With the rapid development of stem cell research in modern times, stem cell-based therapy has opened a new era of tissue regeneration, becoming one of the most promising strategies for currently untreatable retinal diseases. Among the various sources of stem cells, adipose tissue-derived mesenchymal stem cells (ADSCs) have emerged as a promising therapeutic modality due to their characteristics and multiple functions, which include immunoregulation, anti-apoptosis of neurons, cytokine and growth factor secretion, and antioxidative activities. ADSCs can facilitate the replacement of dying cells, promote tissue remodeling and regeneration, and support the survival and growth of retinal cells.
- adipose tissue-derived mesenchymal stem cell
- cell therapy
- regenerative
- retinal diseases
1. Introduction
2. Stem Cells in Ophthalmology
Stem cells (SCs) are unspecialized cells of the human body that are present in the embryonic, fetal, and adult stages of life, and they play a crucial role in embryonic development, tissue repair, and regeneration. This is achieved thanks to their ability to both replicate and proliferate extensively (self-renewal), thereby maintaining a constant pool of undifferentiated cells and differentiating into multiple specialized cell types (potency), forming various tissues and organs [3][4]. SCs can be classified into totipotent, pluripotent, and multipotent. Totipotent SCs (zygote) have the highest differentiation potential and are able to divide and differentiate into any cell of the organism: totipotent SCs are responsible for forming the embryo and all extra-embryonic structures. Pluripotent SCs (PSCs) derive from the totipotent SCs and form cells of all three germ layers but not extraembryonic structures. Examples of PSCs are represented by embryonic stem cells (ESCs), derived from the inner cell mass of preimplantation embryos, and induced pluripotent stem cells (iPSCs), which arise from the epiblast layer of implanted embryos. Multipotent stem cells have a narrower spectrum of differentiation than PSCs and generate distinct and specialized cells of one specific germ layer. Examples of multipotent SCs are mesenchymal stem cells (MSCs) and hematopoietic stem cells. Finally, oligopotent SCs, like myeloid stem cells, can differentiate into several cell types, while unipotent SCs have the narrowest differentiation capabilities and the special ability to divide repeatedly but are only able to form one cell type [4][5][6][5,6,7]. In ophthalmology, SC research is rapidly advancing and has shown great promise in treating a variety of eye conditions. The human eye combines tissues from neuroectodermal (e.g., retina), ectodermal (e.g., lens and cornea), and mesodermal lines and is an optimal target for stem cell transplantation therapies due to the high number of people affected by eye disease and the relative ease of accessibility to ocular tissues [6][7]. The use of SCs has been advocated to restore vision in patients with many eye diseases, and SCs have not gone unnoticed in the context of retinal diseases, with interest in several pathologies, including acquired retinal diseases (e.g., age-related macular degeneration (AMD)) and inherited retinal pathologies [7][8][9,10]. Researchers are exploring the use of ESCs, iPSCs, and adult stem cells to generate RPE cells or photoreceptor cells for transplantation. In particular, iPSCs represent a unique in vitro model, allowing for the generation of retinal progenitor cells [3][4], and human pluripotent stem cells (hPSCs) have shown potential in the replacement of the retinal pigment epithelial cell (RPE) and photoreceptors in the context of AMD. Adult SCs have also been evaluated in various clinical trials in the field of ophthalmology. Among them, neural stem cells (NSCs), bone marrow stem cells (BMSCs), and mesenchymal stem cells (MSCs) have been assessed. NSCs can be isolated from the developing or mature mammalian central nervous system (CNS) and have been shown to secrete trophic factors with the potential for photoreceptor neuroprotection [9][11]. BMSCs may be useful to prevent graft vs. host disease in corneal transplantation and have also been studied in retinal diseases, showing a paracrine trophic effect on the degenerating ischemic retina [10][12]. Finally, MSCs are the most commonly studied multipotent SCs in ophthalmopathies and have been found in various fetal tissues, in extraembryonic tissues, like the placenta, the umbilical cord, and the amniotic fluid, and in adult tissues (bone marrow, peripheral blood, adipose tissue, dermis, synovium, periosteum, cartilage, skeletal muscle, fallopian tube, menstrual blood, gingiva, dental tissue, and eye). Umbilical cord MSCs have shown anti-inflammatory and immune-privilege properties, and they can differentiate into corneal epithelial, stromal, and endothelial cells [11][13], but it is placental and adipose-derived MSCs that have been evaluated most in clinical trials. In particular, human