You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 1 by Hassan Ragab El-Ramady and Version 2 by Peter Tang.
Fibers can originate from two main sources, natural and synthetic fibers. Natural fibers refer to the fibers obtained from plants, animals, and minerals. Natural resources including water, energy, and food have an increase in demand due to the global population increases. The sustainable management of these resources is an urgent global issue. These resources combined in a very vital nexus are called the water–energy–food (WEF) nexus. The field of nanotechnology offers promising solutions to overcome several problems in the WEF nexus.
- WEF nexus
- wastewater treatment
- food packaging
- energy harvesting
- medicinal
- pharmaceutical
- biomedicine
- nanoparticles
1. Introduction
Agriculture is the main source of our food, feed, fiber, and fuel. Fibers are one of the important agro-productivity sources. Fibers can originate from two main sources, natural and synthetic fibers. Natural fibers refer to the fibers obtained from plants, animals, and minerals. Concerning plants, natural fibers may originate from leaves, seeds, bark, fruit, and stalks as sources of fiber, whereas animal ones are derived from silk, wool, and hair as well as mineral fibers like asbestos [1]. The main properties of natural fibers may include renewable, safe, non-polluting, and legitimate sources of fiber, which could be employed for the manufacturing of several composites in the future [1]. Several recent publications issued on different applications of natural fibers, such as important sources for producing polymers (e.g., [1][2][3]). These applications may include the following fields, such as biomedicine [2], 4D printing technology [4], aerospace [5], filtration of water/wastewater [6], textiles [7], additive manufacturing applications [8], the automotive industry [9][10], the construction industry [11], thermal structure engineering [12], food packaging [13], harvesting and the storage of energy [14]. The water–energy–food (WEF) nexus has a strong relationship with nanofibers, which can support their components through mainly the following activities: food packaging [15], energy processing [16], and water handling [17].
Nanofibers are a kind of fiber that can be in natural and synthetic nanoforms (less than 100 nm). These fibers have several distinctive properties compared with natural fibers such as high porosity, large specific surface area, and high size uniformity [6]. Apart from natural fibers, nanofibers have been applied in many major sectors of our life, including agricultural [18], pharmaceutical [19], biomedical [20], and industrial fields [21] of the global water [22][23], energy [24], and food sectors [25]. Biotechnological applications of nanofibers have gained sound interest from scientists and industrial workers due to their promising roles in many fields such as delivering bioactive compounds [26], and the biomedical field [27]. The suggested roles of nanofibers in the main sectors of water, energy, and food are linked to the security of these sectors, and their nexus. Nanofibers in the water sector were discussed in many publications focusing on water treatment [6], water purification [28], and wastewater treatment [23]. Additional studies on energy and food industry applications of nanofibers could be noticed, which mainly focused on harvesting/storage of energy [24] or food packaging [25].
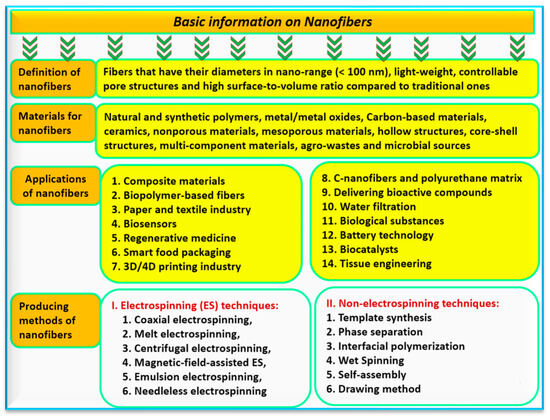
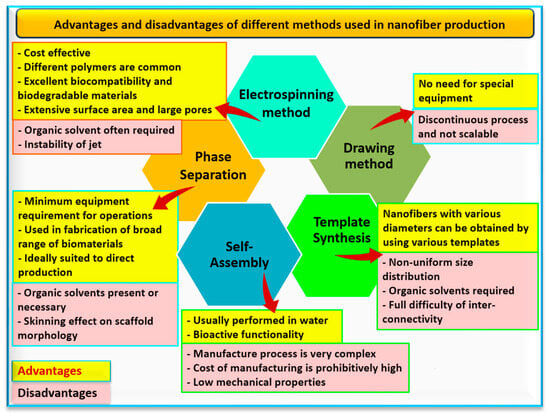
 Due to the previous advantages, nanofibers have many applications such as drug delivery systems [30], industrial building design [31], biomedicine [32][33], 4D printing industry [34], textile industry [35][36], and wastewater remediation [37]. Nanofibers could be produced by electrospinning and non-electrospinning techniques (Figure 2). Several unique properties of electrospun nanofibers are well-known compared with other bulk materials such as adjustability of pore sizes, large surface area, and porosity, as well as the extracellular matrix [26]. These production methods with advantages and disadvantages can be presented in Figure 3. Microbial sources for producing nanofibers are considered an important approach, which is applied to many fields such as bacterial cellulose nanofiber [38], microbial fuel cells [39], microbial polysaccharides for controlled drug release [40], and monitoring of food packaging [41]. These fields will be discussed in the following sections.
Due to the previous advantages, nanofibers have many applications such as drug delivery systems [30], industrial building design [31], biomedicine [32][33], 4D printing industry [34], textile industry [35][36], and wastewater remediation [37]. Nanofibers could be produced by electrospinning and non-electrospinning techniques (Figure 2). Several unique properties of electrospun nanofibers are well-known compared with other bulk materials such as adjustability of pore sizes, large surface area, and porosity, as well as the extracellular matrix [26]. These production methods with advantages and disadvantages can be presented in Figure 3. Microbial sources for producing nanofibers are considered an important approach, which is applied to many fields such as bacterial cellulose nanofiber [38], microbial fuel cells [39], microbial polysaccharides for controlled drug release [40], and monitoring of food packaging [41]. These fields will be discussed in the following sections.
 The electrospinning method is the most widely used compared with other techniques for producing electrospun fibers due to its efficiency, operational simplicity, practicality, cost-effectiveness, and versatility [26]. The production of nanofibers is not only the main limiting factor but also the properties of these fibers, which should be evaluated by scanning electron microscopy (SEM) and other methods. Certain properties of nanofibers, which are important for their functions, will be discussed in the next section including physico-chemical, mechanical, and biological characteristics. On the microscopic level of nanofibers, SEM images of the fabrics revealed that the enhancement in fiber strength can be attributed to the formation of structures resembling bamboo nodes or wheat stem nodes within the fibers. The production of nano-sized fibers and the use of these fibers to create non-woven fabric was achieved by the ouresearchers' lab (Nano Food Lab, Debrecen University, Debrecen, Hungary). These nanofibers were applied as specialized filtering materials for gases (air) and liquids, as well as for manufacturing functional filters. Several scientific attempts for producing nanofibers in the researchers'our lab were developed on different levels including a laboratory scale, a pilot-scale device, and a full-scale manufacturing facility. Design, development, and optimization of nanofiber formation using different parameters (e.g., flow rate, voltage, electrode distance, surface, time, and solution concentration) were evaluated. Construction of a full-scale manufacturing facility included design, implementation, test production, and machine adjustment, as well as mass production of products, quality control, and implementation of a quality assurance system.
