Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Peter Tang and Version 1 by Raffaele Serra.
Chronic kidney disease (CKD), defined as the presence of albuminuria and/or reduction in estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2, is considered a growing public health problem. The implementation of novel biomarkers in clinical practice is crucial, since it could allow earlier diagnosis and lead to an improvement in CKD outcomes.
- end-stage kidney disease (ESKD)
- cardiovascular disease
- epidemiology
- CKD
- biomarkers
1. Introduction
A biomarker is defined, by a collaborative working group involved with both the United States National Institutes of Health (NIH) and the Food and Drug Administration (FDA), as “a characteristic that is measured as an indicator of normal biological processes, pathogenic processes, or responses to an exposure or intervention, including therapeutic interventions” [1]. This working group definition was formed on the initiative of the NIH with the aim of accelerating the development and clinical application of reliable biomarkers based on shared definitions. Indeed, an “ideal” biomarker is defined with the presence of some analytic features: (1) it should be measured and readily available in biological samples, such as blood or urine; (2) it should be reproducible, non-invasive, and not expensive [2]. In addition, several clinical features should also be provided to complete the biomarker’s definition; it needs to allow for an early detection of a disease status, while it also needs to have high sensitivity and specificity, i.e., the biomarker needs to differentiate the pathologic status from the normal one and from other clinical conditions, as accurately as possible [3]. The effort made by the NIH–FDA working group is considerable ever since it forecasted and tried to solve the problems of biomarker development, from discovery to clinical application. Indeed, once a biomarker is found to be involved in one or more pathophysiological mechanisms of a disease, it may be introduced into clinical practice to see whether it offers advantages in clinical management and after completion of the validation phase that is considered a crucial step [4]. An important example of the pitfalls of biomarker development is illustrated in the chronic kidney disease (CKD) scenario. CKD is a chronic disease characterized by a poor prognosis, due to the strong association with the development of cardiovascular events, all-cause mortality, and renal events such as renal replacement therapies (RRT, i.e., dialysis or kidney transplantation) [5,6][5][6]. The Kidney Disease Improving Global Outcomes Work Group (KDIGO), in 2012, defined CKD with the presence of either decreased kidney function (estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2) and/or albuminuria, namely, an abnormal amount of protein excretion with urine, for at least three months [7]. The global dimension of the disease is so important that CKD started to be considered a relevant public health problem. Indeed, according to the Global Burden of Kidney Disease, the CKD incidence and prevalence increased by 88.76% and 86.95%, respectively, from 1990 to 2016 [8]. Moreover, the mortality attributed to CKD increased by 41.5% between 1990 and 2017, a percentage that exceeded the mortality due to several neoplasms or cardiovascular (CV) disease [9]. Hence, great effort is advocated toward improving clinical decision-making and reinforcing treatment and prevention of CKD. The KDIGO working group proposed a classification called “CGA” that incorporates the cause (C) of kidney disease, as well as the eGFR (G) and albuminuria (A) levels, to stratify risk in patients with CKD and to better address the importance of the underlying disease.
2. General Classification of Biomarkers
Depending on the intended use, biomarkers are classified as diagnostic, pharmacodynamic/response, monitoring, prognostic, or predictive [19][10]. WThe researchers focus, in the present review articlesearch, on prognostic and predictive biomarkers, as they represent the most developed biomarkers in CKD patients, while they also incorporate characteristics from other categories of biomarkers. A prognostic biomarker is used to identify the probability of a clinical outcome in patients who are already suffering from the disease of interest [1,20][1][11]. Furthermore, prognostic biomarkers measure the association between the disease and clinical outcome in the absence of therapy or with standard therapy that all patients are likely to receive. On the other hand, predictive biomarkers are used to determine whether a patient is likely to benefit from a particular therapy. The clinical benefit could be either a good response to a drug if the biomarker is positive or, alternatively, a lack of benefit from the same drug, which can save a patient from drug toxicity or unnecessary side effects [1].3. Prognostic Biomarkers in CKD
The importance of prognostic biomarkers in the CKD setting is crucial. Indeed, CKD is a multifactorial disease in which risk factors play different roles in different individuals and in different stages of the disease. It was demonstrated, for example, that the presence of type 2 diabetes leads to the development of CKD in up to 30% of subjects. This means that, in these patients, the deleterious pathogenetic mechanisms of diabetes mellitus are sufficient to damage the kidneys with the onset of typical diabetic glomerulosclerosis. Conversely, it is also possible that, in a portion of diabetic patients, the kidneys are injured by the co-existence of arterial hypertension, which causes different lesions (mainly injuring the kidney vessels), with a completely different prognosis.3.1. Kidney Biomarkers
The assessment of correct risk stratification, i.e., the allocation of CKD patients to the true risk-of-event categories, always represents a difficult challenge for nephrologists and researchers, due to the large variability in etiology and prognosis of CKD [21,22][12][13]. In order to accomplish this aim, a growing number of risk prediction models in CKD patients were developed over the past several years. They show how kidney measures, such as albuminuria (or proteinuria) and eGFR, are strong prognostic biomarkers. Indeed, an eGFR reduction to levels below 60 mL/min/1.73 m2 or even a small increase in albuminuria levels is associated with a significantly increased risk for CV events (CV mortality, coronary heart disease, stroke, heart failure), all-cause mortality, and ESKD (the most advanced stage of CKD that requires referral to renal replacement therapies such as hemodialysis), both in the general population and in patients with an already established CKD (Figure 1) [23,24,25][14][15][16].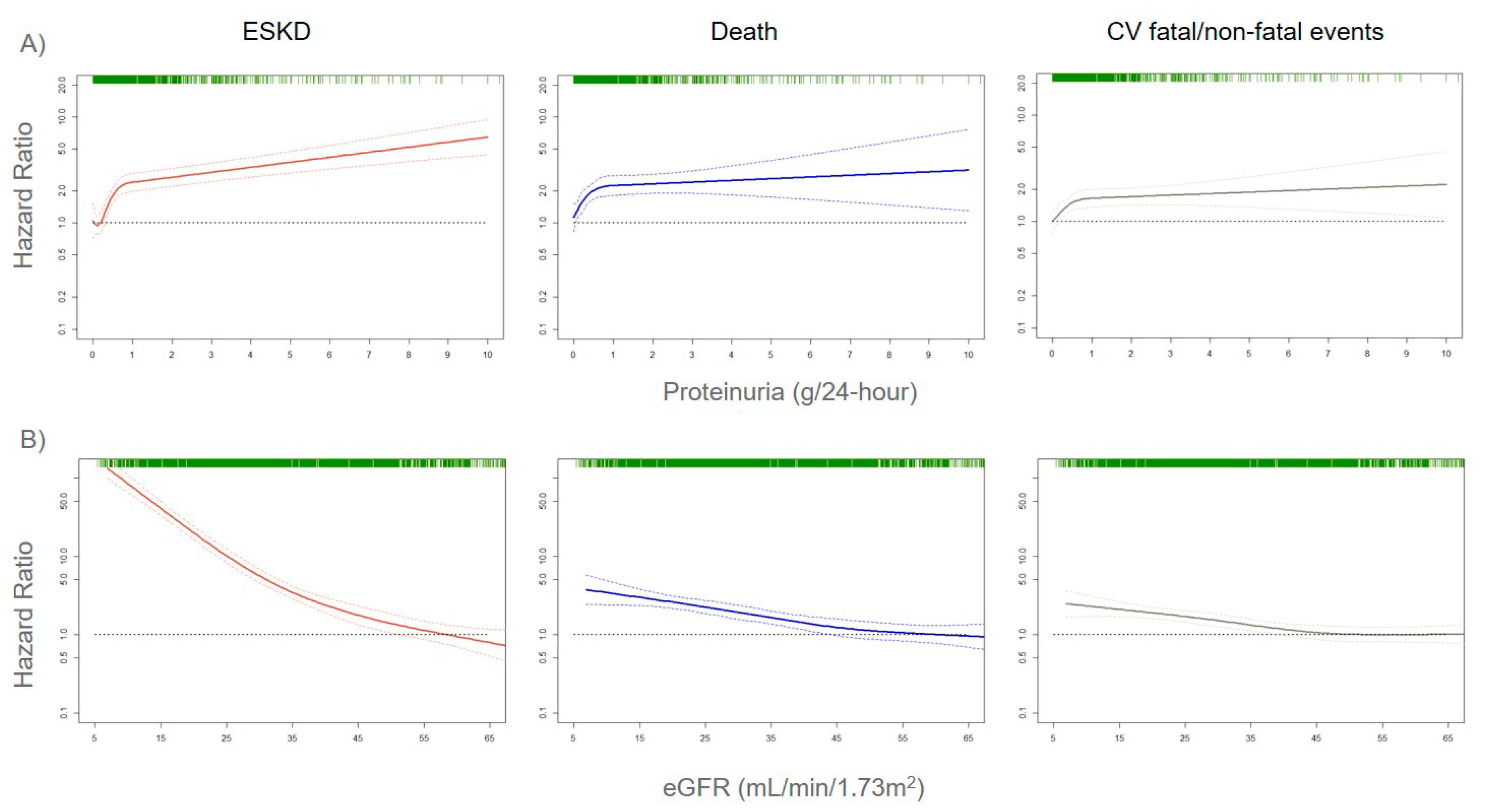
Figure 1. Adjusted risks for end-stage kidney disease (ESKD), death, and cardiovascular (CV) fatal and non-fatal events, by 24-h proteinuria (panel A) or estimated glomerular filtration rate (eGFR) (panel B) levels. Solid lines represent hazard ratios, whereas dashed lines the 95% confidence intervals. Hazard ratios were modeled by means of restricted cubic spline (RCS) due to the non-linear association with the endpoints. Knots are located at the zeroth, 25th, 50th, and 75th percentiles for proteinuria and 15, 30, 45, 60 mL/min/1.73 m2 for eGFR. Risks are adjusted for the four-variable Tangri equation [26][17]: age, gender, eGFR, and proteinuria. Rug plots on the x-axis at the top (colored green) represent the distribution of observations. Data source: pooled analysis of six cohorts of CKD patients referred to Italian nephrology clinics [27][18].
