Lung cancer is the leading cause of cancer deaths in men and women in the United States. Accurate staging is needed to determine prognosis and devise effective treatment plans. The International Association for the Study of Lung Cancer (IASLC) has made multiple revisions to the tumor, node, metastasis (TNM) staging system used by the Union for International Cancer Control and the American Joint Committee on Cancer to stage lung cancer. The eighth edition of this staging system includes modifications to the T classification with cut points of 1 cm increments in tumor size, grouping of lung cancers associated with partial or complete lung atelectasis or pneumonitis, grouping of tumors with involvement of a main bronchus regardless of distance from the carina, and upstaging of diaphragmatic invasion to T4.
- lung cancer
- staging
- TNM
1. Introduction
| T—Primary Tumor | ||
|---|---|---|
| Category | Subcategory | Descriptors |
| T or M Stage | N0 | N1 | N2 | N3 | ||||
|---|---|---|---|---|---|---|---|---|
| TX | Primary tumor cannot be assessed, or tumor is proven by the presence of malignant cells in sputum or bronchial washings but not visualized by imaging or bronchoscopy | |||||||
| T0 | No evidence of primary tumor | |||||||
| IIB | IIIA | IIIB | Tis | Carcinoma in situ: Tis(AIS): adenocarcinoma Tis(SCIS): squamous cell carcinoma |
||||
| T1 | ||||||||
| T1c | IA3 | IIB | IIIA | IIIB | Tumor 3 cm or less in greatest dimension, surrounded by lung or visceral pleura, without bronchoscopic evidence of invasion more proximal than the lobar bronchus (i.e., not in the main bronchus). The uncommon superficial spreading tumor of any size with its invasive component limited to the bronchial wall, which may extend proximal to the main bronchus, is also classified as T1a. | |||
| T2 | T2a | IB | IIB | IIIA | IIIB | T1mi | Minimally invasive adenocarcinoma | |