The electrospinning method is the most widely used compared with other techniques for producing electrospun fibers due to its efficiency, operational simplicity, practicality, cost-effectiveness, and versatility [26]. The production of nanofibers is not only the main limiting factor but also the properties of these fibers, which should be evaluated by scanning electron microscopy (SEM) and other methods. Certain properties of nanofibers, which are important for their functions, will be discussed in the next section including physico-chemical, mechanical, and biological characteristics. On the microscopic level of nanofibers, SEM images of the fabrics revealed that the enhancement in fiber strength can be attributed to the formation of structures resembling bamboo nodes or wheat stem nodes within the fibers. The production of nano-sized fibers and the use of these fibers to create non-woven fabric was achieved by the ouresearchers' lab (Nano Food Lab, Debrecen University, Debrecen, Hungary). These nanofibers were applied as specialized filtering materials for gases (air) and liquids, as well as for manufacturing functional filters. Several scientific attempts for producing nanofibers in the researchers'our lab were developed on different levels including a laboratory scale, a pilot-scale device, and a full-scale manufacturing facility. Design, development, and optimization of nanofiber formation using different parameters (e.g., flow rate, voltage, electrode distance, surface, time, and solution concentration) were evaluated. Construction of a full-scale manufacturing facility included design, implementation, test production, and machine adjustment, as well as mass production of products, quality control, and implementation of a quality assurance system.
2. Nanofibers in Water, Energy and Food
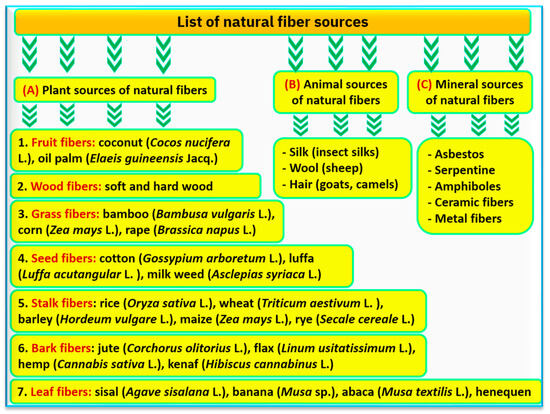
What are the main properties of nanofibers? Nanofibers are polymeric fibers on a nano-scale, which have certain properties and can be produced using both natural and synthetic polymers. Plant natural fibers are the main sources for producing such fibers along with animal and mineral sources, which depend on the used plant fraction such as leaves, stems, stalks, and seeds (Figure 1). The most common plant fiber sources may include seed fibers from cotton (Gossypium arboretum L.), fruit fibers from coconut (Cocos nucifera L.), bark fibers from jute (Corchorus olitorius L.), leaf fibers from sisal (Agave sisalana L.), and banana (Musa sp.). Synthetic or artificial polymers of nanofibers are widely used due to their easy production, low cost, and higher mechanical properties, but they can cause long-term environmental and human health problems due to their nonbiodegradable disposal, toxic nature, and their persistence in the ecosystem for a long time [29]. Nanofibers possess several desired properties such as extremely high porosity, low density, high specific surface area, and a highly porous matrix. Nanofibers also have specific functionalities due to their large specific area, which allow immobilizing nanoparticles (NPs), metal–organic frameworks, and zeolites [29].
Figure 1. The main sources of natural fiber resources that can be used in the agro-productivity, whereas (A) refers to the plant sources of natural fibers, (B) animal sources, and (C) is mineral sources.

Figure 2.
The main basic information on nanofibers, their definition, classification, and applications.

Figure 3.
Suggested advantages (in the yellow boxes) and disadvantages (in the pink boxes) of producing methods of nanofibers.
3. Most Common Applications of Nanofibers
It is well known that natural fibers derived from plants have gained great concern as a sustainable alternative to synthetic materials depending on plant species and the part used. Several cultivated plants have been used in fiber production such as the stem of the following plants Himalayacalamus falconeri [42], Lankaran acacia L. [43], Ficus benjamina L. [44], Waltheria indica L. [45], Myriostachya wightiana [46], and Cyperus platystylis R. Br. [47]. Chemical treatment is the main process for producing natural fibers, which may involve alkaline, acetylation, benzoylation, peroxide, potassium permanganate, silane, and stearic acid in addition to the surface treatments [6]. The biomedical sector has major applications of nanofibers among other different sectors including water, energy, and food. Enormous applications of nanofibers in biomedicine and therapy sectors are well-known and shown in Figure 4. Nanofiber composites can be used to deliver drugs, genes, or other biomolecules. Furthermore, they can be applied for engineering different tissues, from the skin through the blood to neural tissues as summarized in Figure 4. The therapeutic application of nanofiber composites is extensive, they can be used orally, transdermally, or intravenously in different types of therapies (Figure 4).
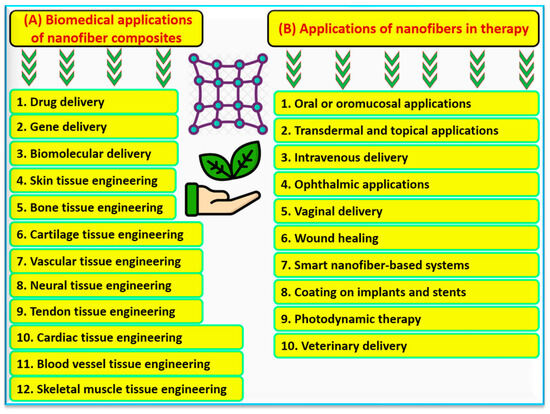

Figure 4.
List of suggested biomedical applications of nanofiber composites (
A
), and list of applications of nanofibers in the therapy sector (
B
).
These applications may involve the delivery of drugs [30], bionanocomposites [48], and genes [49], as well as different applications in the field of tissue engineering for many human organs including bone [50], tendon [51], skin [52], cartilage [53], skeletal muscle [54], cardiac [55], vascular [56], and neural tissues [57]. Nanofibers exist in every branch of the medical sector, which contributes to several fields such as tissue engineering [58][59], regenerative medicine [60], and sensing and biomedical approaches [61]. These applications may involve drug delivery carriers [30], wound dressing [62], and facemasks [63]. These nanofibers are useful for tissue regeneration by mimicking a porous topography, enhancing the adhesion cells, and improving physiological acceptability and mechanical strength [64].
4. Further Applications of Nanofibers
Nanotechnology is one of the fastest growing fields of advanced technology and has been proven to have an essential role in achieving significant development in agricultural systems and many different scopes of science and technology [65]. It has an extremely wide range of applications that could be employed for modern industry, agriculture as nanofertilizers [66], nanopesticides, the food sector, electronics, bioengineering, renewable energy, medicine, pharmacy, cosmetics, and sensor technology [67][68]. Nanotechnology has changed the delivery properties of metal elements into plant cells and has affected the biological systems including plant survival, biosynthesis, antioxidant status, enzyme activity, growth, and development processes, and it has an active interference with the environment [69]. Nanoparticles (NPs) have proven to exhibit catalytic, optical, antibacterial, and antifungal properties [70][71]. With the advancement of nanotechnology, numerous potential applications of nanoparticles have attracted considerable attention due to their unequaled physiochemical, non-toxic properties, and practical effects of their very tiny molecules as well as the low cost and stability at high temperatures [65].