3.2. Markers of Oxidative Stress, Tissue Remodeling, and Metabolism
Myeloperoxidase (MPO) is a biomarker of oxidative stress that fosters nitic oxide consumption and which is associated with the development of atherosclerotic lesions, CV disease, and eGFR decline in CKD [42,43][38][39]. A recent observational analysis of the Chronic Renal Insufficiency Cohort (CRIC), which enrolled approximately 4000 patients with CKD in the United States (US), showed that serum MPO levels were associated with the risk of renal outcome, defined as initiation of RRT, 50% eGFR decline, or eGFR ≤ 15 mL/min/1.73 m2 [44][40]. The key element of this analysis was that the effect of MPO was significant even after adjustment for main confounders, such as baseline eGFR and proteinuria levels. Matrix metalloproteinases (MMPs), endopeptidases involved in tissue development, and homeostasis through the regulation of cell differentiation, apoptosis, and angiogenesis were shown to intervene in inflammatory and fibrotic processes across the kidneys [45,46][41][42]. Blood and urine levels of MMPs were linked to renal and CV disease in previous clinical studies in humans. Serum and urine MMP-2, -8, and -9 levels are increased in patients with diabetic CKD, with MMP-9 being significantly associated with the severity of albuminuria [47,48][43][44]. Increased plasma levels of TIMP-1 (tissue inhibitor of metalloproteinases-1) predicted the incidence of CKD regardless of inflammatory markers such as C-reactive protein [49][45]. MMPs and TIMPs also play a role in accelerating the atherosclerotic process by increasing cell migration to the plaque fibrous cap that in turn determines plaque inflammation and rupture [50][46]. Indeed, the levels of several MMPs (MMP-1, -2, -8, -9) and TIMP-1 were found to be increased in patients with peripheral arterial disease, including those with aneurysms of the arterial wall [51][47]. Fibroblast growth factor-23 (FGF-23), a hormone involved in phosphorus metabolism that increases progressively as kidney function declines, was significantly associated with mortality, atherosclerotic events, heart failure (HF), and ESKD in CKD patients [52,53][48][49].3.3. Cardiac Biomarkers
Several cardiac biomarkers were investigated, mainly for establishing their role in CV and renal risk prediction in CKD patients. Cardiac troponins (high-sensitivity cardiac troponin (hs-cTnT)) and natriuretic peptides (N-terminal pro-B-type natriuretic peptide (NT-proBNP)) are largely used in CV medicine to diagnose coronary artery disease (CAD) and heart failure (HF), respectively. Both these biomarkers are, thus, an expression of subclinical abnormalities in the heart [54,55][50][51]. However, one major problem that makes their introduction in individual risk prediction difficult is that cardiac markers are an expression of both cardiac and kidney dysfunction and cannot discern these two conditions. Natriuretic peptides act by promoting the tubular natriuresis across the kidney and counteracting the effects of renin–angiotensin–aldosterone system, which is triggered by heart failure, as well as renal dysfunction [56][52]. Concerns about the interpretation of hs-cTnT and natriuretic peptides also derive from the evidence that these marker concentrations are influenced by kidney function levels [56,57][52][53]. So far, cardiac markers found more application in the context of prognostic estimation of cardiorenal syndromes (CRS), clinical disorders where an acute or chronic dysfunction of one organ may lead to an acute or chronic dysfunction of the other, thus testifying the strict relationship between the heart and the kidney [58][54]. In the general population, hs-cTnT and NT-proBNP were shown to be strong predictors for incident HF over time [59,60][55][56]. In the setting of CKD, an attempt to evaluate, with appropriate statistical tools, the contribution of cardiac markers to the development of CV events was made using the Atherosclerosis Risk in Communities study (ARIC) population. In this restudyearch, examining 7682 non-CKD and 970 patients with CKD stage 1–5, hs-cTnT and NT-proBNP were associated with the development of CV events (defined as the composite of coronary heart disease, stroke, and HF) independently of kidney measures (eGFR and albuminuria) [61][57]. The finding was confirmed for both CKD and non-CKD patients, as well as for patients with or without previous CV disease. However, the interpretation of these results should be done with caution since the ARIC cohort was stratified, for this analysis, by the presence/absence of CKD, thus limiting the influence of kidney measures on CV risk prediction [61][57]. Results of the association between cardiac markers and renal outcomes in CKD patients are even more conflicting. The CRIC investigators found, in a cohort of over 3000 CKD patients, that increased plasma levels of growth differentiation factor-15 (GDF-15, a member of the transforming growth factor (TGF)-β cytokine family), hs-cTnT, and NT-proBNP were associated with CKD progression. defined as the onset of ESKD or 50% eGFR decline [62][58]. However, when all these parameters were added to the prediction model including traditional CV risk factors, the model discrimination (i.e., the ability of the model to separate individuals who develop events from those who do not; see more details in Section 5) did not improve, meaning that their clinical utility was scarce. Moreover, in the Framingham cohort, hs-cTnT was not associated with a faster eGFR decline or with incident CKD [63][59]. There is, overall, a need for future work to assess the role of cardiac markers in CV and renal risk prediction [61,62,63][57][58][59].3.4. Filtration and Urinary Biomarkers
Filtration biomarkers and urinary markers were also investigated. The use of cystatin C to estimate GFR (eGFRcys) improved the risk stratification for death, death from CV causes, and ESKD with a large proportion (23%) of patients being reclassified toward true risk estimates when compared with eGFR estimated from serum creatinine (eGFRcrea) [64][60]. eGFRcys was also shown to predict the onset of SCD in elderly CKD patients [35][28]. The combination of serum creatinine and cystatin C for estimating eGFR (eGFRcys-crea) allowed clinicians to anticipate the risk prediction of worse outcomes at 85 mL/min, which is well above the 60 mL/min threshold defined by eGFRcrea. β2-microglobulin, another filtration marker, showed a statistical power similar to cystatin C in improving prediction of ESKD, all-cause mortality, and new onset of CV disease beyond eGFRcrea [65][61]. With respect to urinary markers, some evidence, albeit controversial, was provided for the association of urinary markers of tubule damage (interleukin (IL)-18, kidney injury molecule-1 (KIM-1), neutrophil gelatinase-associated lipocalin (NGAL)), repair (human cartilage glycoprotein-40 (YKL-40)), and inflammation (monocyte chemoattractant protein-1 (MCP-1)) with the risk of ESKD [66,67][62][63]. In fact, when risk prediction models were adjusted for baseline eGFR and albuminuria, the associations of these biomarkers with clinical outcome were consistently attenuated. However, the highest values of KIM-1, MCP-1, and YKL-40 also provided useful risk estimation beyond eGFR and albuminuria in a post hoc analysis of the Systolic Blood Pressure Intervention Trial SPRINT trial [68][64]. Interestingly, urinary IL-18 and NGAL levels were shown to predict linear eGFR decline over time, an endpoint of growing interest in clinical research [68][64].3.5. Prognostic Role of Proteomics, Metabolomics, and Genomics
