Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Jason Zhu and Version 1 by Uche Maryann Chukwudulue.
The shift from the terrestrial to the marine environment to discover natural products has given rise to novel bioactive compounds, some of which have been approved for human medicine. However, the ocean, which makes up nearly three-quarters of the Earth’s surface, contains macro- and microorganisms whose natural products are yet to be explored. Among these underexplored marine organisms are macroalgae and their symbiotic microbes, such as Bacillota, a phylum of mostly Gram-positive bacteria previously known as Firmicutes. Macroalgae-associated Bacillota often produce chemical compounds that protect them and their hosts from competitive and harmful rivals.
- seaweeds
- macroalgae
- Firmicutes
- Bacillota
- natural products
1. Introduction
Natural products are molecules produced by organisms that have played a vital role in drug discovery [1]. Generally, the terrestrial environment and its organisms are well-studied for bioactive compounds, and this is due to the relative ease of their sampling [2] and culturing in the laboratory [3]. However, the largest ecosystem, the ocean [4], abundantly endowed with chemical and biological resources [5], is less explored [6,7][6][7]. The challenging conditions of the marine environment, for example, high salt concentration, low temperature, high pressure, low nutrients, and varying light intensities [8], spur marine organisms to synthesise uncommon chemical compounds that help them adapt to their environment [9]. Recently, researchers have been moving away from terrestrial to the marine ecosystem for unique therapeutic molecules with high pharmaceutical and biotechnological potentials [10]. However, many more studies are needed to cover the ocean’s vast biodiversity for this purpose.
So far, about twenty marine natural products (MNPs) are approved as pharmaceutical drugs [11], and twelve of them are specifically for cancer treatments [12]. Despite the vast number of microbes in the marine ecosystem, the number of microbial-derived marine drugs is limited [11]. Investigating more marine microbes, especially symbionts, for drug-like compounds is necessary, since the actual producers of many natural products are microorganisms inhabiting their hosts [13]. A notable example is tetrodotoxin, initially isolated from pufferfish and octopus but later discovered as a secondary metabolite of some symbiotic bacteria [14,15][14][15]. Another example is Taxol, previously obtained from a terrestrial plant, the Pacific yew [16], which was found afterwards to be a product of an endophytic fungus of the plant. Another reason to pursue symbiotic marine microorganisms is their ability to produce a greater variety of novel secondary metabolites than their free-living counterparts [17].
Marine macroalgae (seaweeds) are common hosts of diverse bacterial species [18], and algae–bacterial association is usually host-specific [19,20,21][19][20][21]. Host specificity means that some algae accommodate more microbial species than others [22], and similar microbial species colonise the same algae found in different ecological niches [19]. This algal holobiont, which refers to the host algae and its associated microorganisms, is influenced by the type of nutrients the host provides [23]. In addition to the metabolic duties performed by the bacterial symbionts for their hosts, they also protect them from harmful organisms and substances by producing antimicrobial and antifouling agents [18,24,25][18][24][25]. This advantage is due to the chemical interactions between the host and its associates [26] caused by the quest for survival in a competitive environment [27].
Alongside Actinomycetota and Pseudomonadota, Bacillota has been identified as the bacterial phylum most associative to marine organisms, including algae [28,29,30][28][29][30]. It is a ubiquitous phylum of bacteria found in both terrestrial and aquatic environments. Species of Bacillota are found abundantly in different regions of the human body [31[31][32][33],32,33], animals [34], soil [35[35][36],36], plant stems [37] and rhizospheres [38,39][38][39]. In the aquatic habitat, they can exist in water [40] or sediment [41], or associated with higher marine organisms [24,42,43][24][42][43]. This phylum of bacteria comprises mainly Gram-positive species exhibiting diverse phenotypic forms, most thriving at neutral pH [44]. They form endospores that enable them to adapt to many ecological conditions and are easily cultured in laboratories [45].
Bacillota are among the large genome-sized bacteria that use over 9% of their genomes to encode novel biosynthetic gene clusters (BGCs) that produce new bioactive compounds [46,47][46][47]. These bioactive molecules belong to different chemical classes, such as terpenes, polyketides (PKs), non-ribosomal peptides (NRPs), lasso peptides, bacteriocins, thiopeptides, ectoine, and melanin [28]. Though Bacillota dedicate a significant portion of their genome to producing bioactive compounds, most approved microbial drug products, especially antibiotics, are from a different bacterial phylum, Actinomycetota [48,49][48][49]. So far, Bacillota has only a few products on the market.