Plant tissue cultures are the center of plant biology and play a significant role in the conservation of plant resources, mass propagation, genetic manipulation, bioactive metabolites production, and plant improvement [72]. In recent years, plant tissue culture applications using nanotechnology, called plant nanobiotechnology, have become feasible and more popular [68]. The promising applications in plant tissue culture field include callus induction and proliferation [65][68][69][70], biodiversity preservation of plants by cryopreservation [73], embryogenic callus formation and plant development via somatic embryogenesis [67][74][75], and in vitro shoot multiplication and plant regeneration or organogenesis [65][72][76][77][78][79].
Moreover, NPs have been used in plant tissue cultures to improve in vitro seed germination for commercial production of seedlings by nano-priming [80], antifungal nano-composite [81], copper oxide nanoparticles [82], and iron nanoparticles [83]. NPs also have been applied for enhancing root induction [65][77][81] and stimulating the production of bioactive compounds as they can be used as elicitors in plant tissue cultures [72][75][84][85][86][87][88][89]. On the other hand, NPs may induce or reduce the somaclonal variation of in vitro cultured plants [72]. Genetic and phenotype variations that could be stimulated in vitro by Ag-NPs are very desirable in breeding programs and plant improvement [90]. Furthermore, nanoparticles could be utilized for the genetic transformation of plants via gene delivery “Nanoparticles-based delivery technique” into the plant genome, with the purpose of improving the quality and quantity of agricultural crops [91].
In addition to the direct actions of NPs on in vitro cultures, silver and cobalt nanoparticles (Ag-NPs, Co-NPs) have been used to overcome leaf abscission and enhance the growth, development, and survival rate of the in vitro propagated plantlets. They act as ethylene inhibitors [92]. Ethylene could accumulate in culture vessels and negatively affect the growth of the tissues by increasing the activity of hydrolytic enzymes leading to abnormal phenomena of leaf abscission, yellowing, and hyperhydricity of some species accordingly, which decreases the shoot quality [77]. Ag-NPs were successfully applied to reverse hyperhydricity and regain the normal growth of in vitro shoots [93]. NPs were proven to have the ability to enhance the tolerance of in vitro plants to abiotic stress. The application of iron nanoparticles could significantly mitigate the harmful effects of salinity or drought on the plant tissues cultured in vitro. This is an efficient method to produce plant materials that are tolerant to various abiotic stresses [83][94][95].
Several in vitro studies reported the antimicrobial behavior of NPs. They have the potential use to reduce the fungal and bacterial contamination of tissue-cultured plants [71][81], consequently ensuring aseptic environmental culture conditions. However, they should be used at optimal concentrations, to avoid possible toxicity to plant tissues, due to the cytotoxic behavior of the element itself (i.e., copper). CuO-NPs and Ag-NPs are considered the most effective antimicrobials commonly used in plant tissue cultures mainly for in vitro propagation of plants. They are applied by supplementing the culture media or as an explant disinfectant by surface sterilization [81][92][96][97][98]. Titanium dioxide nanoparticles (TiO2-NPs) showed potential for inhibition of bacterial growth in plant tissue culture media [99].