Proteomics metabolomics and genomics recently provided great input to the implementation of novel biomarkers [69,70,71][65][66][67]. The advantage of these “omics” techniques is to provide a combination of informative peptides/metabolites that are able to classify patients (hence, the appellation of classifiers) into significant clinical or risk categories. A well-depicted classifier in CKD patients is the CKD273, a panel of 273 urine peptides shown to predict, in long-term follow-up cohort studies, eGFR decline with a strong and independent effect to the onset of albuminuria, particularly in diabetic patients [72,73,74][68][69][70]. In further risk prediction models, CKD273 was also able to reclassify about 30% of patients compared with the standard equation that considers eGFR and albuminuria, for the risk of CKD progression [75][71]. The CRIC investigators described, in a recent manuscript, the association between a panel of 13 urine metabolites and CKD progression [76][72]. Results of this analysis are encouraging since the levels of four metabolites, namely, 3-hydroxyisobutyrate (3-HIBA), 3-methylcrotonyglycine, citric acid, and aconitic acid, were associated with eGFR decline, with 3-HIBA and aconitic acid levels also significantly associated with the hard endpoint ESKD. Of particular interest is the prognostic role of the genetic causes of CKD. Of all CKD cases diagnosed at a young age (<25 years), an actual 30% are determined by monogenic disorders, and inherited CKD is globally more prevalent (prevalence ranged between 30% and 75%) than previously thought, particularly in the presence of a family history of CKD [77,78][73][74]. More importantly, the advent of genome-wide association studies (GWAS) allowed the discovery of several single-nucleotide polymorphisms (SNPs) associated with an increased risk for CKD or with a worse prognosis in patients already affected by CKD [78][74]. Polymorphisms in the Uromodulin (UMOD) gene region rs4293393, which codifies the most abundant urinary protein in healthy subjects, namely, uromodulin (also called Tamm–Horsfall protein), are associated with an increased risk of incident CKD [79][75]. A similar role in predicting the onset of CKD was exerted by other SNPs such as Protein Kinase AMP-Activated Non-Catalytic Subunit Gamma 2 (PRKAG2), Longevity Assurance Gene Homologs (LASS2), Disabled Homolog 2 (alias DAB Adaptor Protein 2, DAB2), Dachshund Family Transcription Factor 1 (DACH1), and Stanniocalcin 1 (STC1) [80][76]. Apolipoprotein L1 (APOL1) gene variants were also studied in CKD patients. APOL1 encodes apolipoprotein L1, which is involved in the lysis of Trypanosoma brucei and other trypanosomes [81][77]. The G1 and G2 variants of APOL1 were associated with an increased risk of eGFR decline and disease progression to ESKD in CKD populations [82][78]. Interestingly, information derived from the SNPs were recently combined into a genetic risk score [78][74]. This score was shown to be associated with eGFR decline and kidney outcome regardless of albuminuria and other renal risk factors encompassing diabetes, history of CV disease, and hypertension. A number of studies assessing the associations between SNPs and kidney measures were carried-out by the United Kingdom (UK) biobank, a large cohort of over 500,000 participants enrolled in 2006–2010, from which genotypic information was widely collected [83][79]. Analyses of the UK biobank provided a great contribution to the prognostic research in CKD. For example, genetically predicted testosterone and fasting insulin, with the latter being an expression of insulin resistance, were found to be associated with CKD and worse kidney function in men, thus highlighting the possible reasons for discrepancy in CKD prevalence and CKD progression among men and women [84,85][80][81]. Intriguingly, a genome-wide association study of UK biobank showed that albumin-to-creatinine ratio (ACR) is dependent on multiple pathways and that an ACR genetic risk score may improve the prediction of hypertension and stroke [86][82].4. Predictive Biomarkers in CKD
Predictive biomarkers are used in disparate fields of medicine to assess the likelihood of response to treatments and the individual pathophysiology of the disease. One major example of this strategy is represented by the large use of predictive biomarkers in oncology. Causative mutations of the breast cancer genes 1 and 2 (BRCA1/2) were found to be predictive biomarkers for identifying the response to poly(ADP-ribose) polymerase (PARP) inhibitors [87][83]. Such a discovery is crucial as BRCA1/2 provide information on the best drug for the individual patient in order to improve their prognosis. While, in oncology, a set of pathophysiological mechanisms is crucial for tumor development, what complicates the application of predictive biomarkers in chronic diseases is that different mechanisms are active in different stages of the disease itself and in different patients [88][84]. This means that, if a treatment is started on the basis of a blood/urine biomarker level, the individual prognosis may remain unchanged or even worsen, due to the presence of other active mechanisms of damage, as well as, most importantly, different disease entities that cause the chronic decline of renal function through diverse pathophysiological pathways. Notwithstanding, in chronic disease, great research effort was also started with the aim of personalizing treatments following the methodological concept of “the right drug for the right patient”. Hence, the implementation of predictive biomarkers represents a topic of increasing importance.4.1. Kidney Biomarkers
In nephrology, the most used predictive biomarkers are eGFR and albuminuria. Both these biomarkers can be considered as “dynamic” predictive biomarkers. In fact, their levels change over time with the effects of treatment, such that they can be efficiently used for monitoring the course of CKD and the appropriateness of the therapy followed by the patient. In the past few decades, several interventional studies were carried out testing the effect of nephroprotective drugs on hard endpoints such as mortality, CV events, and ESKD in patients with CKD [89,90,91,92,93,94,95,96][85][86][87][88][89][90][91][92]. Although interventions differed between studies, with principally antihypertensive drugs and albuminuria-lowering agents being tested, all these trials pointed out that the CV, mortality, and ESKD risk reductions were strictly associated with a reduction in albuminuria after the start of treatment. Moreover, the magnitude of treatment effect was greater in patients with higher albuminuria levels at the time of the initial visit [95,96][91][92]. These findings are reinforced by the evidence that albuminuria changes also played a potentially beneficial role in negative clinical trials. In the Aliskiren Trial in Type 2 Diabetes Using Cardiorenal Endpoints (ALTITUDE), which failed in demonstrating the advantage of adding Aliskiren to an angiotensin-converting enzyme inhibitor (ACEi) or an angiotensin-receptor blocker (ARB) on CV and renal outcomes, patients who showed an albuminuria reduction in the Aliskiren arm (37%) were largely protected against CKD progression compared with those who did not show a reduction in albuminuria levels [97][93]. All these pieces of evidence testified that albuminuria has great predictive and prognostic power in CKD patients and, although