In human medicine, a deadly toxin (botulinum toxin type A) produced by Clostridium botulinum, commonly found in soil, has been developed into a vital drug (Botox®) [50]. Botox® is widely used to treat health issues ranging from strabismus to spasticity [51,52][51][52]. Studies have also tested its use in treating bruxism [53] and cosmetic procedures [54], and its anticancer properties [55,56][55][56]. Another FDA-approved product of Bacillota is polymyxin B, a chemical compound first obtained from a soil bacterium, Paenibacillus polymyxa [57]. This antibiotic is the last-resort treatment for multidrug-resistant Gram-negative bacterial infections [58]. Other formulated products from Bacillota are nisin (E 234), an antibiotic food additive isolated from a subspecies of Lactobacillus lactis [59[59][60],60], and two enzymes (protease and carbohydrase) from Bacillus subtilis or Bacillus amyloliquefaciens, considered to be GRAS (generally recognised as safe) by the FDA [61].
Some commercially available plant biofungicides from Bacillota in the agricultural industry include SERENADE®, Double Nickel 55TM and other products. These products are formulated from B. velezensis, a member of the operational group Bacillus amyloliquefaciens (OGBa) made up of B. amyloliquefaciens, B. siamensis, B. velezensis and B. nakamurai [62]. In addition, about 50% of approved plant bacterial biocontrol formulations used in different countries are Bacillus species products [63]. Examples include BioPro®, Rhiso Plus®, Biosubtilin, Botrybel®, and NacillusPro™ [64] and also the animal probiotic Ecobiol Plus® from B. amyloliquefaciens, commercially available in Europe for pigs and chickens, with potential use in aquaculture [65].
2. Aquatic Bacillota
A study by da Silva et al., 2013 identified at least a strain of Bacillota in every analysed sample of sea sediment collected from different depths of the South Atlantic Ocean, in contrast to other phyla [68][66], corroborating the easy cultivation of marine Bacillota in the laboratory [69][67]. Also, while assessing the microbial symbionts of sponges and a soft coral obtained from the Red Sea, Refs. [13,70][13][68] found that Bacillota was the most-encountered bacteria phylum, usually synonymous with bioactive secondary metabolites. For instance, some marine Bacillus species produced many novel natural products, ranging from macrolides [71,72,73][69][70][71] to fatty acids [74][72]. A specific example is Bacillus silvesteris from a marine crab, which synthesised two unknown cyclodepsipeptides with very high cytotoxic effects (GI50s: 0.001–0.01 ng/mL) against human cancer cell lines [75][73]. Unfortunately, with these exciting numbers of novel molecules associated with marine Bacillota, only a few studies covered Bacillota from macroalgae; more studies were on Bacillota of corals and sponges.3. Marine Macroalgae, a Good Source of Bioactive Bacillota
Consistently, marine macroalgae have produced various remarkable molecules [2,26][2][26]. Between 1960 and 2012, more than three thousand natural products were identified in different algae types, and they are still receiving attention for their novel bioactive compounds [76][74]. Some examples of algae chemical compounds include two potential anticancer agents, lophocladines B from a red alga [77][75], dieckol from a brown alga [78][76] and Griffithsin from a red alga, currently in phase I clinical trial for HIV prevention [11,79][11][77]. However, algae’s commercial and ecological applications are linked to the chemical communications between them and their associated microbes [18,80][18][78]. To ascertain this assumption, scientists are investigating algae microbial symbionts for their natural products [19,81,82,83][19][79][80][81]. It appears that Bacillota play more beneficial than pathogenic functions in algae [22[22][79],81], by producing chemical compounds that protect algae from fouling and colonising pathogenic microbes.4. Secondary Metabolites of Marine Macroalgae Bacillota and Their Biosynthetic Gene Clusters
Several chemical compounds have been obtained from marine macroalgae-associated Bacillota in laboratories. However, the type of compounds generated by any microorganism in a conventional laboratory can be influenced by the properties of the growth medium [13]. Different classes of compounds would emerge by varying the composition or condition of the culture media. For instance, growing a symbiotic bacterium in a static culture or one consistently shaken at a particular speed affects the types of chemical compounds the bacterium will produce [82][80]. This is a well-known natural-product drug-discovery approach called the one strain many compounds (OSMAC) approach. Table 1 also shows that most bioactive compounds of the marine macroalgae Bacillota belong to the polyketide class, which aligns with the suggestion by Aleti et al., 2015, regarding the prevalence of a polyketide synthase (pks) gene cluster in Bacillota [83][81]. Of the forty-one compounds isolated from the macroalgae Bacillota, thirty-eight are polyketides, while the remaining three belong to the non-ribosomal peptide–polyketide hybrid. The chemical structures of these molecules (1–41) are shown in Figure 1, Figure 2, Figure 3 and Figure 4, and they are grouped according to their chemical classes, including macrolides, esters, furanoterpenoids and amicoumacins. Apart from the forty-one isolated and characterised compounds, there are forty-seven volatile compounds identified in an extract of a B. amyloliquefaciens strain isolated from a brown alga Zonaria tournefortii [84][82], as well as a YbdN protein isolated from B. licheniformis of Fucus serratus [82][80].Table 1.