Preliminary trials on the possible use of the nanofiber as an antimicrobial in plant tissue cultures were carried out as reported in Figure 5. These experiments were performed in order to answer the following questions: what kind of nanofibers did we apply? What is the main source of these nanofibers? What is the main target of this application? What are the main results of these experiments? To what extent can we use nanofibers in the plant tissue culture field? What are the main limitations and future perspectives of this application? More justifications and validations can be extracted from these experiments by answering the previous questions.
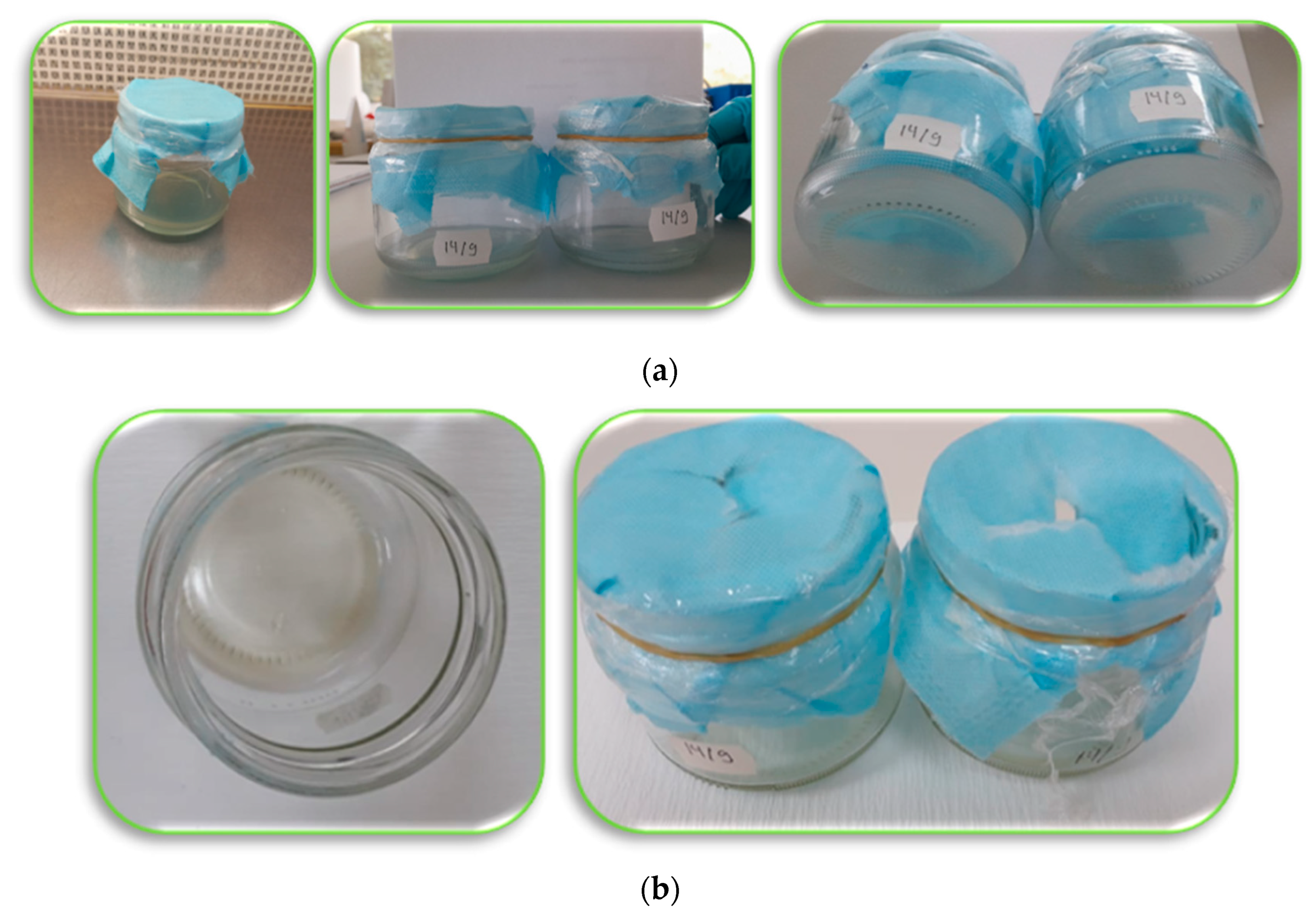

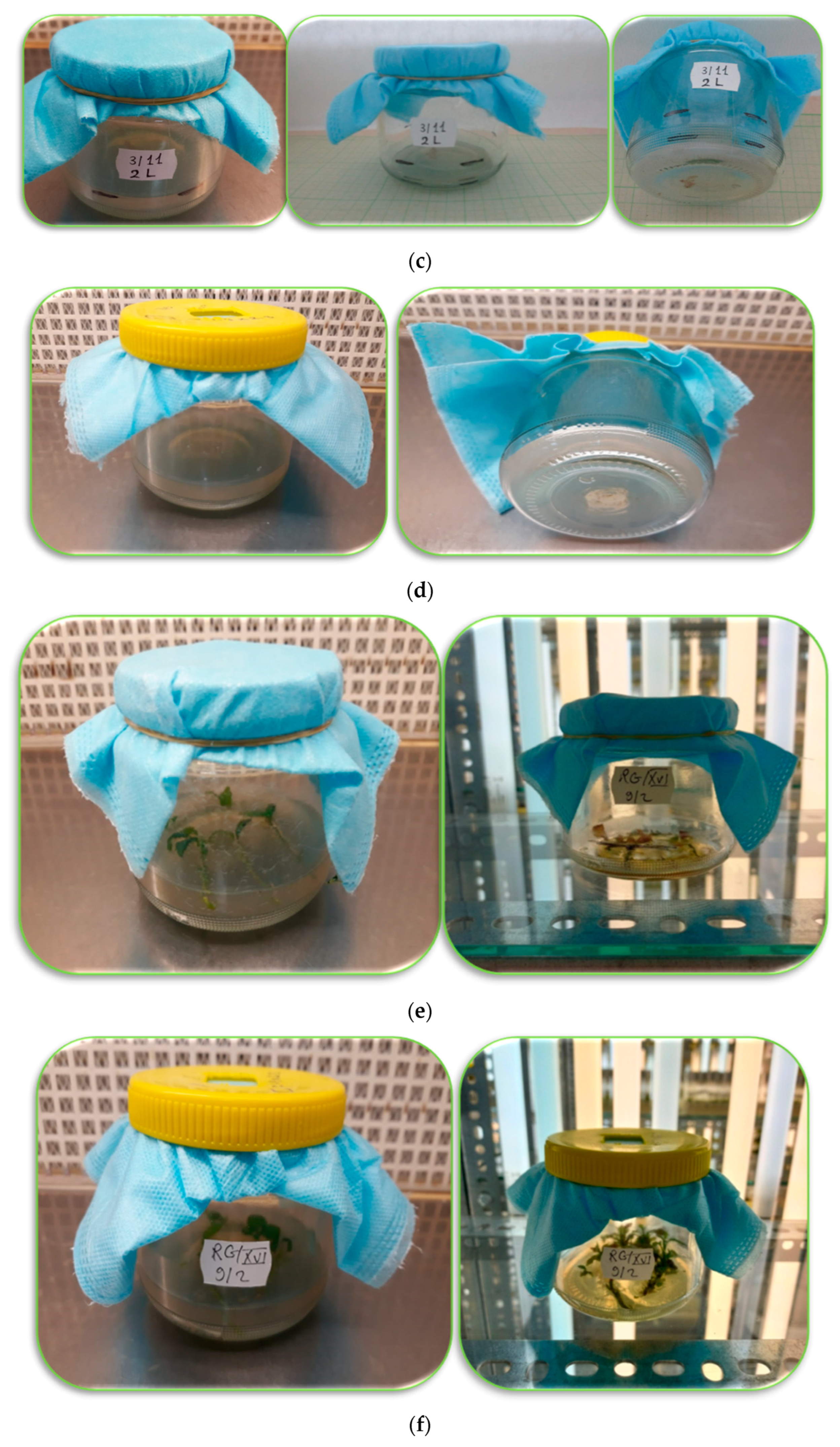




Figure 5. (a) One layer of nanofiber as a closure of the jar containing sterilized medium. (b) Evaporation of the medium and decomposition of the nanofiber after five weeks. (c) Double layers of nanofiber with less evaporation and without contamination. (d) Modified nanofiber (double face with a hole in the plastic cover of the jar of sterilized medium). (e) Double layers nanofiber (drying up the medium and death of the explants (RG apple shoots). (f) Modified nanofiber: the explants started to grow then died due to drying of the medium Photos were taken by Neama Abdalla.
This researchtudy was carried out using the electrospinning method for preparing the nanofibers produced by Dispomedicor Ltd. (Hajdúböszörmény, Hungary). The nanofiber was between two melt-blown nonwoven PE layers. The nanofiber was used as a closure of culture vessels. The researchers aWe aimed to determine whether nanofiber could be used to eliminate microbial contamination in plant tissue culture media or not, and to what extent these nanofibers allow good ventilation for enhancing the growth and quality of tissue-cultured plants as well. The recorded observations for these initial experiments indicated that nanofibers are promising tools and could be used efficiently to eliminate microbial contamination in the culture medium but they need some more modifications in further studies to control the high rate of evaporation from the medium, which may cause stress to the cultured tissues, and to keep the explants alive so they can grow and develop. Accordingly, obtain clean and high-quality tissue-cultured plants. Therefore, future investigations are needed on these kinds of nanofibers for more applicability. The future aim is to use these nanofibers for closing the culture vessels, not only at a small scale in the scientific labs, but also in the tissue culture companies that use big bioreactors, and/or in the nurseries of plant propagation for commercial production in a large scale.
ThWe researchers ssuccessfully developed a laboratory-scale electrospinning device capable of producing nanofibers from different composition solutions. By combining atomization and electrospinning, the researcherswe managed to develop and apply nanofiber-producing heads suitable for industrial-scale production. Various nano-powder additives for the fibers were tested and developed using a 3% graphite, 3% nano-selenium, and 10–12% PVB alcohol solution for nanofiber production (unpublished data). Further remaining tasks may include controlling and depositing the fibers produced by the atomization-based electrospinning technology onto a carrier, further investigation of the filtration efficiency of nano-fabrics, and exploring the potential applications of nanofibers with nanoparticle additives. Industrial optimization of these nanofibers may significantly contribute to the advancement and potential commercial applications of the developed technology.
References
- Kurien, R.A.; Anil, M.M.; Mohan, S.L.S.; Thomas, J.A. Natural fiber composites as sustainable resources for emerging applications—A review. Mater. Today Proc. 2023; in press.
- Dimple Singh, G.P.; Mangal, R. A comprehensive review of natural fiber reinforced composite and their modern application. Mater. Today Proc. 2023; in press.
