distribution: cutaneous mastocytosis, which is more common in children, and systemic mastocytosis, which primarily affects adults
[133][223]. The clinical features of mastocytosis include flushing, pruritus, abdominal aching, looseness, hypotension, syncope, and musculoskeletal pain
[134][224]. MCs and NGF play a role in neuroimmune interactions associated with tissue inflammation. MCs may produce and respond to NGF, and changes in MCs behavior may lead to altered neuroimmune responses, including autoimmune responses
[61][62][2,152]. Neurotrophins (NTs) have been found to promote the differentiation and survival of MCs, making them a significant source of NTs
[63][64][153,154].
Patients with mastocytosis exhibit elevated serum levels of NGF and NT-4, which are related to the load of MCs
[61][2]. Additionally, it has been suggested that the increased expression of modified Trk receptors (TrkA and TrkC) on skin and gut MCs may contribute to the pathophysiology of mastocytosis through autocrine and paracrine loops
[63][153]. Although the precise impact of NGF and its receptors on mastocytosis pathogenesis is not entirely clear, murine models have shown that TrkA activation leads to mastocytosis and is involved in the development of resistance to the receptor tyrosine kinase KIT-targeted therapy, which targets the mast/stem cell growth factor receptor KIT. This suggests that a combined approach targeting both KIT and TrkA might enhance the efficacy of molecular therapy in systemic mastocytosis patients with
KIT mutations
[135][225]. These findings partially explain why treatment with KIT inhibitors alone has been disappointing in most published clinical trials for mastocytosis.
7. Chronic Granulomatous Disease
Chronic granulomatous disease (CGD) is a rare disorder causing loss-of-function in the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX) complex, leading to diminished phagocyte capability in killing microorganisms
[47][138]. Individuals affected by CGD are more vulnerable to infections, excessive inflammation, and autoimmune diseases, as well as experiencing intellectual and cognitive impairment
[48][49][139,140]. In CGD models with NOX2 deficiency, there is a reduced expression of NTs and a decrease in the generation of mature neurons
[47][138]. NGF plays a significant role in developing effective therapeutic strategies for genetic modification
[46].
Most CGD patients are males with hemizygous mutations in the X-linked
CYBB gene coding for gp91-phox (X-CGD). These patients have significantly low levels of superoxide, as only 5 to 10% of neutrophils producing superoxide are enough to protect X-CGD heterozygotes from severe infections. Recently a promising approach using a bicistronic retroviral vector to modify genetic defects and restore superoxide production in phagocytes of CGD patients has been experimented with offering hope for improving the condition of X-linked CGD individuals. In particular, a potential therapeutic approach for X-CGD involves the development of a retroviral vector containing both the coding sequences of gp91-phox and a cytoplasmically truncated version of human p75NTR
[50][51][141,142]. Under optimal conditions, this strategy allows 80% of the CD34+ cells to be transduced, resulting in 70% of normal levels of superoxide synthesis and release in phagocytes derived from transduced cells.