Natural products and pharmacological properties of symbiotic Bacillota from marine macroalgae.
| Algae Species | Growth Medium | Bacterial Species | Biosynthetic Gene Cluster | Extract/ Compounds |
Pharmacological Properties | MIC (µg/mL) | References |
|---|---|---|---|---|---|---|---|
| Brown Algae Bacillota | |||||||
| Sargassum wightii | a ZMA * b NA a NA a ZMA |
Bacillus species Bacillus atrophaeus MW821482 |
pks pks nrps Siderophore |
Ethyl acetate extract Ethyl acetate extract |
Antibacterial Antioxidant Antihypertensive Antihypercholesterolamic Anti-inflammatory Anti-hyperglycemic Cytotoxicity Antioxidant Antibacterial Anti-inflammatory Anti-hyperglycemic Antihypertensive Antioxidant Anti-hypercholesterolemic Antibacterial |
6.25–12.5 ⁑ (133–492.04) ⁑ (498.12–735.42) ⁑ (10.21–24.32) ⁑ (5.22–735.45) ⁑ (92.02–759.24) ♯ 29.5 ♯ (133–4167) 6.25–12.5 ⁑ (9.74–788.8) ⁑ (118.1–513.4) ⁑ 713.6 ⁑ (413.2–429.8) ⁑ 22.23 6.25–12.5 |
[85,86,87,88][83][84][85][86] |
| Anthophycus longifolius | a NA ** NA SWA ZMA * NA MA * NA |
Bacillus subtilis MTCC 10403 | pks pks pks |
(1) (35–38) (2) |
Antibacterial Antibacterial Antibacterial |
3.12–50 3.12–25 ND |
[89,90,91][87][88][89] |
| Sargassum myriocystum | MA * NA |
Bacillus subtilis MTCC 10407 | pks | (26 and 27) | Antibacterial | ND | [92][90] |
| Fucus serratus | a TSA DSTA MA NA * CB |