further studies are needed to find the correct threshold of albuminuria reduction that can confer CV and renal risk protection after an appropriate treatment, there is a general consensus that a 30% reduction in its levels from baseline to six months could be acceptable [98][94]. With respect to eGFR, it was demonstrated that a doubling of serum creatinine level, which corresponds approximately to a 57% eGFR decline, was able to predict CKD progression in previous clinical trials in diabetic CKD patients [99][95]. The importance of that evidence is highlighted by the fact that, in these previous trials, eGFR decline correlated with renal outcomes after exposure to nephroprotective treatments, thus affirming its role as a predictive biomarker, in addition to a prognostic biomarker [100][96]. Since then, the association of lesser eGFR declines with CKD outcomes was tested. A post hoc analysis of the Reduction of End Points in Non-Insulin-Dependent Diabetes with the Angiotensin II Antagonist Losartan (RENAAL) and Irbesartan Diabetic Nephropathy Trial (IDNT) clinical trials, two studies that evaluated the efficacy of ARB treatment in patients with diabetes mellitus and nephropathy, showed that 30% and 40% eGFR declines may improve the power of clinical trials if the drug investigated does not determine an acute (within three months of the start of treatment) drop in eGFR [101][97]. A larger meta-analysis of 37 clinical trials in CKD patients documented a strong association between 30% and 40% eGFR decline in the first 12 months of treatment and the onset of kidney disease progression [100][96].4.2. Biomarkers of Tissue Remodeling
In addition to proteinuria and eGFR, other promising predictive biomarkers in CKD were described. A change in serum levels of MMPs after exposure to BB-1101, a synthetic hydroxamic acid-based inhibitor of MMP, was associated with a reduction in proteinuria in experimental models of glomerular damage [102][98]. A similar effect was observed in diabetic CKD patients who underwent treatment with doxycycline, an antibiotic from the tetracycline family, and who were already treated with renin–angiotensin–aldosterone inhibitors (RAAS-i) [103][99]. Moreover, MMPs are also involved in the mechanism that leads to CV risk reduction exerted by sodium–glucose cotransporter 2 inhibitors (SGLT2-i) through the activation of RECK (reversion-inducing cysteine-rich protein with kazal motifs), an endogenous inhibitor of MMPs [104][100]. That mechanism appeared to be independent of proteinuria levels and could also be useful for selecting high-risk normoalbuminuric CKD patients to be enrolled in future clinical trials, who represent a non-trivial proportion of the CKD cohort [21][12].4.3. Ultrasound Biomarkers
Evidence is also emerging for a possible role of the renal resistive index (RRI) as a dynamic predictive biomarker. RRI is a Doppler ultrasonographic index, whose increase reflects both renal and systemic vascular impairment [105][101]. RRI was also found to predict the onset of CV and kidney outcomes in patients with CKD or essential hypertension [106,107][102][103]. RRI values are changed over time by different drug classes, such as RAAS-i and SGLT2-i; novel studies will hopefully reveal in the future if these treatment-induced modifications could also predict hard CV and renal endpoints [108,109][104][105].4.4. Predictive Role of Proteomics, Metabolomics, and Genomics
A polymorphism of the angiotensin-converting enzyme gene caused by an insertion/deletion (ACE/ID) modifies the systemic and renal activity of the RAAS, which was recognized to be a trigger of kidney damage [110][106]. The ACE/DD–ACE/ID polymorphism was able to predict the response to losartan in type 2 diabetic patients enrolled in the RENAAL trial, that is, patients with worse prognosis (D allele carriers) had the best response to losartan [111][107]. Complex biomarkers and classifiers have a predictive role, in addition to a prognostic role. A set of 21 serum metabolites were selected from a larger panel through a penalized regression analysis, and they were shown to correctly predict the albuminuria response to ARB treatment in type 2 diabetic patients [112][108]. This classifier revealed that the enzyme nitric oxide synthase 3 (NOS3) is crucial to forecast the response to ARB therapy in diabetic CKD, since it is involved in the molecular mechanism of action of these drugs. A proteomic predictive classifier was developed from the Prevention of REnal and Vascular ENd-stage Disease (PREVEND) study, using plasma proteomics profiles of fibrosis and kidney damage that allowed predicting the albuminuria change in patients treated with RAAS-i [113][109].References
- FDA-NIH Biomarker Working Group. BEST (Biomarkers, Endpoints, and other Tools) Resource; Food and Drug Administration (US): Silver Spring, MD, USA; National Institutes of Health (US): Bethesda, MD, USA, 2020. Available online: www.ncbi.nlm.nih.gov/books/NBK326791 (accessed on 25 June 2020).
- Pepe, M.; Janes, H. Methods for Evaluating Prediction Performance of Biomarkers and Tests. In Risk Assessment and Evaluation of Predictions; Lecture Notes in Statistics; Lee, M.L., Gail, M., Pfeiffer, R., Satten, G., Cai, T., Gandy, A., Eds.; Springer: New York, NY, USA, 2013; Volume 215.
- Devarajan, P.; Williams, L.M. Proteomics for biomarker discovery in acute kidney injury. Semin. Nephrol. 2007, 27, 637–651.
- Simon, R. Development and validation of biomarker classifiers for treatment selection. J. Stat. Plan. Inference 2008, 138, 308–320.
- Provenzano, M.; Coppolino, G.; De Nicola, L.; Serra, R.; Garofalo, C.; Andreucci, M.; Bolignano, D. Unraveling cardiovascular risk in renal patients: A new take on old tale. Front. Cell Dev. Biol. 2019, 7, 314.
- Provenzano, M.; Coppolino, G.; Faga, T.; Garofalo, C.; Serra, R.; Andreucci, M. Epidemiology of cardiovascular risk in chronic kidney disease patients: The real silent killer. Rev. Cardiovasc. Med. 2019, 20, 209–220.
- Kidney Disease Improving Global Outcomes Work Group. CVD, medication dosage, patient safety, infections, hospitalizations, and caveats for investigating complications of CKD. Kidney Int. Suppl. 2013, 3, 91–111.
- Xie, Y.; Bowe, B.; Mokdad, A.H.; Xian, H.; Yan, Y.; Li, T.; Maddukuri, G.; Tsai, C.-Y.; Floyd, T.; Al Aly, Z. Analysis of the Global Burden of Disease study highlights the global, regional, and national trends of chronic kidney disease epidemiology from 1990 to 2016. Kidney Int. 2018, 94, 567–581.
- Bikbov, B.T.; A Purcell, C.; Levey, A.S.; Smith, M.; Abdoli, A.; Abebe, M.; Adebayo, O.M.; Afarideh, M.; Agarwal, S.K.; Agudelo-Botero, M.; et al. Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733.
- IOM (Institute of Medicine). Evaluation of Biomarkers and Surrogate Endpoints in Chronic Disease; The National Academies Press: Washington, DC, USA, 2010.
- Serra, R.; Ielapi, N.; Barbetta, A.; Andreucci, M.; De Franciscis, S. Novel biomarkers for cardiovascular risk. Biomark. Med. 2018, 12, 1015–1024.
- De Nicola, L.; Provenzano, M.; Chiodini, P.; Borrelli, S.; Russo, L.; Bellasi, A.; Santoro, D.; Conte, G.; Minutolo, R. Epidemiology of low-proteinuric chronic kidney disease in renal clinics. PLoS ONE 2017, 12, e0172241.
- Minutolo, R.; Gabbai, F.B.; Chiodini, P.; Garofalo, C.; Stanzione, G.; Liberti, M.E.; Pacilio, M.; Borrelli, S.; Provenzano, M.; Conte, G.; et al. Reassessment of Ambulatory blood pressure improves renal risk stratification in nondialysis chronic kidney disease. Hypertension 2015, 66, 557–562.
