Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Sonu M. M. Bhaskar and Version 2 by Catherine Yang.
Atrial fibrillation (AF) is the most prevalent cardiac arrhythmia worldwide and is a major cause of morbidity and mortality. It has a global prevalence of approximately 0.51%, increasing to 10–17% in those over age 80 [4]. AF is characterised by ectopic depolarisations, which lead to asynchronous atrial contractions and irregular ventricular activity
- stroke
- atrial fibrillation
- reperfusion therapy
- thrombectomy
- thrombolysis
1. Diagnosis of Atrial Fibrillation
Considering the association between AF and AIS [1][18], appropriate diagnosis of AF and subsequent treatment is vital to prevent stroke [2][19]. The diagnosis of AF in the setting of AIS is usually based on electrocardiographic (ECG) findings. However, AF may be paroxysmal and not detectable on a single ECG [3][20]. Therefore, prolonged ECG monitoring, such as telemetry or Holter monitoring, may be required to establish the diagnosis of AF [4][21]. Transthoracic or transoesophageal echocardiography may be useful in identifying underlying structural heart disease, such as left atrial enlargement or valvular abnormalities [5][22]. Unfortunately, there are limited organised screening procedures employed across the general population [6][7][23,24]. In patients with suspected AF, a range of ambulatory cardiac monitoring devices may be utilised [8][25] (Figure 12). In-hospital and post-discharge cardiac monitoring is also commonly undertaken following stroke, as detecting AF can help determine the cause of stroke [9][26] and guide treatment to prevent stroke recurrence [10][27]. Following AIS, the identification of AF plays a crucial role in enhancing secondary prevention strategies. A study by Boriani et al. compared AF detection and oral anticoagulant (OAC) initiation in ischemic stroke patients using insertable cardiac monitors (ICMs) versus external cardiac monitors (ECMs) [11][28]. Over 24 months, 33.9% of ICM-monitored patients were diagnosed with new AF compared to 13.3% with ECMs. Consequently, more ICM patients received OAC prescriptions (35.9% vs. 16.8% for ECM patients). Put simply, ICM-monitored ischemic stroke patients were nearly three times more likely to be diagnosed with AF and prescribed OACs. However, this heightened detection and intervention did not translate to a reduction in stroke risk or mortality. Consistent with prior studies [12][13][14][29,30,31], this study highlighted that the use of ICMs may improve AF detection rates and OAC prescription among AIS patients. Nevertheless, it also brings to attention the clinical implications of improved AF detection in an older, medically complex population. Whilst the improvements in mortality or stroke risk were not observed in this study, previous studies have revealed that improved AF detection may lead to better treatment and, hence, reduction in risk of stroke or death [10][14][27,31]. A meta-analysis by Tsivgoulis et al. [15][32] revealed that ICM use, as compared to conventional monitoring in cryptogenic stroke, was associated with improvements in AF detection, increased initiation of OAC, and subsequent lower risks of recurrent stroke. Continuous monitoring for AF proves especially clinically significant in cases where AF occurrences are infrequent, paroxysmal, and asymptomatic [16][17][33,34]. This is particularly relevant for patients with cryptogenic stroke, as detecting AF and administering OACs can have a notable impact due to the influence of silent brain infarcts on cognitive function in AF patients [18][35]. Alongside the diagnostic challenges of AF detection, other factors may also play a role, including peri-hospital systems and workflows [19][20][36,37].