Bacillus licheniformis | ND | YbdN protein | Antibacterial | ND | [82][80] |
| Endarachne binghamiae | MA MB |
Bacillus sp. | ND | Acetone extract | Antibacterial | 188.1–209.7 | [93][91] |
| Sargassum muticum | MA MB |
Bacillus sp. | ND | Acetone extract | Cytotoxicity Antibacterial |
♯ 5.5 174 |
[93][91] |
| Egregia menziesii | MA MB |
Bacillus sp. | ND | Acetone extract | Antibacterial | 203.0–212.3 | [93][91] |
| Padina gymnospora | a NA ** NA SWA ZMA * NA |
Bacillus amyloliquefaciens | pks | (28–31) | Antibacterial | ND | [94][92] |
| Zonaria tournefortii | d LB | Bacillus amyloliquefaciens S13 | ND | Volatile compounds | Antimicrobial | 64–>500 | [84][82] |
| Red algae Bacillota | |||||||
| Hypnea valentiae | a ZMA * MBSA |
Bacillus amyloliquefaciens MB6 (MTCC 12716) | pks pks-nrps ND |
(3–5) and (6–8) (39–41) Ethyl acetate extract |
Antibacterial Antibacterial Antibacterial Anti-inflammatory Anti-hypercholesterolemic Antidiabetic Antioxidant Antibacterial |
0.38–5.00 ¶ (−9.06)–(−10.13) ¶ (−11.33)–(−13.61) 0.78–3.12 3.125–12.5 ⁑ (6.06–675.36) ⁑ 17.30 ⁑ (84.00–639.54) ⁑ (136.78–278.19) 6.25–12.50 |
[95,96,97,98,99,100][93][94][95][96][97][98] |
| Kappaphycus alvarezii | a ZMA * MBSA |
Bacillus amyloliquefaciens MTCC 12713 | pks pks |
(9–12) (22–25) |
Antibacterial Antibacterial |