- Astor, B.C.; Matsushita, K.; Gansevoort, R.T.; Van Der Velde, M.; Woodward, M.; Levey, A.S.; De Jong, P.E.; Coresh, J. Lower estimated glomerular filtration rate and higher albuminuria are associated with mortality and end-stage renal disease. A collaborative meta-analysis of kidney disease population cohorts. Kidney Int. 2011, 79, 1331–1340.
- Gansevoort, R.T.; Matsushita, K.; Van Der Velde, M.; Astor, B.C.; Woodward, M.; Levey, A.S.; De Jong, P.E.; Coresh, J. Chronic kidney disease prognosis consortium lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes. A collaborative meta-analysis of general and high-risk population cohorts. Kidney Int. 2011, 80, 93–104.
- Matsushita, K.; Coresh, J.; Sang, Y.; Chalmers, J.P.; Fox, C.; Guallar, E.; Jafar, T.; Jassal, S.K.; Landman, G.W.; Muntner, P.; et al. Estimated glomerular filtration rate and albuminuria for prediction of cardiovascular outcomes: A collaborative meta-analysis of individual participant data. Lancet Diabetes Endocrinol. 2015, 3, 514–525.
- Tangri, N.; Stevens, L.A.; Griffith, J.; Tighiouart, H.; Djurdjev, O.; Naimark, D.M.; Levin, A.; Levey, A.S. A predictive model for progression of chronic kidney disease to kidney failure. JAMA 2011, 305, 1553–1559.
- Provenzano, M.; Chiodini, P.; Minutolo, R.; Zoccali, C.; Bellizzi, V.; Conte, G.; Locatelli, F.; Tripepi, G.; Del Vecchio, L.; Mallamaci, F.; et al. Reclassification of chronic kidney disease patients for end-stage renal disease risk by proteinuria indexed to estimated glomerular filtration rate: Multicentre prospective study in nephrology clinics. Nephrol. Dial. Transplant. 2018, 35.
- Schroeder, E.B.; Yang, X.; Thorp, M.L.; Arnold, B.M.; Tabano, D.; Petrik, A.F.; Smith, D.H.; Platt, R.W.; Johnson, E.S. Predicting 5-Year Risk of RRT in Stage 3 or 4 CKD: Development and external validation. Clin. J. Am. Soc. Nephrol. 2016, 12, 87–94.
- Grams, M.E.; Sang, Y.; Ballew, S.H. Predicting timing of clinical outcomes in patients with chronic kidney disease and severely decreased glomerular filtration rate. Kidney Int. 2018, 94, 1025–1026.
- De Nicola, L.; Provenzano, M.; Chiodini, P.; Borrelli, S.; Garofalo, C.; Pacilio, M.; Liberti, M.E.; Sagliocca, A.; Conte, G.; Minutolo, R. Independent role of underlying kidney disease on renal prognosis of patients with chronic kidney disease under nephrology care. PLoS ONE 2015, 10, e0127071.
- Haynes, R.; Staplin, N.; Emberson, J.R.; Herrington, W.G.; Tomson, C.; Agodoa, L.; Tesař, V.; Levin, A.; Lewis, D.; Reith, C.; et al. Evaluating the contribution of the cause of kidney disease to prognosis in CKD: Results from the Study of Heart and Renal Protection (SHARP). Am. J. Kidney Dis. 2014, 64, 40–48.
- Abbate, M.; Zoja, C.; Remuzzi, G. How does proteinuria cause progressive renal damage? J. Am. Soc. Nephrol. 2006, 17, 2974–2984.
- Provenzano, M.; Garofalo, C.; Chiodini, P. Ruolo della proteinuria nella ricerca clinica: Per ogni vecchia risposta, una nuova domanda . Recenti Prog Med. 2020, 111, 74–81.
- Nakano, T.; Ninomiya, T.; Sumiyoshi, S.; Fujii, H.; Doi, Y.; Hirakata, H.; Tsuruya, K.; Iida, M.; Kiyohara, Y.; Sueishi, K. Association of kidney function with coronary atherosclerosis and calcification in autopsy samples from Japanese elders: The Hisayama study. Am. J. Kidney Dis. 2010, 55, 21–30.
- Pun, P.H.; Smarz, T.R.; Honeycutt, E.F.; Shaw, L.K.; Al-Khatib, S.M.; Middleton, J.P. Chronic kidney disease is associated with increased risk of sudden cardiac death among patients with coronary artery disease. Kidney Int. 2009, 76, 652–658.
- Deo, R.; Lin, F.; Vittinghoff, E.; Tseng, Z.H.; Hulley, S.B.; Shlipak, M.G. Kidney dysfunction and sudden cardiac death among women with coronary heart disease. Hypertension 2008, 51, 1578–1582.
- Deo, R.; Sotoodehnia, N.; Katz, R.; Sarnak, M.J.; Fried, L.F.; Chonchol, M.; Kestenbaum, B.; Psaty, B.M.; Siscovick, D.S.; Shlipak, M.G. Cystatin C and sudden cardiac death risk in the elderly. Circ. Cardiovasc. Qual. Outcomes 2010, 3, 159–164.
- Meier, P.; Vogt, P.; Blanc, E. Ventricular arrhythmias and sudden cardiac death in end-stage renaldisease patients on chronic hemodialysis. Nephron 2001, 87, 199–214.
- Van Acker, B.A.C.; Stroomer, M.K.J.; Gosselink, M.A.H.E.; Koomen, G.C.M.; Koopman, M.G.; Arisz, L. Urinary protein excretion in normal individuals: Diurnal changes, influence of orthostasis and relationship to the renin-angiotensin system. Contrib. Nephrol. 1993, 101, 143–150.
- Inker, L.A.; Astor, B.C.; Fox, C.H.; Isakova, T.; Lash, J.P.; Peralta, C.A.; Tamura, M.K.; Feldman, H.I. KDOQI US commentary on the 2012 KDIGO Clinical Practice Guideline for the Evaluation and Management of CKD. Am. J. Kidney Dis. 2014, 63, 713–735.
- Glassock, R.J. Con: Thresholds to define chronic kidney disease should not be age dependent. Nephrol. Dial. Transplant. 2014, 29, 774–779.
- Fotheringham, J.; Odudu, A.; McKane, W.; Ellam, T. Modification of the relationship between blood pressure and renal albumin permeability by impaired excretory function and diabetes. Hypertension 2015, 65, 510–516.
- Willows, J.; Odudu, A.; Logan, I.; Sheerin, N.; Tomson, C.; Ellam, T. changing protein permeability with nephron loss: Evidence for a human remnant nephron effect. Am. J. Nephrol. 2019, 50, 152–159.
- Mallamaci, F.; Tripepi, G.; D’Arrigo, G.; Borrelli, S.; Garofalo, C.; Stanzione, G.; Provenzano, M.; De Nicola, L.; Conte, G.; Minutolo, R.; et al. Blood pressure variability, mortality, and cardiovascular outcomes in CKD patients. Clin. J. Am. Soc. Nephrol. 2019, 14, 233–240.
- Zhou, T.L.; Rensma, S.P.; Van Der Heide, F.C.; Henry, R.M.; Kroon, A.A.; Houben, A.J.; Jansen, J.F.; Backes, W.H.; Berendschot, T.T.; Schouten, J.S.; et al. Blood pressure variability and microvascular dysfunction. J. Hypertens. 2020, 38.
- Pena, M.J.; Stenvinkel, P.; Kretzler, M.; Adu, D.; Agarwal, S.K.; Coresh, J.; Feldman, H.I.; Fogo, A.B.; Gansevoort, R.T.; Harris, D.C.; et al. Strategies to improve monitoring disease progression, assessing cardiovascular risk, and defining prognostic biomarkers in chronic kidney disease. Kidney Int. Suppl. 2017, 7, 107–113.
- Xu, G.; Luo, K.; Liu, H.; Huang, T.; Fang, X.; Tu, W. The progress of inflammation and oxidative stress in patients with chronic kidney disease. Ren. Fail. 2014, 37, 45–49.