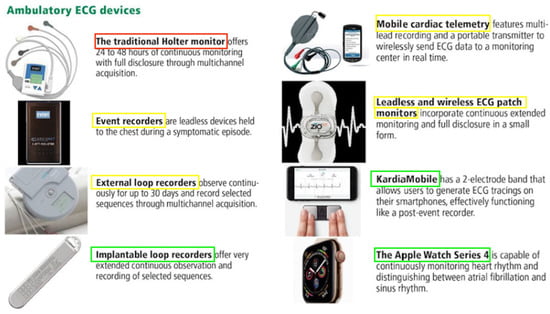
Figure 12. Devices used for ambulatory electrocardiogram monitoring. Red = devices utilised for early secondary stroke prevention via arrhythmia detection, yellow = devices which may be used for secondary stroke prevention in the first 30 days, and green = devices that specialise in long-term secondary stroke prevention over 30 days. Adapted from Sanders et al. Cleveland Clinical Journal of Medicine (2019) [21][38]. Abbreviations: ECG = electrocardiogram.
In addition to the inpatient ECGs that are routinely conducted for patients admitted with palpitations, syncope, or following a stroke, there are multiple devices that may be used to detect or monitor arrhythmias in a real-world scenario [21][38]. Figure 12 depicts some of the common devices that are used and outlines their basic mechanisms. Furthermore, continuous rhythm monitoring allows for a better understanding of various AF patterns over time, shedding light on the progressive remodelling of the atrial substrate or the deterioration of underlying diseases. The Fitbit Heart Study, encompassing 455,699 participants, found that wearable devices leveraging optical photoplethysmography (PPG) sensors and software algorithms demonstrated a high positive predictive value for detecting AF concurrently and in identifying individuals likely to have AF during subsequent ECG patch monitoring [22][39]. This suggests that wearable devices have the potential to assist in identifying individuals with undiagnosed AF. However, there are uncertainties in the literature regarding the optimal approach to AF detection, and these are exacerbated by rapidly developing technologies [23][24][40,41] and a lack of standardised protocols between different centres [7][24]. It is thus essential for clinicians to be aware of the current literature that compares the timescales and detection rates of these monitoring devices (Table 1).
Table 1.
Studies comparing the detection rates of atrial fibrillation between different cardiac monitoring techniques.
| Study | Number of Patients (n) | Study Design | Technique(s) and Timescale | Outcomes (Primarily Regarding AF Detection Rate) |
|---|---|---|---|---|
| Buck et al. [25][42] | 300 | RCT | 12-month ILR vs. 30-day ELR | In ischemic stroke patients monitored for 12 months using an ILR, AF was detected in 15.3% of patients compared to 4.7% of patients monitored with a 30-day ELR (RR 3.29 [95% CI 1.45–7.42], p = 0.03). |
| Koh et al. [26][43] | 203 | RCT | 30 days of KardiaMobile ECG (used for 30 s 3 times per day) vs. one additional round of 24-h Holter monitoring | In patients with a recent cryptogenic stroke or TIA, AF of duration ≥ 30 s was detected in 9.5% of patients in the KardiaMobile group compared to 2.0% in those receiving 24 h Holter monitoring (p = 0.024). |
| Liu et al. [4][21] | 158 | Prospective | 14-day ECG patch vs. 24-h Holter monitoring | AF and/or atrial flutter was detected in 9.5% of patients wearing the 14-day ECG patch compared to 3.8% in patients with 24 h Holter monitoring (p = 0.042). |
| Medic et al. [27][44] | 1000 | Retrospective (economic model) | 30-day MCOT followed by ILR vs. ILR alone over 12 months | In patients with cryptogenic stroke, 30-day MCOT followed by an ILR had an AF detection rate of 20.9% compared to a detection rate of 4.5% when using an ILR alone. Cost-effectiveness analysis revealed 7.72 times lower costs per AF patient detected when using MCOT initially (USD 29,598 per patient with detected AF) compared to when using an ILR only (USD 228,507 per patient with detected AF). |
| Chua et al. [3][20] | 32 | Prospective | 14-day ECG patch vs. 24-h Holter monitoring | Paroxysmal AF and/or atrial flutter was detected in 19% of patients wearing the ECG patch compared to 3% in patients with 24 h Holter monitoring (p < 0.05). |