adipose-derived stem cells (hADSCs) resemble BMSCs in terms of morphology, proliferation, and multipotency and have been shown to have neuroprotective and neurogenerative properties in the treatment of retinal degeneration [12][14] and corneal disease [13][15]. Dental pulp stem cells (DPSCs) are another category of SCs that have been studied for corneal repair [14][16] and for the treatment of glaucoma and retinal pathologies [15][17]. An interesting type of adult SC capable of producing both CNS and mesoderm-associated lineages is represented by RPE stem cells (RPESCs), which proliferate pathologically in specific conditions and result in retinal disease [16][18]. In the field of corneal regeneration, SCs derived from various sources, such as limbal stem cells (LSCs) or iPSCs, can differentiate into corneal epithelial cells, and can be used to regenerate and repair damaged corneal tissue and help restore vision [17][19]. In terms of optic nerve regeneration, research is underway to develop methods to differentiate SCs into retinal ganglion cells (RGCs), which make up the optic nerve, and integrate them into the existing neural circuitry [18][22]. iPSCs/ESCs represent the first source studied for optical nerve regeneration: hESCs have shown the ability to propagate indefinitely and to differentiate into many different types of cell lineages, including retinal ganglion cells (RGCs) [3][4].3. Adipose-Derived Mesenchymal Stem Cells (ADSCs)
Adipose tissue is present in all mammalians and some non-mammalian species, where it mainly serves as an energy reservoir in lipidic form, exerting this endocrine function throughout the body, in subcutaneous tissues, as visceral fat in the intraperitoneal compartment, and padding many vital structures. Like the dermis, bone, cartilage, and blood vessels, fat tissue develops from the embryonic mesoderm. Mature adipocytes are produced by a process known as adipogenesis, where precursor cells called preadipocytes proliferate and differentiate under the control of complex hormonal, neuronal, and paracrine pathways. MSCs studied for regenerative applications derive from white adipose tissue and can be easily collected via liposuction. This minimally invasive procedure can provide a high number of multipotent SCs, which makes it particularly advantageous when compared to scavenging from other sources, such as bone marrow and dental pulp [19][20][24,25]. In particular, ADSCs are multipotent cells naturally residing in the adipose stroma that can be isolated in large quantities through enzymatic digestion and centrifugation of the lipoaspirate material, and they can be subsequently separated and cultured. ADSCs present fibroblastic morphology and, differently from other lipoaspirate cells, bear the potential to differentiate in all three mesodermal lineages (osteogenic, adipogenic, and chondrogenic). ADSCs have also been found to bear anti-inflammatory effects by secreting immunomodulatory cytokines and growth factors [21][27] and to present migratory and homing abilities targeting their action in tissue remodeling and regeneration [22][23][24][28,29,30]. Interestingly, ADSCs express low levels of oxygen reactive species (ROS) and high levels of glutathione, conferring in them significant resistance to oxidative stress. This represents a considerable advantage for potential applications of ADSCs in the treatment of various forms of retinal degeneration where oxidation plays a relevant role [25][31] (Figure 1).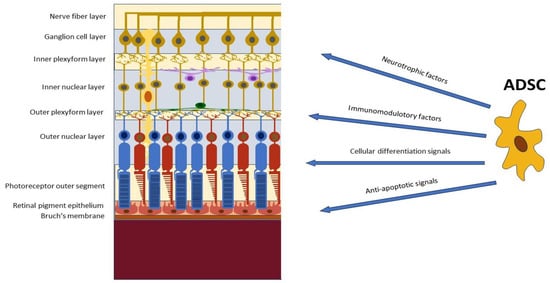
45. Adipose-Derived Stem Cells (ADSCs) in Retinal Diseases
4.1. Modulation of Retinal Inflammation and Immune Responses
5.1. Modulation of Retinal Inflammation and Immune Responses
Retinal diseases often involve chronic inflammation and immune dysregulation, leading to progressive tissue damage. The immunomodulatory properties of ADSCs may attenuate inflammation and modulate immune responses [38][39][40][41][50,51,52,53]. Several research groups have demonstrated that ADSCs regulate the immune system through two primary mechanisms, directly via cell-to-cell communication and indirectly through the secretion of soluble mediators, growth factors, and extravascular vesicles [42][43][44][54,55,56]. In particular, ADSCs have been shown to exert immunomodulatory effects both in vitro and in vivo [45][46][47][57,58,59] by releasing various immunomodulatory proteins, including indoleamine 2, 3-dioxygenase 1 (IDO) and prostaglandin E2 (PGE2) [48][49][60,61]. ADSCs can also inhibit the activation and proliferation of immune cells, such as T cells and macrophages, and suppress the secretion of pro-inflammatory cytokines. Finally, they can also promote the generation of regulatory T cells and anti-inflammatory cytokines, creating an immune-regulatory environment that supports tissue healing and regeneration [50][62]. ADSCs are also able to act on vascular inflammatory responses and endothelial dysfunction. An excess of reactive oxygen