- Ismael, F.O.; Proudfoot, J.M.; Brown, B.E.; Van Reyk, D.M.; Croft, K.D.; Davies, M.J.; Hawkins, C.L. Comparative reactivity of the myeloperoxidase-derived oxidants HOCl and HOSCN with low-density lipoprotein (LDL): Implications for foam cell formation in atherosclerosis. Arch. Biochem. Biophys. 2015, 573, 40–51.
- Correa, S.; Pena-Esparragoza, J.K.; Scovner, K.M.; Waikar, S.S.; Mc Causland, F.R. Myeloperoxidase and the risk of CKD Progression, cardiovascular disease, and death in the Chronic Renal Insufficiency Cohort (CRIC) Study. Am. J. Kidney Dis. 2020, 76, 32–41.
- Provenzano, M.; Andreucci, M.; Garofalo, C.; Faga, T.; Michael, A.; Ielapi, N.; Grande, R.; Sapienza, P.; De Franciscis, S.; Mastroroberto, P.; et al. The association of matrix metalloproteinases with chronic kidney disease and peripheral vascular disease: A light at the end of the tunnel? Biomolecules 2020, 10, 154.
- Butrico, L.; Barbetta, A.; Ciranni, S.; Andreucci, M.; Mastroroberto, P.; De Franciscis, S. Role of metalloproteinases and their inhibitors in the development of abdominal aortic aneurysm: Current insights and systematic review of the literature. Chirurgia 2017, 30, 151–159.
- Lauhio, A.; Sorsa, T.; Srinivas, R.; Stenman, M.; Tervahartiala, T.; Stenman, U.-H.; Grönhagen-Riska, C.; Honkanen, E. Urinary matrix metalloproteinase -8, -9, -14 and their regulators (TRY-1, TRY-2, TATI) in patients with diabetic nephropathy. Ann. Med. 2008, 40, 312–320.
- Tashiro, K.; Koyanagi, I.; Ohara, I.; Ito, T.; Saitoh, A.; Horikoshi, S.; Tomino, Y. Levels of urinary matrix metalloproteinase-9 (MMP-9) and renal injuries in patients with type 2 diabetic nephropathy. J. Clin. Lab. Anal. 2004, 18, 206–210.
- Lieb, W.; Song, R.J.; Xanthakis, V.; Vasan, R.S. Association of circulating tissue inhibitor of Metalloproteinases-1 and Procollagen type III Aminoterminal peptide levels with incident heart failure and chronic kidney disease. J. Am. Hear. Assoc. 2019, 8, e011426.
- Forough, R.; Koyama, N.; Hasenstab, D.; Lea, H.; Clowes, M.; Nikkari, S.T.; Clowes, A.W. Overexpression of tissue inhibitor of matrix metalloproteinase-1 inhibits vascular smooth muscle cell functions In Vitro and In Vivo. Circ. Res. 1996, 79, 812–820.
- Newman, K.M.; Jean-Claude, J.; Li, H.; Scholes, J.V.; Ogata, Y.; Nagase, H.; Tilson, M. Cellular localization of matrix metalloproteinases in the abdominal aortic aneurysm wall. J. Vasc. Surg. 1994, 20, 814–820.
- Scialla, J.J.; Xie, H.; Rahman, M.; Anderson, A.H.; Isakova, T.; Ojo, A.; Zhang, X.; Nessel, L.; Hamano, T.; Grunwald, J.E.; et al. Fibroblast Growth Factor-23 and cardiovascular events in CKD. J. Am. Soc. Nephrol. 2013, 25, 349–360.
- Isakova, T.; Xie, H.; Yang, W.; Xie, D.; Anderson, A.H.; Scialla, J.J.; Wahl, P.; Gutiérrez, O.M.; Steigerwalt, S.; He, J.; et al. Fibroblast Growth Factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA 2011, 305, 2432–2439.
- Defilippi, C.R.; De Lemos, J.A.; Christenson, R.H.; Gottdiener, J.S.; Kop, W.J.; Zhan, M.; Seliger, S. Association of serial measures of cardiac troponin T using a sensitive assay with incident heart failure and cardiovascular mortality in older adults. JAMA 2010, 304, 2494–2502.
- Ballew, S.H.; Matsushita, K. Cardiovascular risk prediction in CKD. Semin. Nephrol. 2018, 38, 208–216.
- Srisawasdi, P.; Vanavanan, S.; Charoenpanichkit, C.; Kroll, M.H. The Effect of renal dysfunction on BNP, NT-proBNP, and their ratio. Am. J. Clin. Pathol. 2010, 133, 14–23.
- Matsushita, K.; Ballew, S.H.; Coresh, J. Cardiovascular risk prediction in people with chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 2016, 25, 518–523.
- Rangaswami, J.; Bhalla, V.; Blair, J.E.; Chang, T.I.; Costa, S.; Lentine, K.L.; Lerma, E.V.; Mezue, K.; Molitch, M.; Mullens, W.; et al. Cardiorenal syndrome: Classification, pathophysiology, diagnosis, and treatment strategies: A scientific statement from the american heart association. Circulation 2019, 139, e840–e878.
- Saunders, J.T.; Nambi, V.; De Lemos, J.A.; Chambless, L.E.; Virani, S.S.; Boerwinkle, E.; Hoogeveen, R.C.; Liu, X.; Astor, B.C.; Mosley, T.H.; et al. Cardiac troponin T measured by a highly sensitive assay predicts coronary heart disease, heart failure, and mortality in the Atherosclerosis Risk in Communities study. Circulation 2011, 123, 1367–1376.
- Agarwal, S.K.; Chambless, L.E.; Ballantyne, C.M.; Astor, B.; Bertoni, A.G.; Chang, P.P.; Folsom, A.R.; He, M.; Hoogeveen, R.C.; Ni, H.; et al. Prediction of incident heart failure in general practice: The Atherosclerosis Risk in Communities (ARIC) study. Circ. Hear. Fail. 2012, 5, 422–429.
- Matsushita, K.; Sang, Y.; Ballew, S.H.; Astor, B.C.; Hoogeveen, R.C.; Solomon, S.D.; Ballantyne, C.M.; Woodward, M.; Coresh, J. Cardiac and kidney markers for cardiovascular prediction in individuals with chronic kidney disease. Arter. Thromb. Vasc. Boil. 2014, 34, 1770–1777.
- Bansal, N.; Zelnick, L.; Shlipak, M.G.; Anderson, A.; Christenson, R.; Deo, R.; Defilippi, C.; Feldman, H.; Lash, J.; He, J.; et al. Cardiac and stress biomarkers and chronic kidney disease progression: The CRIC study. Clin. Chem. 2019, 65, 1448–1457.
- Ho, J.E.; Hwang, S.-J.; Wollert, K.C.; Larson, M.G.; Cheng, S.; Kempf, T.; Vasan, R.S.; Januzzi, J.L.; Wang, T.J.; Fox, C.S. Biomarkers of cardiovascular stress and incident chronic kidney disease. Clin. Chem. 2013, 59, 1613–1620.
- Shlipak, M.G.; Matsushita, K.; Ärnlöv, J.; Inker, L.A.; Katz, R.; Polkinghorne, K.R.; Rothenbacher, D.; Sarnak, M.J.; Astor, B.C.; Coresh, J.; et al. Cystatin C versus creatinine in determining risk based on kidney function. N. Engl. J. Med. 2013, 369, 932–943.
- Foster, M.C.; Coresh, J.; Hsu, C.-Y.; Xie, D.; Levey, A.S.; Nelson, R.G.; Eckfeldt, J.H.; Vasan, R.S.; Kimmel, P.L.; Schelling, J.; et al. Serum β-trace protein and β2-microglobulin as predictors of ESRD, mortality, and cardiovascular disease in adults with CKD in the Chronic Renal Insufficiency Cohort (CRIC) study. Am. J. Kidney Dis. 2016, 68, 68–76.