- Mohammed, M.; Oleiwi, J.K.; Mohammed, A.M.; Jawad, M.A.; Osman, A.F.; Adam, T.; Betar, B.O.; Gopinath, S.C.B.; Dahham, O.S.; Jaafar, M. Comprehensive Insights on Mechanical Attributes of Natural-Synthetic Fibres in Polymer Composites. J. Mater. Res. Technol. 2023, 25, 4960–4988.
- Kishore, S.R.; Sridharan, A.P.; Chadha, U.; Narayanan, D.; Mishra, M.; Selvaraj, S.K.; Patterson, A.E. Natural fiber biocomposites via 4D printing technologies: A review of possibilities for agricultural bio-mulching and related sustainable applications. Prog. Addit. Manuf. 2023.
- Bhadra, D.; Dhar, N.R. Selection of the natural fiber for sustainable applications in aerospace cabin interior using fuzzy MCDM model. Materialia 2022, 21, 101270.
- Badgar, K.; Abdalla, N.; El-Ramady, H.; Prokisch, J. Sustainable Applications of Nanofibers in Agriculture and Water Treatment: A Review. Sustainability 2022, 14, 464.
- De Vos, B.; De Souza, M.F.; Michels, E. Industrial hemp (Cannabis sativa L.) field cultivation in a phytoattenuation strategy and valorization potential of the fibers for textile production. Environ. Sci. Pollut. Res. 2023, 30, 41665–41681.
- Adapa, S.K. Jagadish Prospects of Natural Fiber-Reinforced Polymer Composites for Additive Manufacturing Applications: A Review. J. Miner. 2023, 75, 920–940.
- Akter, M.; Uddin, M.H.; Anik, H.R. Plant fiber-reinforced polymer composites: A review on modification, fabrication, properties, and applications. Polym. Bull. 2023.
- Dua, S.; Khatri, H.; Naveen, J.; Jawaid, M.; Jayakrishna, K.; Norrrahim, M.N.F.; Rashedi, A. Potential of natural fiber based polymeric composites for cleaner automotive component production—A comprehensive review. J. Mater. Res. Technol. 2023, 25, 1086–1104.
- Abdalla, J.A.; Hawileh, R.A.; Bahurudeen, A.; Jyothsna, G.; Sofi, A.; Shanmugam, V.; Thomas, B.S. A comprehensive review on the use of natural fibers in cement/geopolymer concrete: A step towards sustainability. Case Stud. Constr. Mater. 2023, 19, e02244.
- Sekhar, V.C.; Dasore, A.; Yalamasetti, B.; Madhuri, K.S.; Narendar, G. Flexural behavior of natural fiber epoxy composites. Mater. Today Proc. 2023; in press.
- Bangar, S.P.; Ilyas, R.A.; Chaudhary, N.; Dhull, S.B.; Chowdhury, A.; Lorenzo, J.M. Plant-Based Natural Fibers for Food Packaging: A Green Approach to The Reinforcement of Biopolymers. J. Polym. Environ. 2023.
- Manjakkal, L.; Jain, A.; Nandy, S.; Goswami, S.; Carvalho, J.T.; Pereira, L.; See, C.H.; Pillai, S.C.; Hogg, R.A. Sustainable electrochemical energy storage devices using natural bast fibres. Chem. Eng. J. 2023, 465, 142845.
- Al-Gharrawi, M.Z.; Wang, J.; Bousfield, D.W. Improving water vapor barrier of cellulose based food packaging using double layer coatings and cellulose nanofibers. Food Packag. Shelf Life 2022, 33, 100895.
- Deerattrakul, V.; Sakulaue, P.; Bunpheng, A.; Kraithong, W.; Pengsawang, A.; Chakthranont, P.; Iamprasertkun, P.; Itthibenchapong, V. Introducing hydrophilic cellulose nanofiber as a bio-separator for “water-in-salt” based energy storage devices. Electrochim. Acta 2023, 453, 142355.
- Yu, M.; Jiang, G.; Demir, M.; Sun, Y.; Wang, R.; Liu, T. PTFE-based composite nanofiber membranes for solar-driven interfacial water evaporation. Mater. Today Commun. 2022, 32, 104019.
- Kandeh, S.H.; Amini, S.; Ebrahimzadeh, H. PVA/Stevia/MIL-88A@AuNPs composite nanofibers as a novel sorbent for simultaneous extraction of eight agricultural pesticides in food and vegetable samples followed by HPLC-UV analysis. Food Chem. 2022, 386, 132734.
- Ravindran, J.; Kumar, P.S.; Saravanan, A.; Lenin, N.; Baskaran, A. Fabrication and characterization of polyvinyl-alcohol-combined bromelain nanofiber and assessment of its antimicrobial potencies. Appl. Nanosci. 2023, 13, 4157–4165.
- Valachová, K.; Švík, K.; Jurčík, R.; Ondruška, Ľ.; Biró, C.; Šoltés, L. Enhanced healing of skin wounds in ischemic rabbits using chitosan/hyaluronan/edaravone composite membranes: Effects of laponite, carbon and silver-plated carbon nanofiber fillers. Chem. Pap. 2023, 77, 1835–1841.
- Bhuyan, C.; Konwar, A.; Bora, P.; Rajguru, P.; Hazarika, S. Cellulose nanofiber-poly(ethylene terephthalate) nanocomposite membrane from waste materials for treatment of petroleum industry wastewater. J. Hazard. Mater. 2023, 442, 129955.
- Khurana, D.; Sadashiva, S.; Dey, B.; Guruprasad, K.P.; Bhat, S.N.; Singh, B.N. Recent advancement in development and modification of nanofibrous matrix for the application in sensing and remediation of water pollutants. Appl. Nanosci. 2023, 13, 6115–6132.
- Xu, X.; Lv, H.; Zhang, M.; Wang, M.; Zhou, Y.; Liu, Y.; Yu, D.G. Recent progress in electrospun nanofibers and their applications in heavy metal wastewater treatment. Front. Chem. Sci. Eng. 2023, 17, 249–275.
- Venkatesan, M.; Chandrasekar, J.; Liang, F.C.; Lin, W.C.; Chen, W.C.; Cho, C.J.; Chen, Y.-T.; Lee, W.-Y.; Su, C.; Zhou, Y.; et al. Surface-enhanced fully nanofiber-based self-cleanable ultraviolet resistive triboelectric energy harvester for wearable smart garments. Nano Energy 2023, 113, 108556.
- Nkede, F.N.; Wardana, A.A.; Phuong, N.T.H.; Takahashi, M.; Koga, A.; Wardak, M.H.; Fanze, M.; Tanaka, F.; Tanaka, F. Preparation and Characterization of Chitosan/Lemongrass Oil/Cellulose Nanofiber Pickering Emulsions Active Packaging and Its Application on Tomato Preservation. J. Polym. Environ. 2023, 31, 4930–4945.
- El-Aassar, M.R.; Ibrahim, O.M.; Al-Oanzi, Z.H. Biotechnological Applications of Polymeric Nanofiber Platforms Loaded with Diverse Bioactive Materials. Polymers 2021, 13, 3734.