species (ROS) is involved in the process of apoptosis, whilst low cell levels of ROS in the cells activate receptor types and signaling pathways that influence proliferation. It has been demonstrated that the functional properties of ADSCs are under redox control [51][63], and although ADSCs are responsive to several stimuli, the primary factor is oxygen tension, and low oxygen levels (2–8% O2) are a key aspect of the cell niche. Absolute oxygen tension within adipose tissue is very low, and ADSCs exist in low-oxygen conditions in the body; [52][64] it is now clear that ROS generation associated with hypoxia increases the proliferation and survival of human ADSCs [53][65], and when a local injury occurs, ROS and endogenous factors such as chemokines are produced from damaged cells, inducing ADSC migration. This highlights the multiple roles of ADSCs in the protection of cells by modulating inflammation and immunity [54][66].4.2. Promotion of Retinal Cell Survival and Regeneration
5.2. Promotion of Retinal Cell Survival and Regeneration
ADSCs have been shown to enhance retinal cell survival and regeneration in various retinal disease models. The ADSC-secreted paracrine factors can promote the survival of retinal cells, including PRs and RGCs, commonly affected in degenerative conditions. Furthermore, ADSCs can stimulate endogenous retinal progenitor cells, promoting their proliferation and differentiation into functional retinal cells. This regenerative potential of ADSCs contributes to the replacement of damaged cells and the restoration of retinal structure and function. The results obtained in a large number of experimental and clinical studies demonstrated that either systemic or local (intracerebral or intraocular) injection of ADSCs had beneficial effects in the treatment of neural and retinal diseases. ADSCs engraft in injured tissues and produce neurotrophins, angio-modulatory, and immunoregulatory factors that suppress the detrimental immune response and promote the regeneration of injured neural and retinal cells [55][56][57][58][67,68,69,70].4.3. Modulation of Retinal Vascularization and Neuroprotection
5.3. Modulation of Retinal Vascularization and Neuroprotection
In retinal vascular diseases, such as DR, ADSCs have demonstrated the ability to enhance retinal vascularization by secreting pro-angiogenic factors that stimulate the formation of new blood vessels, thus improving retinal blood flow. It has been found that ADSCs differentiate into pericytes that can stabilize retinal vessels in multiple pre-clinical models of retinal vasculopathy, suggesting they may be useful as a protective and regenerative cellular therapy for retinal vascular disease [59][60][76,77]. ADSCs also secrete neurotrophic factors, such as brain-derived neurotrophic factor (BDNF) and hepatocyte growth factor, exerting neuroprotective effects by reducing oxidative stress, inhibiting apoptosis, and promoting the survival of retinal neurons [56][68]. It has been demonstrated in various animal models that ADSCs represent a potential tool in order to prevent diabetic retinopathy and provide an effective cytoprotective microenvironment in the retina of diabetic mice [59][60][61][62][39,76,77,79].56. In Vitro Studies
In vitro studies explore ADSCs’ potential therapeutic applications. They can involve cell culture models, differentiation assays, and molecular analysis. In cell culture models, ADSC cultures are primarily used to evaluate their potential to differentiate into specific cell types and their overall behavior. Protocols that induce ADSCs to differentiate into RPE cells, PR-like cells, and other retinal cell types have been developed, allowing researchers to quantify the differentiation efficiency, integration potential, and functional properties of ADSC-derived retinal cells. ADSCs are known to play a role in retinal and photoreceptor cell proliferation [63][82]. Human ADSCs (hADSCs) have shown a trilineage potential to proliferate, migrate, and differentiate into RPE cells when exposed to an RPE-cell-conditioned medium [36][48]. Moreover, paired box 6 protein (5a) (PAX6 (5a)), a highly conserved transcription factor expression that has an essential role in the development of the vertebrate visual system, paired with a fibronectin-supplemented medium, is able to induce the differentiation of hADSCs into retinal progenitors, RPE cells, and PRs [37][49]. In differentiation assays, immunocytochemistry, gene expression analysis, and functional assays can be used to check the expression of retina-specific markers or the acquisition of functional properties of ADSCs, thus establishing the likeliness these cells have of integrating into the retina and replacing damaged tissue. Mannino et al. showed that the presence of growth factors produced by an RPE cell line (ARPE-19 cells) in tissue culture induces ADSCs to express neural differentiation markers typical of the neuronal and glial cells of the retina [64][84].5.1. Evaluation of Trophic and Paracrine Effects on Retinal Cells