| Perez et al. [28][45] | 419,297 | Prospective | 8 months of monitoring with an Apple Watch | Of Apple Watch wearers among the general population, 0.52% received a notification regarding an irregular pulse over 8 months. Of the patients who were notified to have an irregular pulse and subsequently wore and returned an ECG patch, AF was confirmed in 34%. Comparatively, in those who did not receive a notification regarding an irregular pulse, a diagnosis of AF was established in 1.0% of patients. |
| Derkac et al. [29][46] | 78,490 | Retrospective | MCOT vs. ILR analysed over 8 months | AF was diagnosed in 23.5% of patients with MCOT compared to 11.3% of patients with an ILR. It should be noted that the median prescription time for the MCOT group was 20 days compared to 30 days in those with the ILR group, despite the latter device having the potential to be used for a considerably longer duration. |
| Gladstone et al. [30][47] | 572 | RCT | 30 days of event-triggered loop recorder vs. 24 h ECG | AF was detected in 16.1% of patients with the event-triggered loop recorder compared to 3.2% of patients with 24 h ECG monitoring (p < 0.001). Episodes of AF spanning 150 s or longer were recorded in 9.9% of patients with event-triggered recorders compared to 2.5% of those with standard 24 h ECG monitoring (p < 0.001). These differences had clinical implications as 18.6% of patients in the loop recorder group had commenced anticoagulant therapy compared to 11.1% of those in the 24 h ECG monitoring group (p = 0.01). |
| Sanna et al. [31][48] | 441 | RCT | 6 months of ILR vs. conventional follow-up (ECG assessment at follow-up visits, with exact protocol at the discretion of each site) | AF was detected in 8.9% of patients with an ILR by 6 months compared to 1.4% in patients receiving conventional follow-up following a cryptogenic stroke (HR 6.4 [95% CI 1.9 to 21.7], p < 0.001). By 12 months, AF was detected in 12.4% of patients with an ILR compared to 2.0% in those with conventional follow-up (HR 7.3 [95% CI 2.6 to 20.8], p < 0.001). In the patients followed up for 36 months, these rates grew to 30% and 3.0% respectively (HR 8.8 [95% CI 3.5 to 22.2], p < 0.001). |
Abbreviations: AF = atrial fibrillation, HR = hazard ratio, RR = relative risk, CI = confidence interval, ECG = electrocardiogram, ILR = implantable loop recorder, ELR = external loop recorder, MCOT = mobile cardiac outpatient telemetry, RCT = randomised controlled trial, USD = United States dollar, TIA = transient ischemic attack.
2. Treatment of Atrial Fibrillation
The management of AF in the setting of AIS should be guided by the underlying aetiology of the stroke, the severity of the stroke, and the risk of recurrent stroke. The primary goals of management are to prevent recurrent stroke [20][37] and to maintain cardiac hemodynamic stability [4][21].
Anticoagulation: Anticoagulation is the cornerstone of stroke prevention in patients with AF. The decision to initiate anticoagulation should be based on the risk of stroke and bleeding [32][49]. The CHA2DS2-VASc score is commonly used to assess stroke risk in patients with AF [8][25]. For patients with a CHA2DS2-VASc score of 2 or more, anticoagulation with a vitamin K antagonist (VKA) or a direct-acting oral anticoagulant (DOAC) is recommended [8][25]. VKAs such as Warfarin are indicated in patients with valvular AF, which refers to those with moderate-to- severe mitral stenosis or mechanical heart valves [32][49]. However, most AF patients are recommended DOACs such as dabigatran, apixaban, rivaroxaban, and edoxaban. The shift in guidelines of recommending DOACs in most AF patients was prompted by seminal clinical trials comparing warfarin with the four aforementioned DOACs, respectively [33][34][35][36][50,51,52,53]. A recent real-world study from Japan demonstrated