- Ix, J.H.; Katz, R.; Bansal, N.; Foster, M.; Weiner, D.E.; Tracy, R.P.; Jotwani, V.; Hughes-Austin, J.; McKay, D.; Gabbai, F.; et al. Urine fibrosis markers and risk of allograft failure in kidney transplant recipients: A Case-Cohort Ancillary Study of the FAVORIT Trial. Am. J. Kidney Dis. 2017, 69, 410–419.
- Puthumana, J.; Hall, I.E.; Reese, P.P.; Schroppel, B.; Weng, F.L.; Thiessen-Philbrook, H.; Doshi, M.D.; Rao, V.; Lee, C.G.; Elias, J.A.; et al. YKL-40 associates with renal recovery in deceased donor kidney transplantation. J. Am. Soc. Nephrol. 2017, 28, 661–670.
- Malhotra, R.; Katz, R.; Jotwani, V.; Ambrosius, W.T.; Raphael, K.L.; Haley, W.; Rastogi, A.; Cheung, A.K.; Freedman, B.I.; Punzi, H.; et al. Urine markers of kidney tubule cell injury and kidney function decline in SPRINT Trial participants with CKD. Clin. J. Am. Soc. Nephrol. 2020, 15, 349–358.
- Sun, L.; Zou, L.-X.; Chen, M.-J. Make precision medicine work for chronic kidney disease. Med Princ. Pr. 2016, 26, 101–107.
- Serra, R.; Ssempijja, L.; Provenzano, M.; Andreucci, M. Genetic biomarkers in chronic venous disease. Biomark Med. 2020, 14, 75–80.
- Serra, R.; Ielapi, N.; Barbetta, A.; Buffone, G.; Bevacqua, E.; Andreucci, M. Biomarkers for precision medicine in phlebology and wound care: A systematic review. Acta. Phlebol. 2017, 18, 52–56.
- Good, D.M.; Zürbig, P.; Argilés, A.; Bauer, H.W.; Behrens, G.; Coon, J.J.; Dakna, M.; Decramer, S.; Delles, C.; Dominiczak, A.F.; et al. Naturally occurring human urinary peptides for use in diagnosis of chronic kidney disease. Mol. Cell. Proteom. 2010, 9, 2424–2437.
- Argiles, A.; Siwy, J.; Duranton, F.; Gayrard, N.; Dakna, M.; Lundin, U.; Osaba, L.; Delles, C.; Mourad, G.; Weinberger, K.M.; et al. A new proteomics classifier assessing CKD and its prognosis. PLoS ONE 2013, 8, e62837.
- Roscioni, S.S.; De Zeeuw, D.; Hellemons, M.E.; Mischak, H.; Zürbig, P.; Bakker, S.J.L.; Gansevoort, R.T.; Reinhard, H.; Persson, F.; Lajer, M.; et al. A urinary peptide biomarker set predicts worsening of albuminuria in type 2 diabetes mellitus. Diabetologia 2012, 56, 259–267.
- Schanstra, J.P. Diagnosis and prediction of progression of chronic kidney disease by assessment of urinary peptides. J. Am. Soc. Nephrol. 2014, 26, 1999–2010.
- Kwan, B.; Fuhrer, T.; Zhang, J.; Darshi, M.; Van Espen, B.; Montemayor, D.; De Boer, I.H.; Dobre, M.; Hsu, C.-Y.; Kelly, T.N.; et al. Metabolomic markers of kidney function decline in patients with diabetes: Evidence From the Chronic Renal Insufficiency Cohort (CRIC) study. Am. J. Kidney Dis. 2020.
- Connaughton, D.M.; Hildebrandt, F. Personalized medicine in chronic kidney disease by detection of monogenic mutations. Nephrol. Dial. Transplant. 2019, 35, 390–397.
- Thio, C.H.; Van Der Most, P.J.; Nolte, I.M.; Van Der Harst, P.; Bültmann, U.; Gansevoort, R.T.; Snieder, H. Evaluation of a genetic risk score based on creatinine-estimated glomerular filtration rate and its association with kidney outcomes. Nephrol. Dial. Transplant. 2017, 33, 1757–1764.
- Köttgen, A.; Hwang, S.-J.; Larson, M.G.; Van Eyk, J.E.; Fu, Q.; Benjamin, E.J.; Dehghan, A.; Glazer, N.L.; Kao, W.L.; Harris, T.B.; et al. Uromodulin levels associate with a common UMOD variant and risk for incident CKD. J. Am. Soc. Nephrol. 2009, 21, 337–344.
- Böger, C.A.; Gorski, M.; Li, M.; Hoffmann, M.M.; Huang, C.; Yang, Q.; Teumer, A.; Krane, V.; O’Seaghdha, C.M.; Kutalik, Z.; et al. Association of eGFR-related loci identified by GWAS with incident CKD and ESRD. PLoS Genet. 2011, 7, e1002292.
- Pays, E.; Vanhollebeke, B. Human innate immunity against African trypanosomes. Curr. Opin. Immunol. 2009, 21, 493–498.
- Parsa, A.; Kao, W.L.; Xie, D.; Astor, B.C.; Li, M.; Hsu, C.-Y.; Feldman, H.I.; Parekh, R.S.; Kusek, J.W.; Greene, T.H.; et al. APOL1 risk variants, race, and progression of chronic kidney disease. N. Engl. J. Med. 2013, 369, 2183–2196.
- Sudlow, C.; Gallacher, J.; Allen, N.; Beral, V.; Burton, P.; Danesh, J.; Downey, P.; Elliott, P.; Green, J.; Landray, M.; et al. UK Biobank: An open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015, 12, e1001779.
- Zhao, J.; Schooling, C.M. Sex-specific associations of insulin resistance with chronic kidney disease and kidney function: A bi-directional Mendelian randomisation study. Diabetologia 2020, 63, 1–10.
- Zhao, J.; Schooling, C.M. The role of testosterone in chronic kidney disease and kidney function in men and women: A bi-directional Mendelian randomization study in the UK Biobank. BMC Med. 2020, 18, 122.
- Casanova, F.; Tyrrell, J.; Beaumont, R.N.; Ji, Y.; Jones, S.E.; Hattersley, A.T.; Weedon, M.N.; Murray, A.; Shore, A.C.; Frayling, T.M.; et al. A genome-wide association study implicates multiple mechanisms influencing raised urinary albumin–creatinine ratio. Hum. Mol. Genet. 2019, 28, 4197–4207.
- Ledermann, J.A.; Harter, P.; Gourley, C.; Friedlander, M.; Vergote, I.; Rustin, G.; Scott, C.; Meier, W.; Shapira-Frommer, R.; Safra, T.; et al. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N. Engl. J. Med. 2012, 366, 1382–1392.
- Perco, P.; Pena, M.; Heerspink, H.J.; Mayer, G. Multimarker panels in diabetic kidney disease: The way to improved clinical trial design and clinical practice? Kidney Int. Rep. 2018, 4, 212–221.
- Brenner, B.M.; Cooper, M.E.; De Zeeuw, D.; Keane, W.F.; Mitch, W.E.; Parving, H.-H.; Remuzzi, G.; Snapinn, S.M.; Zhang, Z.; Shahinfar, S. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N. Engl. J. Med. 2001, 345, 861–869.
- The GISEN Group (Gruppo Italiano di Studi Epidemiologici in Nefrologia). Randomised placebo-controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non-diabetic nephropathy. Lancet 1997, 349, 1857–1863.
- Heerspink, H.J.L.; Ninomiya, T.; Perkovic, V.; Woodward, M.; Zoungas, S.; Cass, A.; Cooper, M.; Grobbee, D.E.; Mancia, G.; Mogensen, C.E.; et al. Effects of a fixed combination of perindopril and indapamide in patients with type 2 diabetes and chronic kidney disease. Eur. Hear. J. 2010, 31, 2888–2896.