6.1. Evaluation of Trophic and Paracrine Effects on Retinal Cells
As mentioned previously, ADSCs have been shown to promote a supportive microenvironment for retinal cell survival and function by means of paracrine abilities and the secretion of growth factors, cytokines, and chemokines. ADSCs can secrete a wide variety of neurotrophic factors (NTFs), such as hepatocyte growth factor (HGF), ciliary neurotrophic factor (CNTF), insulin-like growth factor (IGF) [65][89], basic fibroblast growth factor (FGF2), epidermal growth factor (EGF) [66][90], vascular endothelial growth factor (VEGF), nerve growth factor (NGF), brain-derived growth factor (BDNF), glial cell-derived neurotrophic factor (GDNF), neurotrophin-3 (NT-3), and platelet-derived growth factor (PDGF) [67][91]. These factors have a role in the survival, proliferation, and differentiation of retinal cells [68][92]. Dov et al. demonstrated that the transplantation of ADSCs significantly improved the recovery of retinal RGCs in organotypic ischemic retinas, probably via paracrine pathways, and advocated the potential of using these cells in future studies of regenerative therapy for ischemic retinas and ischemic RGCs [69][37]. Conditioned media from human ADSCs have also been shown to protect RPE and PR cells from oxidative stress-mediated cell death [70][38], and ADSCs have been found to have a protective role against retinal ischemic damage [71][85] and not only mediate angiogenesis via paracrine mechanisms in retinal endothelial cells but also promote retinal regeneration in vitro [72][86].5.2. Exploration of ADSC-Mediated Immunomodulation
6.2. Exploration of ADSC-Mediated Immunomodulation
The mechanism of immunosuppression by ADSCs involves the cell-to-cell contact-mediated repression of function and maturation of T cells (CD4+ and CD8+ cells), B cells, dendritic cells (DCs), NK cells, neutrophils, and macrophages [73][95]. The secretion of immune-modulatory cytokines, such as nitric oxide (NO), indoleamine 2,3-dioxygenase (IDO), tumor necrosis factor-stimulated gene 6 (TSG6), prostaglandin E2 (PGE2), thrombospondin type 1 (TSP1), interleukins 6, 10 (IL6, IL10), TGFβ1, and HGF, has been shown to regulate these immune cells and anti-inflammatory responses by MSCs [74][96].67. Animal Studies
Animal studies are a mandatory step between in vitro investigations and human clinical trials to evaluate the safety and efficacy of ADSC-based therapies using animal models of retinal diseases. Animal models (rodents or non-human primates) mimic retinal diseases and enable researchers to study the therapeutic effects of ADSC transplantation, assess cell survival and integration, and investigate functional outcomes in the diseased retina. ADSCs can be delivered into the eyes of animal models through various routes, like intravitreal injection, subretinal injection, or transplantation, depending on the specific research objectives and the targeted retinal cell layer. Intravitreal injections allow the ADSC paracrine effects on retinal cells to take effect; subretinal injections and transplantations place ADSCs in close proximity to the RPE and photoreceptor layers, facilitating direct interactions. Transplanted ADSCs can also be labeled with fluorescent dyes or genetic markers to track their “journey” within the eye and retina. The therapeutic effects of ADSCs within the retina can be evaluated through several means, including functional assessments (for example, with electrophysiological studies) or structural analysis, especially via optical coherence tomography (OCT), histological examination, and immunohistochemistry to detect cell markers and analyze gene expression. Transplanted ADSCs may differentiate into retinal cell types (RPE cells; PRs), thereby contributing to tissue regeneration. By investigating the fate and behavior of transplanted ADSCs, animal studies can explore the integration of transplanted ADSCs into the host retina and their ability to establish functional connections with existing retinal cells. Moreover, animal studies provide valuable insights for optimizing protocols, understanding the therapeutic effects, and identifying potential/ideal targets for ADSC-based therapies in retinal diseases [75][76][77][108,109,110]. In Kadkhodaeian et al.’s work on albino Sprague-Dawley rats, which used a sodium iodate model for the RPE injury, ADSCs were found to survive for 4 weeks after transplantation and to migrate into the RPE layer in the injured retina [78][101]. More recently, Dov et al. examined the transplantation of ADSCs in a co-culture system with organotypic ischemic retinas, and RGC recovery was demonstrated. Since there was no advantage from the direct contact of ADSCs with RGCs, the beneficial effect seen is likely to be related to the paracrine activity of ADSCs, and the data correlated well with the secretion profile of ADSCs’ anti-apoptotic and pro-proliferative cytokines [69][37].78. Human Studies