that DOACs exhibit notably superior safety and effectiveness profiles when compared to warfarin in elderly patients diagnosed with nonvalvular AF [37][54]. The current literature reinforces this shift in anticoagulant choice, with recent meta-analyses demonstrating superior efficacy and safety with DOACs (Table 2). In cases of ischemic stroke with AF, anticoagulation therapy is preferred over antiplatelet therapy for thromboprophylaxis [38][55]. However, for ischemic strokes unrelated to AF, anticoagulant therapy does not show incremental benefits and may carry higher risks compared to antiplatelet therapy [39][56]. The main concern with antithrombotic therapy is the risk of bleeding, especially when combining antiplatelets and anticoagulants, particularly in patients with bleeding history or other risk factors. The question arises about combining these therapies for ischemic stroke patients with AF and large-artery atherosclerosis. A study by Kim et al. [40][57] in South Korea found no additional benefit and a greater risk associated with combining antithrombotic and antiplatelet therapy in such patients, indicating OAC monotherapy as an optimal antithrombotic regimen in preventing recurrent stroke in ischemic stroke due to AF and large-vessel atherosclerosis. However, the study had limitations, including the inclusion of individuals treated with VKAs, known for higher bleeding risk compared to direct oral anticoagulants. Another observational study examined the impact of antithrombotic medications in patients with both AF and cerebral microbleeds [41][58]. It found that combining antiplatelets and anticoagulants can be detrimental in patients with cerebral microbleeds and AF, confirming previous research that such combination therapy does not provide added protection against ischemic stroke in AF patients. Additional research is necessary to optimise stroke prevention strategies for at-risk populations such as those with AF and cerebral microbleeds. Future trials are needed to explore novel combinations of antiplatelets with DOACs or other drug classes (e.g., factor XII antagonists) to determine their effectiveness and safety.
Table 2.
Meta-analyses comparing outcomes in atrial fibrillation patients prescribed different types of antithrombotic medications.
| Study | Number of Patients (n) | Study Design | Treatment(s) | Outcomes (Primarily Regarding the Incidence of Stroke) | Haemorrhagic Adverse Events |
|---|---|---|---|---|---|
| Carnicelli et al. [42][59] | 71,683 | Meta-analysis | Standard-dose DOACs vs. lower-dose DOACs vs. warfarin | Relative to warfarin, standard-dose DOACs were linked to significant decreases in the risk of stroke or systemic embolism (HR 0.81 [95% CI 0.74–0.89]) and mortality (HR 0.92 [95% CI 0.87–0.97]). When compared to warfarin, lower-dose DOACs were not associated with a significant difference in the risk of stroke or systemic embolism (HR 1.06 [95% CI 0.95–1.19]). However, there was a significant decrease in mortality (HR 0.90, [95% CI 0.83–0.97]. |
Relative to warfarin, standard-dose DOACs were linked to a significant decrease in the risk of intracranial bleeding (HR 0.45 [95% CI 0.37–0.56]) but not in the risk of major bleeding (HR 0.86, 95% CI [0.74–1.01]). On the other hand, when compared to warfarin, lower dose DOACs were associated with a lower risk of both intracranial bleeding (HR 0.28 [95% CI 0.21–0.37]) and major bleeding (HR 0.63 [95% CI 0.45–0.88]). |
| Erdem et al. [43][60] | 73,122 | Meta-analysis | DOACs vs. warfarin | Compared to warfarin, there was a decreased risk of stroke or systemic embolism when taking DOACs in patients ≥ 75 years old (RR 0.57 [95% CI 0.42–0.76]) and patients < 75 years old (RR 0.74, 95% CI [0.43, 1.27]). This was statistically significant for ages ≥75 years but not ages <75 years. | Compared to warfarin, there was a significantly lower risk of major bleeding in patients taking DOACs who were ≥75 years old (RR 0.74 [95% CI 0.63–0.87]) as well as in those <75 years old (RR 0.64 [95% CI 0.44–0.93]). |