- Langwald, S.V.; Ehrmann, A.; Sabantina, L. Measuring Physical Properties of Electrospun Nanofiber Mats for Different Biomedical Applications. Membranes 2023, 13, 488.
- Shang, M.; Ma, B.; Hu, X.; Liu, L.; Wang, J.; Zhang, X. Biomimetic Core-Shell-Structured Nanofiber Membranes for Rapid and Portable Water Purification. ACS Appl. Mater. Interfaces 2022, 14, 44849–44858.
- Agrawal, S.; Ranjan, R.; Lal, B.; Rahman, A.; Singh, S.P.; Selvaratnam, T.; Nawaz, T. Synthesis and Water Treatment Applications of Nanofibers by Electrospinning. Processes 2021, 9, 1779.
- Vojoudi, E.; Babaloo, H. Application of Electrospun Nanofiber as Drug Delivery Systems: A Review. Pharm. Nanotechnol. 2023, 11, 10–24.
- Liu, T. Application of Carbon Nanofiber-Modified Concrete in Industrial Building Design. Int. J. Anal. Chem. 2023, 2023, 2587551.
- Abadi, B.; Goshtasbi, N.; Bolourian, S.; Tahsili, J.; Adeli-Sardou, M.; Forootanfar, H. Electrospun hybrid nanofibers: Fabrication, characterization, and biomedical applications. Front. Bioeng. Biotechnol. 2022, 10, 986975.
- El-Seedi, H.R.; Said, N.S.; Yosri, N.; Hawash, H.B.; El-Sherif, D.M.; Abouzid, M.; Abdel-Daim, M.M.; Yaseen, M.; Omar, H.; Shou, Q.; et al. Gelatin nanofibers: Recent insights in synthesis, bio-medical applications and limitations. Heliyon 2023, 9, e16228.
- Khalid, M.Y.; Arif, Z.; Noroozi, R.; Zolfagharian, A.; Bodaghi, M. 4D printing of shape memory polymer composites: A review on fabrication techniques, applications, and future perspectives. J. Manuf. Process. 2022, 81, 759–797.
- Mallakpour, S.; Radfar, Z.; Hussain, C.M. Current advances on polymer-layered double hydroxides/metal oxides nanocomposites and bionanocomposites: Fabrications and applications in the textile industry and nanofibers. Appl. Clay Sci. 2021, 206, 106054.
- Zheng, H.; Li, X.; Liu, L.; Bai, C.; Liu, B.; Liao, H.; Yan, M.; Liu, F.; Han, P.; Zhang, H.; et al. Preparation of nanofiber core-spun yarn based on cellulose nanowhiskers/quaternary ammonium salts nanocomposites for efficient and durable antibacterial textiles. Compos. Commun. 2022, 36, 101388.
- Tian, G.; Duan, C.; Zhou, B.; Tian, C.; Wang, Q.; Chen, J. Lignin-based electrospun nanofiber membrane decorated with photo-Fenton Ag@MIF-100(Fe) heterojunctions for complex wastewater remediation. Front. Chem. Sci. Eng. 2023, 17, 930–941.
- Fernandes, A.; Cruz-Lopes, L.; Esteves, B.; Evtuguin, D. Nanotechnology Applied to Cellulosic Materials. Materials 2023, 16, 3104.
- Wu, X.; Li, X.; Shi, Z.; Wang, X.; Wang, Z.; Li, C.M. Electrospinning Mo-Doped Carbon Nanofibers as an Anode to Simultaneously Boost Bioelectrocatalysis and Extracellular Electron Transfer in Microbial Fuel Cells. Materials 2023, 16, 2479.
- Bayer, I.S. Controlled Drug Release from Nanoengineered Polysaccharides. Pharmaceutics 2023, 15, 1364.
- Min, T.; Zhou, L.; Sun, X.; Du, H.; Bian, X.; Zhu, Z.; Wen, Y. Enzyme-responsive food packaging system based on pectin-coated poly (lactic acid) nanofiber films for controlled release of thymol. Food Res. Int. 2022, 157, 111256.
- Pokhriyal, M.; Rakesh, P.K.; Rangappa, S.M.; Siengchin, S. Effect of alkali treatment on novel natural fiber extracted from Himalayacalamus falconeri culms for polymer composite applications. Biomass Conv. Biorefin. 2023.
- Maguteeswaran, R.; Prathap, P.; Satheeshkumar, S.; Madhu, S. Effect of alkali treatment on novel natural fiber extracted from the stem of Lankaran acacia for polymer composite applications. Biomass Conv. Biorefin. 2023.
- Joe, M.S.; Sudherson, D.P.S.; Suyambulingam, I.; Siengchin, S.; Rajeshkumar, G. Characterization of novel cellulosic plant fiber reinforced polymeric composite from Ficus benjamina L. stem for lightweight applications. Biomass Conv. Biorefin. 2023, 13, 14267–14280.
- Priyadharshini, G.S.; Velmurugan, T.; Suyambulingam, I.; Sanjay, M.R.; Siengchin, S.; Vishnu, R. Characterization of cellulosic plant fiber extracted from Waltheria indica Linn. stem. Biomass Conv. Biorefin. 2023.
- Parida, P.K.; Pradhan, A.K.; Pandit, M.K. Characterization of Cellulose Fiber Extracted from Stems of Myriostachya wightiana (MW) Plants: A Viable Reinforcement for Polymer Composite. Fibers Polym. 2023, 24, 489–503.
- Bhunia, A.K.; Mondal, D.; Parui, S.M.; Mondal, A.K. Characterization of a new natural novel lignocellulose fiber resource from the stem of Cyperus platystylis R.Br. Sci. Rep. 2023, 13, 9699.
- Shazleen, S.S.; Sabaruddin, F.A.; Ando, Y.; Ariffin, H. Optimization of Cellulose Nanofiber Loading and Processing Conditions during Melt Extrusion of Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) Bionanocomposites. Polymers 2023, 15, 671.
- Ranamalla, S.R.; Porfire, A.S.; Tomuță, I.; Banciu, M. An Overview of the Supramolecular Systems for Gene and Drug Delivery in Tissue Regeneration. Pharmaceutics 2022, 14, 1733.
- Yan, X.; Yao, H.; Luo, J.; Li, Z.; Wei, J. Functionalization of Electrospun Nanofiber for Bone Tissue Engineering. Polymers 2022, 14, 2940.
- Zhu, S.; He, Z.; Ji, L.; Zhang, W.; Tong, Y.; Luo, J.; Zhang, Y.; Li, Y.; Meng, X.; Bi, Q. Advanced Nanofiber-Based Scaffolds for Achilles Tendon Regenerative Engineering. Front. Bioeng. Biotechnol. 2022, 10, 897010.
- Baran, E.T.; Tahmasebifar, A.; Yilmaz, B. Co-axial electrospinning of PLLA shell, collagen core nanofibers for skin tissue engineering. J. Biomater. Appl. 2023, 37, 1645–1666.
- Ju, Y.X.; Song, P.; Wang, P.Y.; Chen, X.X.; Chen, T.; Yao, X.H.; Zhao, W.G.; Zhang, D.Y. Cartilage structure-inspired elastic silk nanofiber network hydrogel for stretchable and high-performance supercapacitors. Int. J. Biol. Macromol. 2023, 242 Pt 2, 124912.