| T2b | IIA | IIB | IIIA | IIIB | T1a | Tumor 1 cm or less in greatest dimension | ||
| T3 | T3 | IIB | IIIA | IIIB | T1b | Tumor more than 1 cm but not more than 2 cm in greatest dimension | ||
| IIIC | ||||||||
| T4 | T4 | IIIA | IIIA | IIIB | IIIC | T1c | Tumor more than 2 cm but not more than 3 cm in greatest dimension | |
| M1 | M1a | IVA | IVA | IVA | IVA | T2 | Tumor more than 3 cm but not more than 5 cm; or tumor with any of the following features. T2 tumors with these features are classified T2a if 4 cm or less, or if size cannot be determined; and T2b if greater than 4 cm but not larger than 5 cm.
| |
| T2b | Tumor more than 4 cm but not more than 5 cm in greatest dimension | |||||||
| T3 | Tumor more than 5 cm but not more than 7 cm in greatest dimension or one that directly invades any of the following: parietal pleura (PL3), chest wall (including superior sulcus tumors), phrenic nerve, parietal pericardium; or associated separate tumor nodule(s) in the same lobe as the primary | |||||||
| T4 | ||||||||
| T1 | T1a | IA1 | IIB | IIIA | IIIB | |||
| Tumors more than 7 cm or one that invades any of the following: diaphragm, mediastinum, heart, great vessels, trachea, recurrent laryngeal nerve, esophagus, vertebral body, carina; separate tumor nodule(s) in a different ipsilateral lobe to that of the primary | ||||||||
| T1b | IA2 | |||||||
| ||||||||
| M1b | IVA | IVA | IVA | IVA | T2a | Tumor more than 3 cm but not more than 4 cm in greatest dimension | ||
| M1c | IVB | IVB | IVB | IVB | ||||
| N—Regional Lymph Nodes | ||||||||
| NX | Regional lymph nodes cannot be assessed | |||||||
| N0 | No regional lymph node metastasis | |||||||
| N1 | Metastasis in ipsilateral peribronchial and/or ipsilateral hilar lymph nodes and intrapulmonary nodes, including involvement by direct extension | |||||||
| N2 | Metastasis in ipsilateral mediastinal and/or subcarinal lymph node(s) | |||||||