| Zeng et al. [44][61] | 835,520 | Meta-analysis | DOACs vs. warfarin | Relative to warfarin, DOACs were associated with a significantly lower risk of ischemic stroke (HR 0.79 [95% CI 0.71–0.87]) and mortality (HR 0.90 [95% CI 0.84–0.96]). | Relative to warfarin, DOACs were associated with a significantly lower risk of intracranial bleeding (HR 0.58 [95% CI 0.52–0.65]) and major bleeding (HR 0.79 [95% CI 0.64–0.97]) but no significant decrease in the risk of gastrointestinal bleeding (HR 0.97 [95% CI 0.73–1.29]). |
| Tereshchenko et al. [45][62] | 96,017 | Meta-analysis | Aspirin vs. VKAs vs. DOACs vs. placebo | After adjusting for other variables, treatment with VKAs and DOACs led to significantly lower rates of stroke or systemic embolism compared to placebo. However, the odds were not significantly lower for patients taking aspirin compared to placebo (aOR 0.77 [95% CI 0.53–1.11]). | After adjusting for other variables, there was no significant difference in the rates of major bleeding between treatments with aspirin, VKAs, and DOACs. |
Abbreviations: AF = atrial fibrillation, DOAC = direct-acting oral anticoagulant, RR = relative risk, CI = confidence interval, HR = hazard ratio, OR = odds ratio, aOR = adjusted odds ratio, VKA = vitamin K-antagonists.
However, continuing anticoagulation in the acute setting of ischemic stroke is controversial, as it may increase the risk of haemorrhagic transformation (HT) [46][63]. The American Heart Association/American Stroke Association (AHA/ASA) recommends withholding anticoagulation for at least 24 h after IVT or EVT in patients with AF-related AIS, with early initiation of anticoagulation reserved for high-risk patients [47][13]. However, recent randomised controlled trials (RCTs) suggest that early initiation of DOACs may be safe and effective in selected patients with AF-related AIS [48][49][64,65].
AF known before an ischemic stroke (KAF) is postulated to be an independent category with a higher recurrence risk compared to AF detected after stroke (AFDAS) [50][51][52][53][54][55][66,67,68,69,70,71]. It raises the possibility that the difference in risk may be influenced by pre-existing anticoagulation, which is more common in KAF and signifies a heightened risk of ischemic stroke recurrence [56][72]. A recent study by Lyrer et al. [57][73] challenged the idea that KAF and AFDAS are distinct prognostic entities and suggest that, rather than solely attributing a high stroke recurrence risk to KAF, future research should concentrate on understanding the causes of stroke in patients despite anticoagulation treatment to develop more effective preventive measures.
Alongside stroke prevention and the management of AF risk factors, there are various methods of treating AF directly (Figure 23). These treatments are broadly categorised into the aims of restoring sinus rhythm (rhythm control) or controlling the heart rate (rate control) [58][74]. Despite the wide range of options, a large portion of AF patients remains undertreated [59][75]. Both undertreatment and underdiagnosis are particularly common in developing nations [60][76]. These regions are further plagued by a lack of local studies investigating the epidemiology and clinical outcomes in AF patients experiencing AIS.
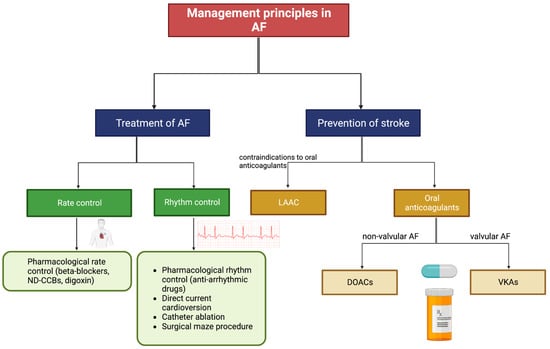
Figure 23. Patient management options for AF. Abbreviations: AF = atrial fibrillation, ND-CCBs = non-dihydropyridine calcium channel blockers, LAAC = left atrial appendage closure, DOACs = direct-acting oral anticoagulants, VKAs = vitamin K antagonists. Adapted from Li et al. (2020) Cardiovascular Journal of Africa [58][74].