- Perkovic, V.; Jardine, M.J.; Neal, B.; Bompoint, S.; Heerspink, H.J.; Charytan, D.M.; Edwards, R.; Agarwal, R.; Bakris, G.; Bull, S.; et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N. Engl. J. Med. 2019, 380, 2295–2306.
- Wanner, C.; E Inzucchi, S.; Lachin, J.M.; Fitchett, D.; Von Eynatten, M.; Mattheus, M.; Johansen, O.E.; Woerle, H.J.; Broedl, U.C.; Zinman, B. Empagliflozin and progression of kidney disease in type 2 diabetes. N. Engl. J. Med. 2016, 375, 323–334.
- Heerspink, H.J.L.; Parving, H.H.; Andress, D.L. SONAR Committees and Investigators. Atrasentan and renal events in patients with type 2 diabetes and chronic kidney disease (SONAR): A double-blind, randomised, placebo-controlled trial. Lancet 2019, 393, 1937–1947.
- Perkovic, V.; Ninomiya, T.; Arima, H.; Gallagher, M.; Jardine, M.; Cass, A.; Neal, B.; MacMahon, S.; Chalmers, J.P. Chronic kidney disease, cardiovascular events, and the effects of perindopril-based blood pressure lowering: Data from the PROGRESS study. J. Am. Soc. Nephrol. 2007, 18, 2766–2772.
- De Zeeuw, D.; Remuzzi, G.; Parving, H.-H.; Keane, W.F.; Zhang, Z.; Shahinfar, S.; Snapinn, S.; Cooper, M.E.; Mitch, W.E.; Brenner, B.M. Albuminuria, a therapeutic target for cardiovascular protection in type 2 diabetic patients with nephropathy. Circulation 2004, 110, 921–927.
- Heerspink, H.J.L.; Ninomiya, T.; Persson, F.; Brenner, B.M.; Brunel, P.; Chaturvedi, N.; Desai, A.S.; Haffner, S.M.; McMurray, J.J.; Solomon, S.D.; et al. Is a reduction in albuminuria associated with renal and cardiovascular protection? A post hoc analysis of the ALTITUDE trial. Diabetes Obes. Metab. 2016, 18, 169–177.
- Heerspink, H.J.L.; Gansevoort, R.T. Albuminuria is an appropriate therapeutic target in patients with CKD: The pro view. Clin. J. Am. Soc. Nephrol. 2015, 10, 1079–1088.
- Levey, A.S.; Inker, L.A.; Matsushita, K.; Greene, T.; Willis, K.; Lewis, E.; De Zeeuw, D.; Cheung, A.K.; Coresh, J. GFR decline as an end point for clinical trials in CKD: A scientific workshop sponsored by the National Kidney Foundation and the US Food and Drug Administration. Am. J. Kidney Dis. 2014, 64, 821–835.
- Heerspink, H.J.L.; Tighiouart, H.; Sang, Y.; Ballew, S.H.; Mondal, H.; Matsushita, K.; Coresh, J.; Levey, A.S.; Inker, L.A. GFR decline and subsequent risk of established kidney outcomes: A Meta-analysis of 37 Randomized Controlled Trials. Am. J. Kidney Dis. 2014, 64, 860–866.
- Heerspink, H.J.L.; Weldegiorgis, M.; Inker, L.A.; Gansevoort, R.; Parving, H.-H.; Dwyer, J.P.; Mondal, H.; Coresh, J.; Greene, T.; Levey, A.S.; et al. Estimated GFR decline as a surrogate end point for kidney failure: A post hoc analysis from the reduction of end points in non–insulin-dependent diabetes with the Angiotensin II Antagonist Losartan (RENAAL) Study and Irbesartan Diabetic Nephropathy Trial (IDNT). Am. J. Kidney Dis. 2014, 63, 244–250.
- Steinmann-Niggli, K.; Ziswiler, R.; Küng, M.; Marti, H.P. Inhibition of matrix metalloproteinases attenuates anti-Thy1.1 nephritis. J. Am. Soc. Nephrol. 1998, 9, 397–407.
- Aggarwal, H.K.; Jain, D.; Talapatra, P.; Yadav, R.K.; Gupta, T.; Kathuria, K.L. Evaluation of role of doxycycline (a matrix metalloproteinase inhibitor) on renal functions in patients of diabetic nephropathy. Ren. Fail. 2010, 32, 941–946.
- Das, N.A.; Carpenter, A.J.; Belenchia, A.; Aroor, A.R.; Noda, M.; Siebenlist, U.; Chandrasekar, B.; Demarco, V.G. Empagliflozin reduces high glucose-induced oxidative stress and miR-21-dependent TRAF3IP2 induction and RECK suppression, and inhibits human renal proximal tubular epithelial cell migration and epithelial-to-mesenchymal transition. Cell. Signal. 2020, 68, 109506.
- Provenzano, M.; Rivoli, L.; Garofalo, C.; Faga, T.; Pelagi, E.; Perticone, M.; Serra, R.; Michael, A.; Comi, N.; Andreucci, M. Renal resistive index in chronic kidney disease patients: Possible determinants and risk profile. PLoS ONE 2020, 15, e0230020.
- Doi, Y.; Iwashima, Y.; Yoshihara, F.; Kamide, K.; Hayashi, S.-I.; Kubota, Y.; Nakamura, S.; Horio, T.; Kawano, Y. Response to renal resistive index and cardiovascular and renal outcomes in essential hypertension. Hypertension 2013, 61, e23.
- Sugiura, T.; Wada, A. Resistive index predicts renal prognosis in chronic kidney disease. Nephrol. Dial. Transplant. 2009, 24, 2780–2785.
- Solini, A.; Giannini, L.; Seghieri, M.; Vitolo, E.; Taddei, S.; Ghiadoni, L.; Bruno, R.M. Dapagliflozin acutely improves endothelial dysfunction, reduces aortic stiffness and renal resistive index in type 2 diabetic patients: A pilot study. Cardiovasc. Diabetol. 2017, 16, 138.
- Leoncini, G.; Martinoli, C.; Viazzi, F.; Ravera, M.; Parodi, D.; Ratto, E.; Vettoretti, S.; Tomolillo, C.; Derchi, L.E.; Deferrari, G.; et al. Changes in renal resistive index and urinary albumin excretion in hypertensive patients under long-term treatment with lisinopril or nifedipine GITS. Nephron 2002, 90, 169–173.
- Parving, H.-H.; Mauer, M.; Ritz, E. Diabetic nephropathy. In Brenner and Rector’s the Kidney, 7th ed.; Brenner, B.M., Ed.; Boston Saunders: Cambridge, UK, 2004; pp. 1777–1818.
- Parving, H.-H.; De Zeeuw, D.; Cooper, M.E.; Remuzzi, G.; Liu, N.; Lunceford, J.; Shahinfar, S.; Wong, P.H.; Lyle, P.A.; Rossing, P.; et al. ACE gene polymorphism and losartan treatment in type 2 diabetic patients with nephropathy. J. Am. Soc. Nephrol. 2008, 19, 771–779.
- Pena, M.J.; Heinzel, A.; Rossing, P.; Parving, H.-H.; Dallmann, G.; Rossing, K.; Andersen, S.; Mayer, B.; Heerspink, H.J.L. Serum metabolites predict response to angiotensin II receptor blockers in patients with diabetes mellitus. J. Transl. Med. 2016, 14, 203.
- Pena, M.J.; Jankowski, J.; Heinze, G.; Kohl, M.; Heinzel, A.; Bakker, S.J.; Gansevoort, R.T.; Rossing, P.; De Zeeuw, D.; Heerspink, H.J.L.; et al. Plasma proteomics classifiers improve risk prediction for renal disease in patients with hypertension or type 2 diabetes. J. Hypertens. 2015, 33, 2123–2132.
More