| N3 | Metastasis in contralateral mediastinal, contralateral hilar, ipsilateral or contralateral scalene, or supraclavicular lymph node(s) | |||||||
| M—Distant Metastasis | ||||||||
| M0 | No distant metastasis | |||||||
| M1 | Distant metastasis | |||||||
| M1a | Separate tumor nodule(s) in a contralateral lobe; tumor with pleural nodules or malignant pleural or pericardial effusion. Most pleural (pericardial) effusions with lung cancer are due to tumor. In a few patients, however, multiple microscopic examinations of pleural (pericardial) fluid are negative for tumor, and the fluid is non-bloody and is not an exudate. Where these elements and clinical judgment dictate that the effusion is not related to the tumor, the effusion should be excluded as a staging descriptor. |
|||||||
| M1b | Single extrathoracic metastasis in a single organ and involvement of a single distant (non-regional) node | |||||||
| M1c | Multiple extrathoracic metastases in one or several organs | |||||||
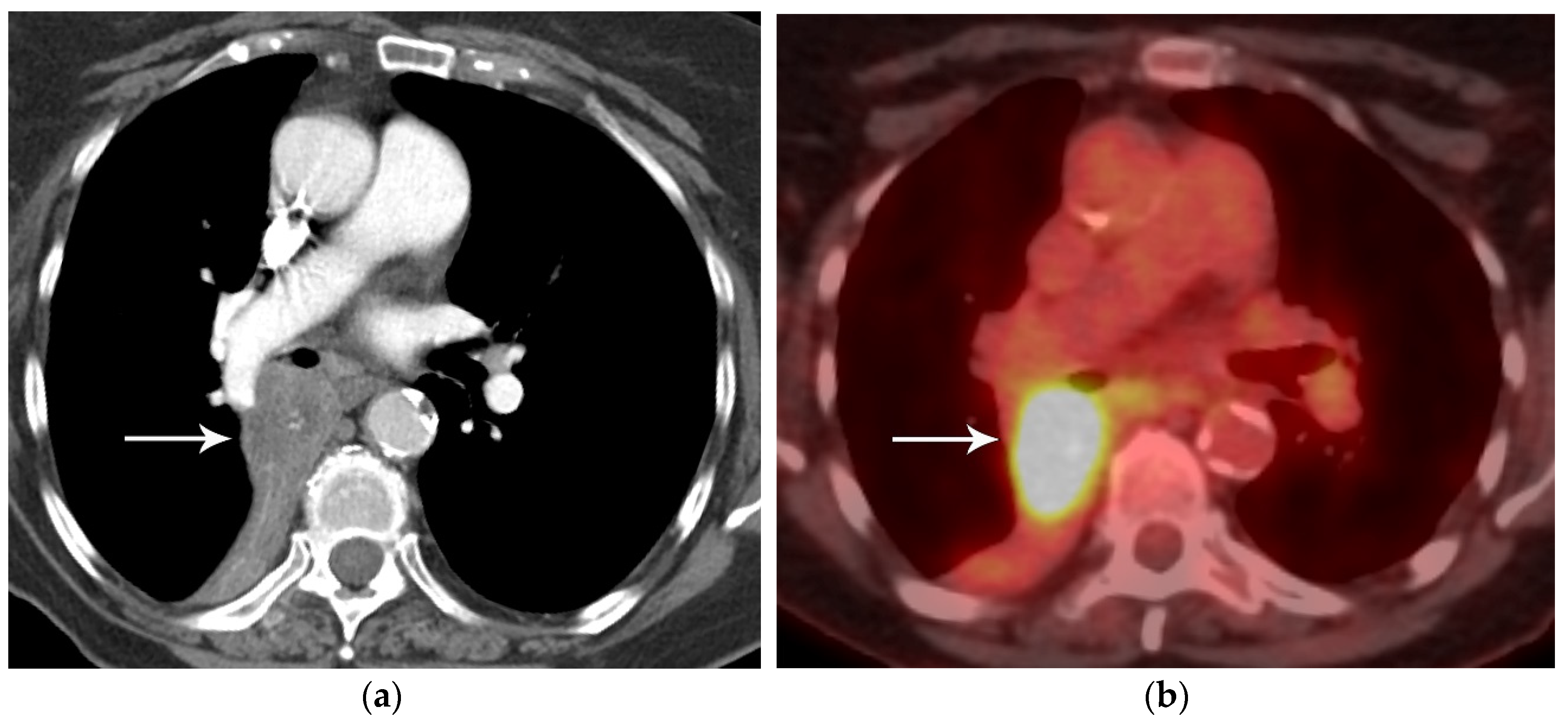
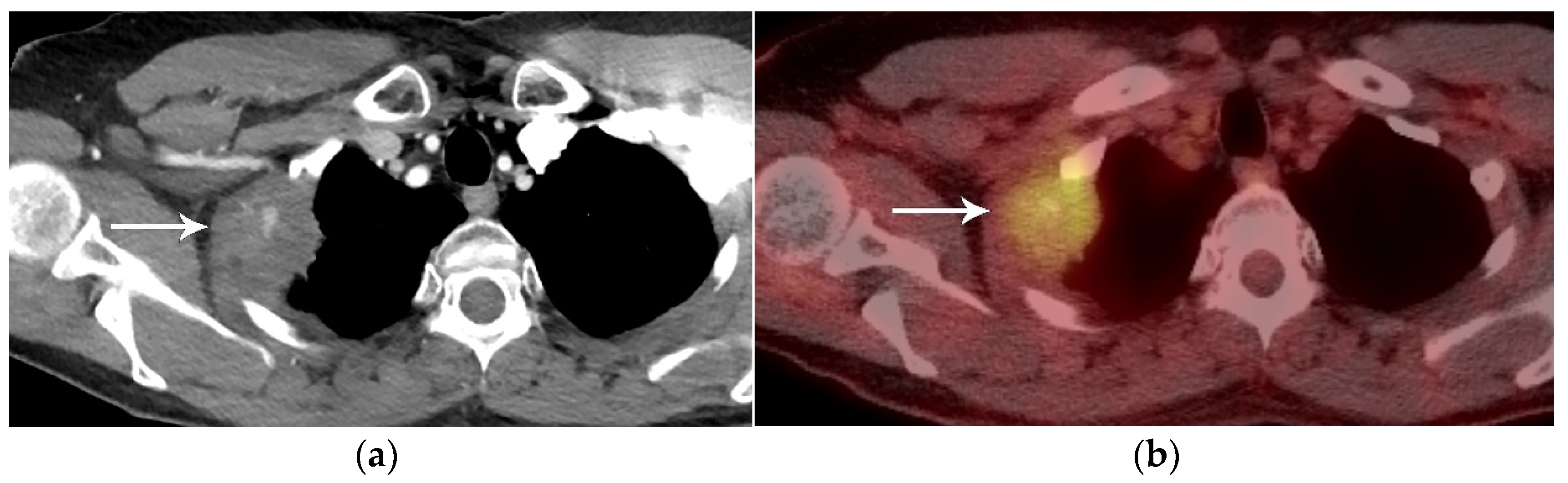
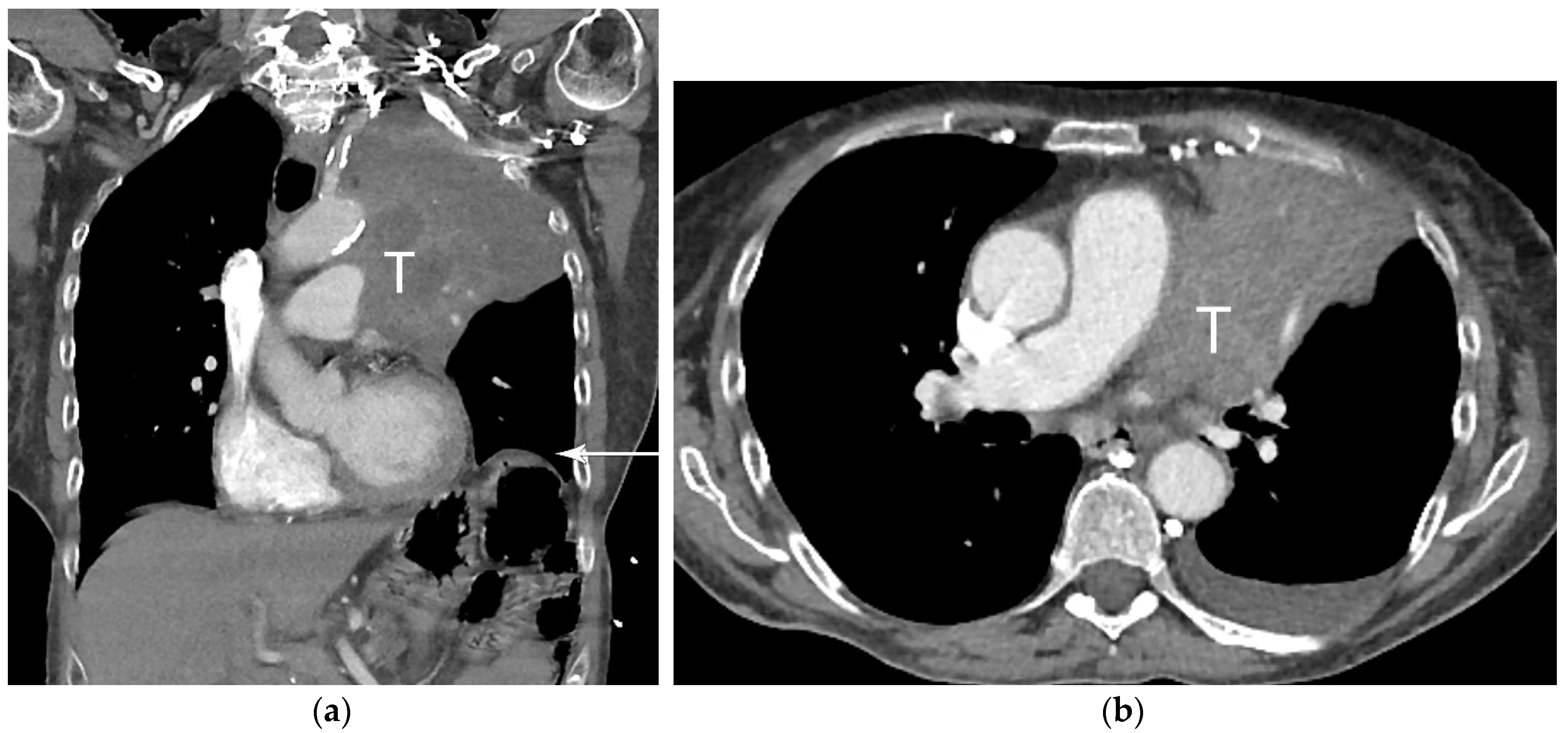
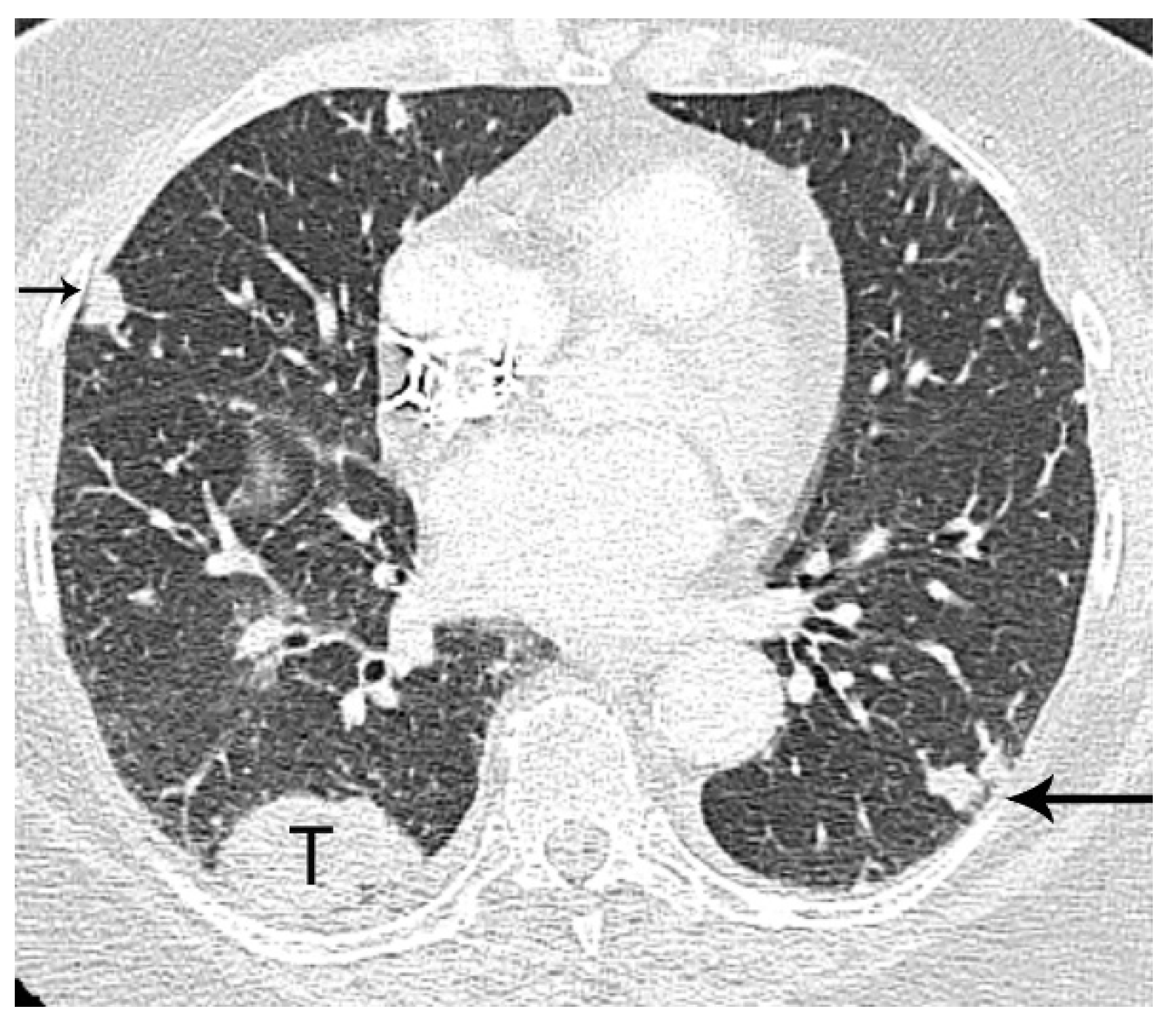
2. T Classification
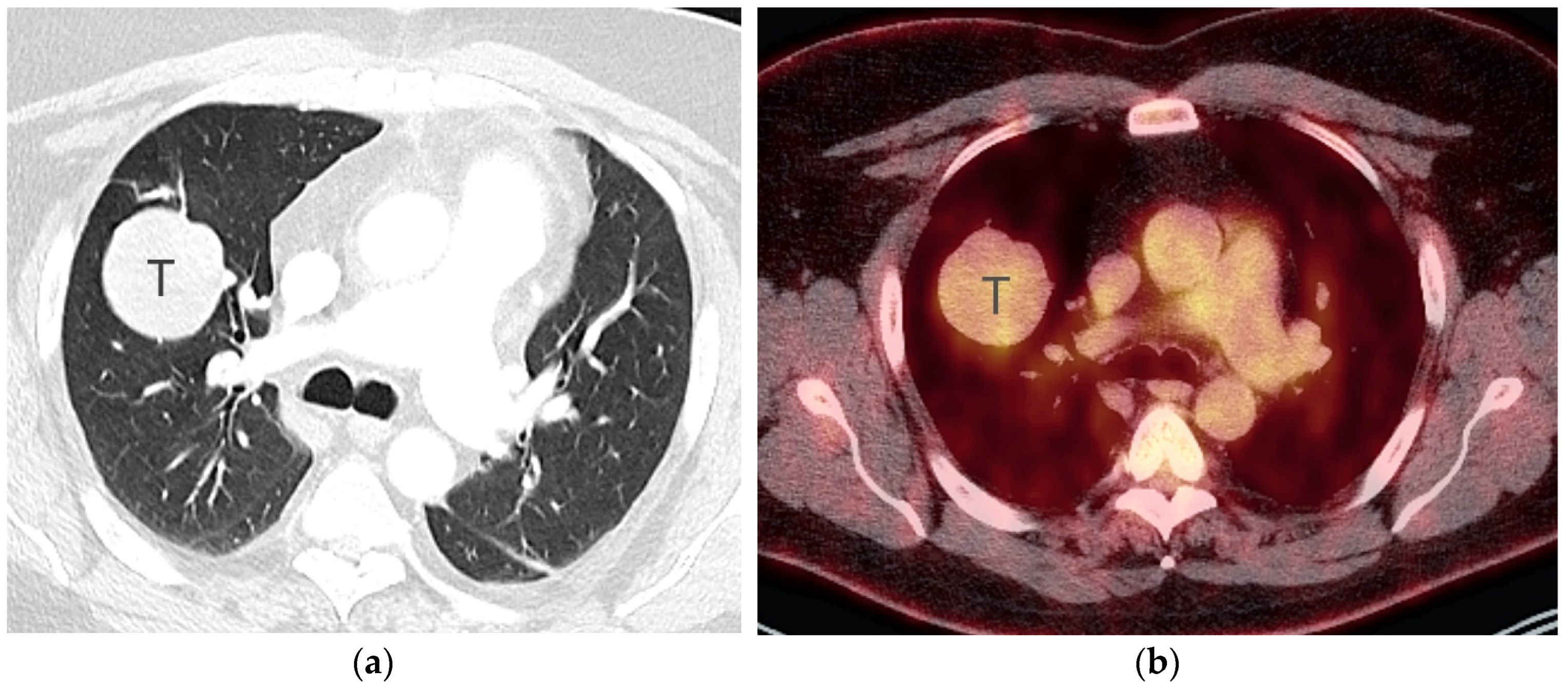
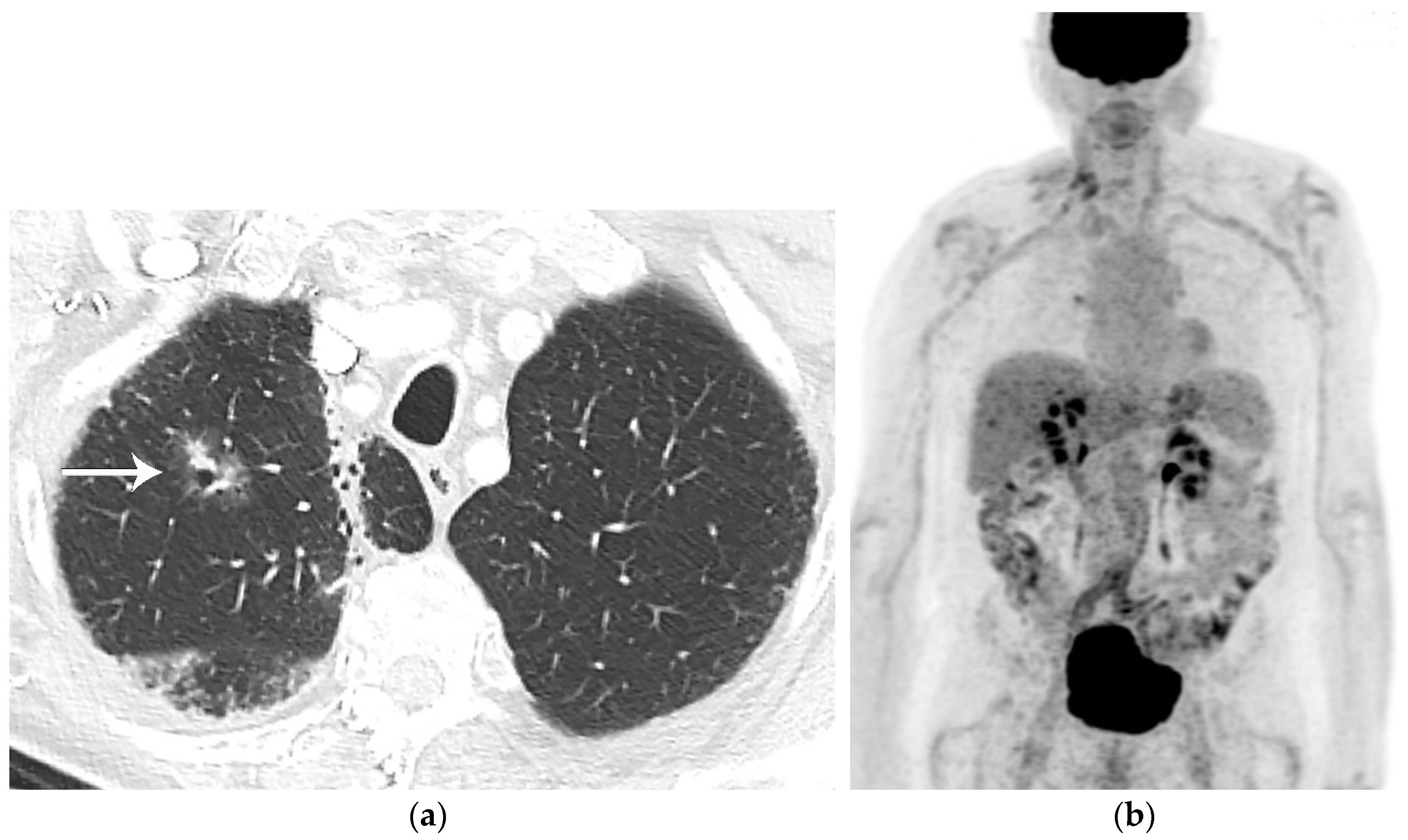
References
- NCCN Clinical Practice Guidelines in Oncology, Non-Small Cell Lung Cancer, Version 4. 2023. Available online: https://www.nccn.org/ (accessed on 20 September 2023).
- Thai, A.A.; Solomon, B.J.; Sequist, L.V.; Gainor, J.F.; Heist, R.S. Lung cancer. Lancet 2021, 398, 535–554.
- Carter, B.W.; Lichtenberger, J.P., III; Benveniste, M.K.; de Groot, P.M.; Wu, C.C.; Erasmus, J.J.; Truong, M.T. Revisions to the TNM Staging of Lung Cancer: Rationale, Significance, and Clinical Application. Radiographics 2018, 38, 374–391.
- Goldstraw, P.; Chansky, K.; Crowley, J.; Rami-Porta, R.; Asamura, H.; Eberhardt, W.E.; Nicholson, A.G.; Groome, P.; Mitchell, A.; Bolejack, V.; et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2016, 11, 39–51.
- Nicholson, A.G.; Chansky, K.; Crowley, J.; Beyruti, R.; Kubota, K.; Turrisi, A.; Eberhardt, W.E.; van Meerbeeck, J.; Rami-Porta, R.; Staging and Prognostic Factors Committee; et al. The International Association for the Study of Lung Cancer Lung Cancer Staging Project: Proposals for the Revision of the Clinical and Pathologic Staging of Small Cell Lung Cancer in the Forthcoming Eighth Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2016, 11, 300–311.
- Galgano, S.J.; Wei, B.; Rose, J.B. PET Imaging of Neuroendocrine Tumors. Radiol. Clin. N. Am. 2021, 59, 789–799.
- Yoon, J.Y.; Sigel, K.; Martin, J.; Jordan, R.; Beasley, M.B.; Smith, C.; Kaufman, A.; Wisnivesky, J.; Kim, M.K. Evaluation of the Prognostic Significance of TNM Staging Guidelines in Lung Carcinoid Tumors. J. Thorac. Oncol. 2019, 14, 184–192.
- Kutob, L.; Schneider, F. Lung Cancer Staging. Surg. Pathol. Clin. 2020, 13, 57–71.