‡ 2–9 × 10−3 1.56–6.25 ¶ (−9.06)–(−12.61) |
[101,102][99][100] |
| Laurencia papillosa | a NA c ZMA * NA a ZMA a NA * NA a NA ** NA SWA ZMA * NA |
Bacillus velezensis MBTDLP1 MTCC 13048 Bacillus velezensis MBTDLP1 Bacillus amyloliquefaciens |
pks ND pks |
(34) Ethyl acetate extract (32 and 33) |
Antibacterial Antibacterial Anti-inflammatory Cytotoxicity Antidiabetic Antioxidant Antibacterial |
0.38 7.5–15 ♯ 17 ♯ (32.3–200) ♯ (120–420) ♯ (107–4127) ND |
[103,104,105][101][102][103] |
| Laurencia pacifica | MA MB |
Bacillus sp. | ND | Acetone extract | Antibacterial | 288.1 | [93][91] |
| Centroceras clavulatum | MA MB |
Bacillus sp. | ND | Acetone extract | Antibacterial | 217.1 | [93][91] |
| Schizymenia dubyi | MB | Bacillus sp. PP19-H3 | pks | (13–21) | Antibacterial | 10–>100 | [73][71] |
| Green Algae Bacillota | |||||||
| Codium fragile | MA MB |
Bacillus sp. | ND | Acetone extract | Antibacterial | 196 | [93][91] |
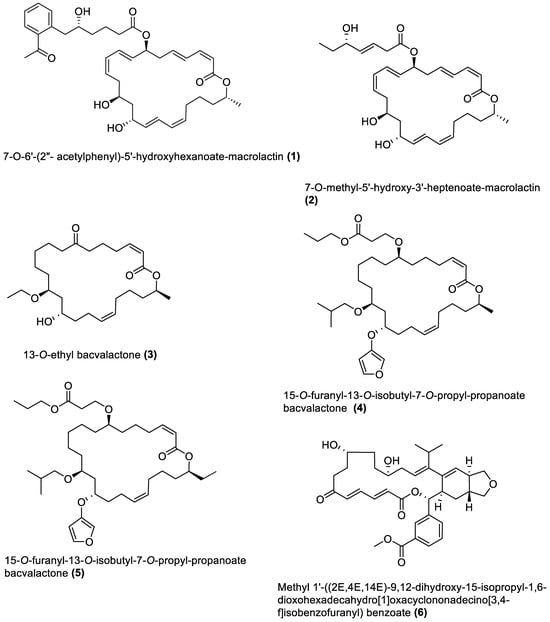
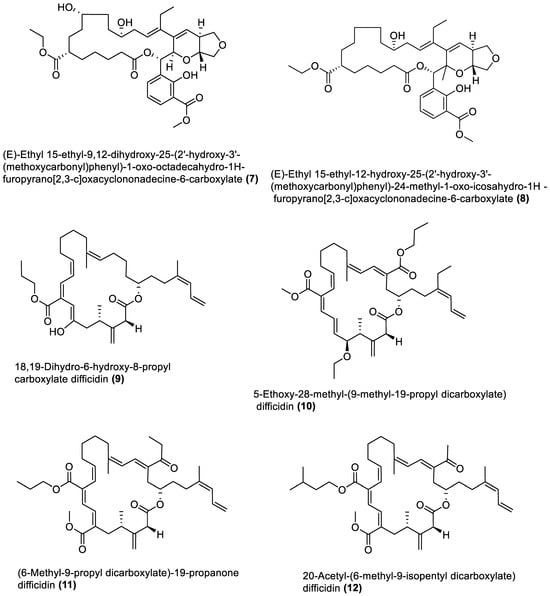
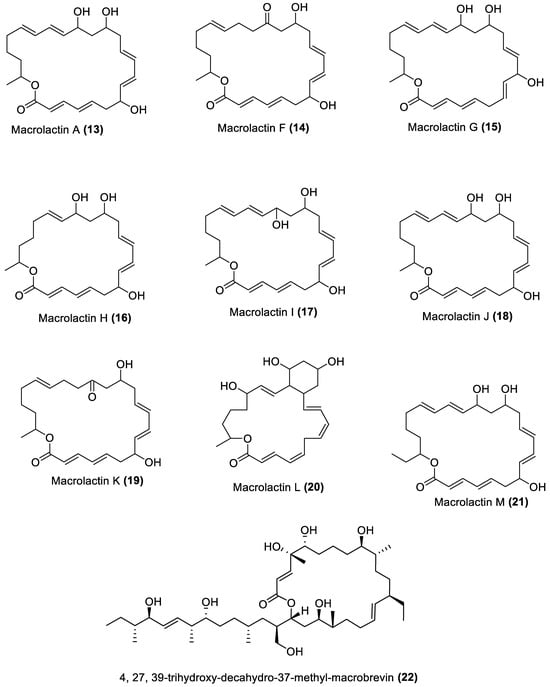
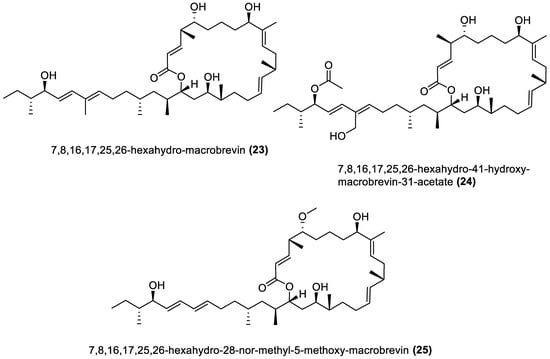
Figure 1.
Macrolides from marine macroalgae Bacillota.
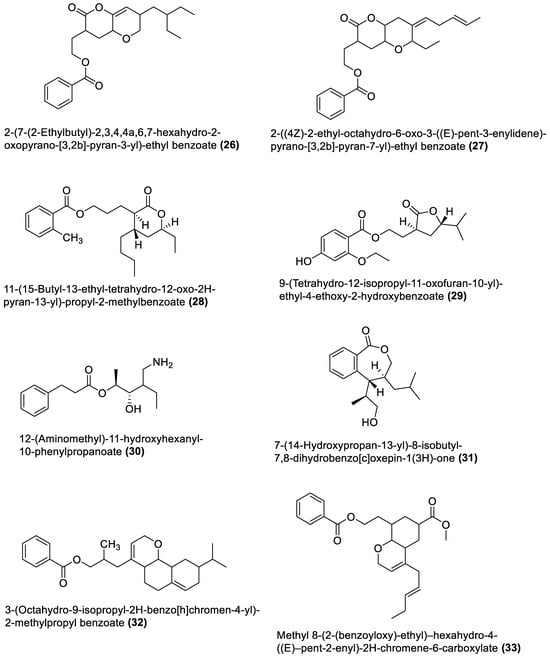
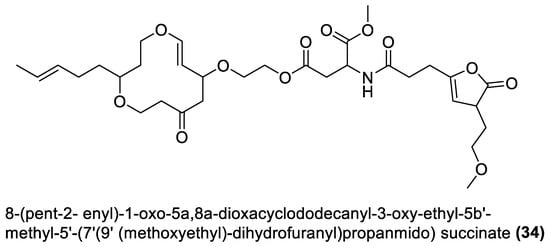
Figure 2.
Esters from marine macroalgae Bacillota.
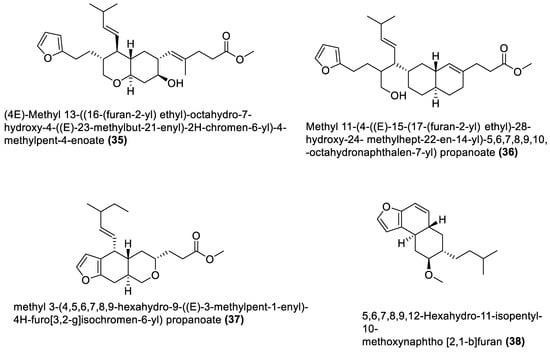
Figure 3.