- Dos Santos, A.E.A.; Cotta, T.; Santos, J.P.F.; Camargos, J.S.F.; do Carmo, A.C.C.; Alcântara, E.G.A.; Fleck, C.; Copola, A.G.L.; Nogueira, J.M.; Silva, G.A.B.; et al. Bioactive cellulose acetate nanofiber loaded with annatto support skeletal muscle cell attachment and proliferation. Front. Bioeng. Biotechnol. 2023, 11, 1116917.
- Dorkhani, E.; Noorafkan, Y.; Salehi, Z.; Ghiass, M.A.; Tafti, S.H.A.; Heirani-Tabasi, A.; Tavafoghi, M. Design and fabrication of polyvinylidene fluoride-graphene oxide/gelatine nanofibrous scaffold for cardiac tissue engineering. J. Biomater. Sci. Polym. Ed. 2023, 34, 1195–1216.
- Jin, C.; Chen, D.; Zhu, T.; Chen, S.; Du, J.; Zhang, H.; Dong, W. Poly(ferulic acid)-hybrid nanofibers for reducing thrombosis and restraining intimal hyperplasia in vascular tissue engineering. Biomater. Adv. 2023, 146, 213278.
- Sahrayi, H.; Hosseini, E.; Ramazani Saadatabadi, A.; Atyabi, S.M.; Bakhshandeh, H.; Mohamadali, M.; Aidun, A.; Farasati Far, B. Cold atmospheric plasma modification and electrical conductivity induction in gelatin/polyvinylidene fluoride nanofibers for neural tissue engineering. Artif. Organs. 2022, 46, 1504–1521.
- Choe, J.A.; Uthamaraj, S.; Dragomir-Daescu, D.; Sandhu, G.S.; Tefft, B.J. Magnetic and Biocompatible Polyurethane Nanofiber Biomaterial for Tissue Engineering. Tissue Eng. Part. A. 2023, 29, 413–423.
- Hama, R.; Reinhardt, J.W.; Ulziibayar, A.; Watanabe, T.; Kelly, J.; Shinoka, T. Recent Tissue Engineering Approaches to Mimicking the Extracellular Matrix Structure for Skin Regeneration. Biomimetics 2023, 8, 130.
- Phutane, P.; Telange, D.; Agrawal, S.; Gunde, M.; Kotkar, K.; Pethe, A. Biofunctionalization and Applications of Polymeric Nanofibers in Tissue Engineering and Regenerative Medicine. Polymers 2023, 15, 1202.
- Kanjwal, M.A.; Ghaferi, A.A. Graphene Incorporated Electrospun Nanofiber for Electrochemical Sensing and Biomedical Applications: A Critical Review. Sensors 2022, 22, 8661.
- Huang, C.; Xu, X.; Fu, J.; Yu, D.G.; Liu, Y. Recent Progress in Electrospun Polyacrylonitrile Nanofiber-Based Wound Dressing. Polymers 2022, 14, 3266.
- Tahir, R.; Albargi, H.B.; Ahmad, A.; Qadir, M.B.; Khaliq, Z.; Nazir, A.; Khalid, T.; Batool, M.; Arshad, S.N.; Jalalah, M.; et al. Development of Sustainable Hydrophilic Azadirachta indica Loaded PVA Nanomembranes for Cosmetic Facemask Applications. Membranes 2023, 13, 156.
- Ghajarieh, A.; Habibi, S.; Talebian, A. Biomedical Applications of Nanofibers. Russ. J. Appl. Chem. 2021, 94, 847–872.
- Al-Mayahi, A.M.W. The effect of humic acid (HA) and zinc oxide nanoparticles (ZnO-NPS) on in vitro regeneration of date palm (Phoenix dactylifera L.) cv. Quntar. Plant Cell Tissue Organ. Cult. 2021, 145, 445–456.
- El-Bialy, S.M.; El-Mahrouk, M.E.; Elesawy, T.; Alaa El-Dein Omara, A.E.D.; Elbehiry, F.; El-Ramady, H.; Áron, B.; Prokisch, J.; Brevik, E.C.; Solberg, S.Ø. Biological Nanofertilizers to Enhance Growth Potential of Strawberry Seedlings by Boosting Photosynthetic Pigments, Plant Enzymatic Antioxidants, and Nutritional Status. Plants 2023, 12, 302.
- Nalci, O.B.; Nadaroglu, H.; Pour, A.H.; Gungor, A.A.; Haliloglu, K. Effects of ZnO, CuO and γ-Fe3O4 nanoparticles on mature embryo culture of wheat (Triticum aestivum L.). Plant Cell Tissue Organ. Cult. 2019, 136, 269–277.
- Dağlioğlu, Y.; Açikgöz, M.A.; Özcan, M.M.; Kara, S.M. Impact of Al2O3 NPs on Callus Induction, Pigment Content, Cell Damage and Enzyme Activities in Ocimum basilicum Linn. J. Int. Environ. Appl. Sci. 2022, 17, 22–33.
- Mazaheri-Tirani, M.; Dayani, S. In vitro effect of zinc oxide nanoparticles on Nicotiana tabacum callus compared to ZnO micro particles and zinc sulfate (ZnSO4). Plant Cell Tissue Organ. Cult. 2020, 140, 279–289.
- Yoshihara, S.; Yamamoto, K.; Nakajima, Y.; Takeda, S.; Kurahashi, K.; Tokumoto, H. Absorption of zinc ions dissolved from zinc oxide nanoparticles in the tobacco callus improves plant productivity. Plant Cell Tissue Organ. Cult. 2019, 138, 377–385.
- Hasanin, M.; El-Henawy, A.; Eisa, W.H.; El-Saied, H.; Sameeh, M.J. Nano-amino acid cellulose derivatives: Eco-synthesis, characterization, and antimicrobial properties. Int. J. Biol. Macromol. 2019, 132, 963–969.
- Kim, D.H.; Gopal, J.; Sivanesan, I. Nanomaterials in plant tissue culture: The disclosed and undisclosed. RSC Adv. 2017, 7, 36492–36505.
- Kulus, D.; Tymoszuk, A. Gold nanoparticles affect the cryopreservation efficiency of in vitro-derived shoot tips of bleeding heart. Plant Cell Tissue Organ. Cult. 2021, 146, 297–311.
- Ibrahim, A.S.; Fahmy, A.H.; Ahmed, S.S. Copper nanoparticles elevate regeneration capacity of (Ocimum basilicum L.) plant via somatic embryogenesis. Plant Cell Tissue Organ. Cult. 2019, 136, 41–50.
- Cuong, D.M.; Du, P.C.; Tung, H.T.T.; Ngan, H.T.M.; Luan, V.Q.; Phong, T.H.; Khai, H.D.; Phuong, T.T.B.; Nhut, D.T. Silver nanoparticles as an effective stimulant in micropropagation of Panax vietnamensis—A valuable medicinal plant. Plant Cell Tissue Organ. Cult. 2021, 146, 577–588.