AF is linked to cognitive decline and various forms of dementia, often attributed to brain injuries resulting from macro- and microembolic events [61][62][77,78]. Emerging evidence supports the strategic utilisation of anticoagulation in AF patients, including the timing and appropriate administration, to specifically reduce the likelihood of dementia [63][64][65][79,80,81]. In a large observational cohort study involving over 142,000 patients aged 50 and older with nonvalvular atrial fibrillation (AF), the use of oral anticoagulants was linked to a decreased risk of dementia, especially in patients aged 75 or older (Hazard Ratio, 0.84) [66][82]. These findings emphasize the benefits of anticoagulation in reducing dementia risk, even in the absence of a significant prior stroke. This study reinforces the importance of early AF diagnosis, ongoing anticoagulant use, and its potential role in lowering the risks of stroke, cognitive decline, and dementia.
Managing cardiovascular, metabolic, and lifestyle risk factors, as well as upstream therapies that indirectly target the mechanisms of AF, aids in the prevention of AF [58][74]. When AF is diagnosed, there are various options to achieve rhythm control (restoring sinus rhythm) and/or rate control (slowing the heart rate). Pharmacological rhythm control is useful in preventing AF progression and harmful remodelling, whilst rate control reduces hospitalisation but is less effective at resolving the arrhythmia itself [67][83]. Catheter ablation is a common procedure used to treat AF and is reported to have a higher success rate than pharmacological therapy [68][69][70][71][84,85,86,87]. The invasive nature of ablation carries risks, but the overall rate of adverse events is comparable to that of pharmacological treatments [69][70][85,86]. AF patients are commonly prescribed oral anticoagulants, which prevent stroke by restraining thrombosis [42][59]. Patients who have contraindications to oral anticoagulants may undergo LAAC, which prevents stroke by blocking thrombi from exiting the left atrial appendage [72][88].
Early vs. later anticoagulation: When to initiate anticoagulation for long-term secondary stroke prevention, especially in individuals with AF, is a critical question [73][89]. It revolves around determining the point at which the risk of haemorrhagic complications resulting from anticoagulation is balanced by the positive impact it has in preventing recurrent strokes. Hospital-based cohort studies have indicated the feasibility of early DOAC initiation within 1, 2, 3, or 4 days according to stroke severity in reducing the risk of recurrent stroke or systemic embolism without an increase in major bleeding [74][90]. In a recent international open-label trial involving 2013 patients with AF who had experienced an AIS, the introduction of DOACs within 2 days after a minor/moderate stroke and 6–7 days after a major stroke, as determined by imaging assessments, resulted in a similar risk for a combined safety and efficacy outcome at both 30 days (Odds ratio [OR], 0.57) and 90 days (OR, 0.60) [75][91]. These findings indicate that the early administration of systemic anticoagulation with a DOAC following an AIS is safe when guided by severity classification based on imaging.
Rhythm control: To control the irregular rhythm in AF, treatment strategies such as antiarrhythmic drugs and cardioversion may be employed [71][76][87,92]. A meta-analysis of 447,202 AF patients demonstrated that early rhythm control was associated with significant reductions in the risk of stroke or systemic embolism [77][93]. Compared to patients receiving only pharmacological treatments, those treated with catheter ablation have reported lower rates of stroke and mortality [71][87]. Recent studies suggest that early rhythm control is also safe and effective in selected patients with AF-related AIS [78][79][80][94,95,96].
Rate control: Treatments to slow the heart rate in AF patients, such as beta-blockers, non-dihydropyridine calcium channel blockers, and digoxin, provide symptom relief whilst reducing the risk of thromboembolic complications [81][97]. Despite their inability to restore sinus rhythm, a meta-analysis revealed no significant differences in the odds of stroke and all-cause mortality in patients receiving medications to achieve rate control compared to pharmacological rhythm control [82][98].