Furanoterpenoids from marine macroalgae Bacillota.
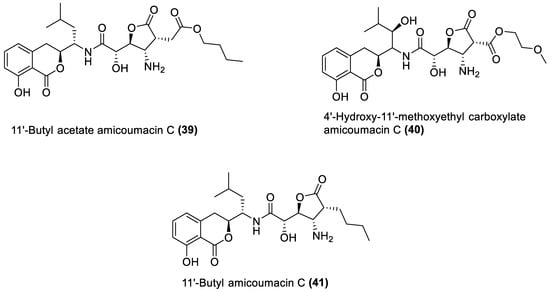
Figure 4.
Amicoumacins from marine macroalgae Bacillota.
4.1. Macrolides
These are polyketide macrocyclic lactones of varying ring sizes [106][104]. They are highly oxygenated polyenes [107][105] with a broad spectrum of antibacterial effects against pathogenic bacteria, which they achieve by binding to the 50S ribosomal subunit of bacteria and disrupting protein synthesis. Erythromycin and azithromycin, with 14- and 15-membered rings, belong to the first- and second-generation macrolide antibiotics [108][106]. Twenty-five macrolides belonging to different classes have been isolated from marine macroalgae Bacillota, including derivatives of macrolactin (1, 2, 13–21) [73[71][89],91], bacvalactone (3–5), elansolid (6–8) [95,96,97][93][94][95], difficidin (9–12) [101][99], and macrobrevin (22–25) [102][100]. Compounds 1 and 2 are 24-membered macrolactins isolated from B. subtilis of a brown alga, Anthophycus longifolius [89,91][87][89]. They are biosynthesised by the type-1 pks gene cluster through decarboxylative Claisen condensation, ketoreduction, dehydration and cyclisation reaction steps for 1. The bacvalactones (3–5) and elansolids (6–8), which are also biosynthesised by several decarboxylative Claisen condensation reactions, were isolated from a strain of B. amyloliquefaciens of a red alga, Hypnea valentiae. The bacvalactones are 24-membered macrolactones with 13-O-ethyl (3) and 15-O-furanyl (4 and 5) substituents. The elansolids are 19-membered macrocyclic lactones which contain octahydroisobenzofuran derivative (6), 4a,5,7,7a-tetrahydro-2H-furo[3,4-b]pyran derivative (7) and hexahydro-2H-furo[3,4-b]pyran derivative (8) in their structure. In addition, 22-membered difficidin (9–12) and macrobrevin (22–25) analogues, isolated from Kappaphycus alvarezii’s (a red alga) B. amyloliquefaciens, are also the products of repeated decarboxylative Claisen condensation reactions of the pks gene clusters. Other macrolides of marine algae Bacillota are a set of macrolactins (13–21) isolated from a red alga (Schizymenia dubyi) Bacillus sp. All these macrolides were tested only for antibacterial properties, even though they have been reported elsewhere to possess anticancer, neuroprotective, antidiabetic and anti-inflammatory properties [107][105].4.2. Esters
Different previously undescribed derivatives of heterocyclic (26–29 and 31–34) and aliphatic (30) esters have been isolated and characterised from Bacillota of macroalgae (Figure 2). For example, compounds (26 and 27), isolated from B. subtilis of Sargassum myriocystum, are pks-1 gene products and members of the pyranyl benzoate analogues [92][90]. They are synthesised through the Claisen condensation, dehydration and ketoreduction pathways, and resemble two compounds isolated from the alga host. On the other hand, B. amyloliquefaciens, isolated from Padina gymnospora (a brown alga), produced polyketides (28–31) through the pks-1 gene cluster [94][92]. The heterocyclic esters (32 and 33), which are octahyrobenzopyran derivatives and the secondary metabolites of B. amyloliquefaciens, isolated from a red alga, Laurencia papillosa, are also pks gene products [105][103]. In addition, the macrocyclic diester (34), isolated from B. velezensis of Laurencia papillosa (a red alga), also belongs to the pks-1 gene products [103][101]. Like the macrolides, these nine esters were only tested for antibacterial properties.4.3. Furanoterpenoids
Furanoterpenoids are a class of terpenoids containing at least a furan ring [109][107]. Many furan-containing compounds, including furanoterpenoids, are toxic to humans. Their toxicity is through the cytochrome P450-catalysed oxidation of the furan ring to two reactive electrophilic intermediates that can bond with macromolecules and cause toxicity [110,111][108][109]. However, furanoterpenoids have been reported to elicit anti-inflammatory [112][110], antimalarial [113][111], and other properties [111][109]. Furanoterpenoids (35–38) isolated from B. subtilis of red alga (Anthophycus longifolius) showed in vitro antibacterial effects [90][88]. Two were sesterpenoid-type compounds (35, 36), and the others were furan annulation compounds. However, considering the toxicity concern regarding this group of compounds, an in vivo toxicity assay of any furan-containing molecule would be necessary, using an animal model, to ascertain their safety. The four furanoterpenoids are biosynthesised by the pks gene cluster.4.4. Amicoumacin C Derivatives