- Venkatachalam, P.; Malar, S.; Thiyagarajan, M. Effect of phycochemical coated silver nanocomplexes as novel growth stimulating compounds for plant regeneration of Alternanthera sessilis L. J. Appl. Phycol. 2017, 29, 1095–1106.
- Ha, N.T.M.; Do, C.M.; Hoang, T.T.; Ngo, N.D.; Bui, L.V.; Nhut, D.T. The effect of cobalt and silver nanoparticles on overcoming leaf abscission and enhanced growth of rose (Rosa hybrida L. ‘Baby Love’) plantlets cultured in vitro. Plant Cell Tissue Organ. Cult. 2020, 141, 393–405.
- Jadczak, P.; Kulpa, D.; Bihun, M.; Przewodowski, W. Positive effect of AgNPs and AuNPs in in vitro cultures of Lavandula angustifolia Mill. Plant Cell Tissue Organ. Cult. 2019, 139, 191–197.
- Abbas, H.K.; Abdulhussein, M.A.A. Improving Shoot Multiplication of Strawberry (Fragaria ananassa L. Cv. Roby Gem) In Vitro By Using AgNPs and Iron Nanoparticles. Nat. Volatiles Essent. Oils 2021, 8, 2521–2530.
- Dehkourdi, E.H.; Mosavi, M. Effect of Anatase Nanoparticles (TiO2) on Parsley Seed Germination (Petroselinum crispum) In Vitro. Biol. Trace Elem. Res. 2013, 155, 283–286.
- Hasanin, M.; Hashem, ·A.H.; Lashin, I.; Hassan, S.A.M. In Vitro improvement and rooting of banana plantlets using antifungal nanocomposite based on myco-synthesized copper oxide nanoparticles and starch. Biomass Convers. Biorefin. 2023, 13, 8865–8875.
- Elakkiya, T.V.; Meenakshi, R.V.; Kumar, S.P.; Karthik, V.; Shankar, R.K.; Sureshkumar, P.; Hanan, A. Green synthesis of copper nanoparticles using Sesbania aculeata to enhance the plant growth and antimicrobial activities. Int. J. Environ. Sci. Technol. 2022, 19, 1313–1322.
- Mozafari, A.; Havas, F.; Ghaderi, N. Application of iron nanoparticles and salicylic acid in in vitro culture of strawberries (Fragaria × ananassa Duch.) to cope with drought stress. Plant Cell Tiss. Organ. Cult. 2018, 132, 511–523.
- Ghanti, F.; Somayeh, B. Effect of methyl jasmonate and silver nanoparticles on production of secondary metabolites by Calendula officinalis L. (Asteraceae). Trop. J. Pharm. Res. 2014, 13, 1783–1789.
- Javed, R.; Mohamed, A.; Yücesan, B.; Gürel, E.; Kausar, R.; Zia, M. CuO nanoparticles significantly influence in vitro culture, steviol glycosides, and antioxidant activities of Stevia rebaudiana Bertoni. Plant Cell Tissue Organ. Cult. 2017, 131, 611–620.
- Chung, M.; Rekha, K.; Rajakumar, G.; Thiruvengadam, M. Production of bioactive compounds and gene expression alterations in hairy root cultures of Chinese cabbage elicited by copper oxide nanoparticles. Plant Cell Tissue Organ. Cult. 2018, 134, 95–106.
- Khan, A.K.; Kousar, S.; Tungmunnithum, D.; Hano, C.; Abbasi, B.H.; Anjum, S. Nano-Elicitation as an Effective and Emerging Strategy for In Vitro Production of Industrially Important Flavonoids. Appl. Sci. 2021, 11, 1694.
- Iqbal, Z.; Javad, S.; Naz, S.; Shah, A.A.; Shah, A.N.; Paray, B.A.; Gulnaz, A.; Abdelsalam, N.R. Elicitation of the in vitro Cultures of Selected Varieties of Vigna radiata L. With Zinc Oxide and Copper Oxide Nanoparticles for Enhanced Phytochemicals Production. Front. Plant Sci. 2022, 13, 908532.
- Rahmawati, M.; Mahfud, C.; Risuleo, G.; Jadid, N. Nanotechnology in Plant Metabolite Improvement and in Animal Welfare. Appl. Sci. 2022, 12, 838.
- Tymoszuk, A.; Kulus, D. Silver nanoparticles induce genetic, biochemical, and phenotype variation in chrysanthemum. Plant Cell Tissue Organ. Cult. 2020, 143, 331–344.
- Niazian, M.; Nalousi, A.M.; Azadi, P.; Ma’mani, L.; Chandler, S.F. Perspectives on new opportunities for nano-enabled strategies for gene delivery to plants using nanoporous materials. Planta 2021, 254, 83.
- Sarmast, M.K.; Salehi, H. Silver Nanoparticles: An Influential Element in Plant Nanobiotechnology. Mol. Biotechnol. 2016, 58, 441–449.
- Sreelekshmi, R.; Siril, E.A.; Muthukrishnan, S. Role of Biogenic Silver Nanoparticles on Hyperhydricity Reversion in Dianthus chinensis L. an In Vitro Model Culture. J. Plant Growth Regul. 2021, 41, 23–39.
- Mozafari, A.; Dedejani, S.; Ghaderi, N. Positive responses of strawberry (Fragaria × ananassa Duch.) explants to salicylic and iron nanoparticle application under salinity conditions. Plant Cell Tiss. Organ. Cult. 2018, 134, 267–275.
- Asl, A.G.; Mozafari, A.; Ghaderi, N. Iron nanoparticles and potassium silicate interaction effect on salt-stressed grape cuttings under in vitro conditions: A morphophysiological and biochemical evaluation. Cell Dev. Biol—Plant 2019, 55, 510–518.
- Ramirez-Mosqueda, M.A.; Sanchez-Segura, L.; Hernandez-Valladolid, S.L.; Bello-Bello, E.; Bello-Bello, J.J. Influence of silver nanoparticles on a common contaminant isolated during the establishment of Stevia rebaudiana Bertoni culture. Plant Cell Tissue Organ. Cult. 2020, 143, 609–618.
- Timoteo, C.O.; Paiva, R.; dos Reis, M.V.; Claro, P.I.C.; da Silva, D.P.C.; Marconcini, J.M.; de Oliveira, J.E. Silver nanoparticles in the micropropagation of Campomanesia rufa (O. Berg) Nied. Plant Cell Tissue Organ. Cult. 2019, 137, 359–368.
- Tung, H.T.; Thuong, T.T.; Cuong, D.M.; Luan, V.Q.; Hien, V.T.; Hieu, T.; Nam, N.B.; Phuong, H.T.N.; Vinh, B.V.T.; Khai, H.D.; et al. Silver nanoparticles improved explant disinfection, in vitro growth, runner formation and limited ethylene accumulation during micropropagation of strawberry (Fragaria × ananassa). Plant Cell Tissue Organ. Cult. 2021, 145, 393–403.
- Safavi, K. Effect of Titanium Dioxide Nanoparticles in Plant Tissue Culture Media for Enhance Resistance to Bacterial Activity. Bull. Env. Pharmacol. Life Sci. 2014, 3, 163–166.
More