Current ADSC-based human studies in retinal disease are primarily focused on safety and preliminary efficacy assessments and, therefore, typically involve a limited number of patients. Patients with severe retinal diseases, such as advanced AMD or retinitis pigmentosa (RP), are monitored for adverse events, functional changes (visual acuity, visual field, contrast sensitivity, electrophysiology), and structural improvements (i.e., retinal thickness and morphology on OCT) following transplantation of autologous or allogeneic ADSCs. Human clinical trials are crucial for evaluating the safety, feasibility, and efficacy of ADSCs in retinal disease, in order to assess safety, visual outcomes, and functional improvements.7.1. Clinical Trials Investigating ADSC Transplantation in Retinal Disease
8.1. Clinical Trials Investigating ADSC Transplantation in Retinal Disease
ADSCs can be delivered to the human retina via intravitreal injection, subretinal injection, or transplantation with a carrier scaffold, and their therapeutic potential has been tested in various retinal diseases, including AMD, RP, and DR [79][113]. The trials aim to determine the safety and feasibility of ADSC transplantation, evaluate its therapeutic effects, and establish optimal transplantation protocols. They often involve careful patient selection and follow-up examinations to monitor the progression of the disease and treatment outcomes. Several trials on ADSC administration for the treatment of retinal degenerative diseases exist; most of them are in phase 1 or 2 and are focused on safety. These cell applications were studied on patients suffering from various retinal diseases, ranging from retinitis pigmentosa and other rare inherited retinal disorders to more common retinopathies, such as DR, retinal vasculopathies, and macular pathologies of medical and surgical interest, such as AMD, RP and macular holes [80][81][82][114,115,116]. Only one study applied cells obtained from adipose tissue. Oner et al. injected ADSCs under the retina of 11 patients affected by RP and followed them for 6 months. The experimental procedure consisted of complete vitrectomy, subretinal injection of cells, air tamponade, and face-down posturing for 1 day after surgery to spread the cells in the subretinal space. Significant improvements in BCVA (from 20/2000 to 20/200), perimetry, and ERG were observed in one patient. Slight improvements in BCVA and brighter color vision were reported by three patients, whereas the remaining seven patients showed no BCVA changes, with five of them having light perception at baseline.7.2. Safety and Feasibility Assessments
8.2. Safety and Feasibility Assessments
Safety assessments involve monitoring for adverse events, such as infections, inflammation, or immune reactions, associated with the transplantation procedure or the presence of transplanted cells. Feasibility assessments evaluate the practicality and effectiveness of ADSC transplantation in the clinical setting. Factors, such as cell viability, survival, and integration within the host retina, as well as the surgical technique and delivery method, are evaluated to determine the feasibility of ADSC transplantation as a viable treatment option. Limoli et al. observed favorable outcomes among elderly patients with non-exudative AMD after undergoing the suprachoroidal grafting of mature adipocytes and ADSCs in the stromal vascular fraction (SVF), which was enriched with platelet-rich plasma (PRP). The study showed no complications and an improvement in scotopic electroretinographic scores in these patients [83][118].7.3. Visual Outcomes and Functional Improvements
8.3. Visual Outcomes and Functional Improvements
Human studies also aim to assess visual outcomes and functional/structural improvements following ADSC transplantation. Functional electrophysiological assessments provide objective data on the improvement in or preservation of retinal function following ADSC transplantation. Visual acuity measurements and visual-field testing can be utilized to evaluate subjective measures, whilst imaging techniques such as OCT are used to evaluate structural changes in the retina.89. Conclusions
Studies reinforced the potential of ADSCs as a promising therapeutic approach for retinal diseases. The regenerative and immunomodulatory properties of this class of SCs offer a multi-faceted approach to address the complex pathophysiology of retinal diseases. Combining ADSC-based therapies with other treatment modalities, such as anti-VEGF therapy or gene therapy, may further enhance the treatment outcomes. However, further research and well-designed clinical trials are necessary to establish the long-term efficacy, safety, and optimal protocols for ADSC transplantation in retinal diseases. Large-scale randomized controlled trials with standardized outcome measures are needed to validate the promising results observed in preclinical and early clinical studies.