- Travis, W.D.; Asamura, H.; Bankier, A.A.; Beasley, M.B.; Detterbeck, F.; Flieder, D.B.; Goo, J.M.; MacMahon, H.; Naidich, D.; Nicholson, A.G.; et al. The IASLC Lung Cancer Staging Project: Proposals for Coding T Categories for Subsolid Nodules and Assessment of Tumor Size in Part-Solid Tumors in the Forthcoming Eighth Edition of the TNM Classification of Lung Cancer. J. Thorac. Oncol. 2016, 11, 1204–1223.
- Choi, Y.; Kim, S.H.; Kim, K.H.; Choi, Y.; Park, S.G.; Sohn, I.; Kim, H.S.; Um, S.W.; Lee, H.Y. Clinical T category for lung cancer staging: A pragmatic approach for real-world practice. Thorac. Cancer 2020, 11, 3555–3565.
- Wu, D.Y.; de Hoyos, A.; Vo, D.T.; Hwang, H.; Spangler, A.E.; Seiler, S.J. Clinical Non-Small Cell Lung Cancer Staging and Tumor Length Measurement Results From U.S. Cancer Hospitals. Acad. Radiol. 2021, 28, 753–766.
- Oh, J.; Piao, Z.; Cho, H.J.; Chong, Y.; Kim, S.S.; Kim, J.H.; Kang, M.W. CT-based three-dimensional invasiveness analysis of adenocarcinoma presenting as pure ground-glass nodules. Transl. Cancer Res. 2023, 12, 765–773.
- Ridge, C.A.; Huang, J.; Cardoza, S.; Zabor, E.C.; Moskowitz, C.S.; Zakowski, M.F.; Ginsberg, M.S. Comparison of multiplanar reformatted CT lung tumor measurements to axial tumor measurement alone: Impact on maximal tumor dimension and T stage. AJR Am. J. Roentgenol. 2013, 201, 959–963.
- Ahn, H.; Lee, K.W.; Lee, K.H.; Kim, J.; Kim, K.; Chung, J.H.; Lee, C.T. Effect of computed tomography window settings and reconstruction plane on 8th edition T-stage classification in patients with lung adenocarcinoma manifesting as a subsolid nodule. Eur. J. Radiol. 2018, 98, 130–135.
- Rami-Porta, R.; Bolejack, V.; Crowley, J.; Ball, D.; Kim, J.; Lyons, G.; Rice, T.; Suzuki, K.; Thomas, C.F., Jr.; Travis, W.D.; et al. The IASLC Lung Cancer Staging Project: Proposals for the Revisions of the T Descriptors in the Forthcoming Eighth Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2015, 10, 990–1003.
- Mallum, A.; Mkhize, T.; Akudugu, J.M.; Ngwa, W.; Vorster, M. The Role of Positron Emission Tomography and Computed Tomographic (PET/CT) Imaging for Radiation Therapy Planning: A Literature Review. Diagnostics 2022, 13, 53.
- Lococo, F.; Guerrera, F.; Rena, O.; Ampollini, L.; Vannucci, J.; Bertoglio, P.; Ventura, L.; Lyberis, P.; Marchese, V.; Arena, V.; et al. Accuracy of (18)F-FDG in Detecting Stage I Lung Adenocarcinomas According to IASLC/ATS/ERS Classification. Heart Lung Circ. 2022, 31, 726–732.
- Ichinose, J.; Kohno, T.; Fujimori, S.; Harano, T.; Suzuki, S.; Fujii, T. Invasiveness and malignant potential of pulmonary lesions presenting as pure ground-glass opacities. Ann. Thorac. Cardiovasc. Surg. 2014, 20, 347–352.
- Shao, X.; Niu, R.; Jiang, Z.; Shao, X.; Wang, Y. Role of PET/CT in Management of Early Lung Adenocarcinoma. AJR Am. J. Roentgenol. 2020, 214, 437–445.
- Pijl, J.P.; Nienhuis, P.H.; Kwee, T.C.; Glaudemans, A.; Slart, R.; Gormsen, L.C. Limitations and Pitfalls of FDG-PET/CT in Infection and Inflammation. Semin. Nucl. Med. 2021, 51, 633–645.