Amicoumacins are derivatives of the dihydroisocoumarin class of compounds biosynthesised by bacterial non-ribosomal peptide–polyketide (nrp-pk) hybrid biosynthetic pathway [114][112]. Out of the forty-one natural products of the macroalgae Bacillota, the amicoumacins (39–41) are the only chemical compounds produced by another gene cluster other than the pks, even though non-ribosomal peptides synthetase (nrps) gene clusters are quite common in Bacillus species [101,115][99][113]. There is, therefore, a need to explore different biosynthetic pathways of macroalgae Bacillota for diverse bioactive chemical species. The structures of 39–41 are shown in Figure 4, and they were isolated from the B. amyloliquefaciens of a red alga, Hypnea valentiae [98][96].5. Pharmacological Properties of the Secondary Metabolites of Marine Macroalgae Bacillota
In addition to the chemical constituents of the marine macroalgae Bacillota, Table 1 also captures the biological activities of the compounds numbered 1–41 and the extracts. Among the marine Bacillota, Bacillus species are the most common symbionts of macroalgae, and they often showcase higher antibacterial properties than their counterparts [117][114]. Furthermore, species of Bacillus dedicate more than 7% of their genomes to producing compounds with antimicrobial properties [118][115]. These two assertions can be seen clearly in Table 1: Bacillus were the only species isolated from all the macroalgae included in the table for bioactive natural products, and mainly elicited antibacterial properties. Researchers realised that some authors carried out preliminary bioassays only, using bacterial cultures instead of extracts or isolated chemical compounds from the bacteria [81,117,119,120,121,122,123,124,125,126,127,128,129][79][114][116][117][118][119][120][121][122][123][124][125][126]. Another scenario is where the antimicrobial effects of bacterial extracts/fractions were checked without determining the basic bioassay parameters like MIC, IC50 or GI50 [130,131,132,133,134,135][127][128][129][130][131][132]. The studies mentioned above were omitted in Table 1. However, the table captures chemical constituents with low therapeutic properties—MIC/IC50/GI50 values greater than 100 and 10 µg/mL—for crude extracts and pure compounds, respectively, and compounds whose basic bioassay parameters were not determined.5.1. Antibacterial Property of Marine Macroalgae Bacillota
Mostly in vitro antibacterial properties were reported for macroalgae Bacillota, according to data in Table 1. The high frequency of documented antibacterial properties might be due to the ease of carrying out this assay in the laboratory, as opposed to other bioassays. Another potential explanation could be the author’s intention to demonstrate the antifouling properties attributed to symbiotic bacteria associated with macroalgae. The compounds isolated from the macroalgae Bacillota showed varied levels of antibacterial effects against human bacterial pathogens. The best in vitro antibacterial activity (with the MIC value of 2–9 × 10−3 µM) was exhibited by the difficidin analogues (9–12) isolated from B. amyloliquefaciens associated with a red alga, Kappaphycus alvarezii [101][99]. These compounds showed bactericidal activities against a broad spectrum of pathogenic bacteria, including methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus faecalis [101][99]. Other compounds with appreciable antibacterial activity are 7 and 34. At an MIC value of 0.38 µg/mL, compound 7, a product of B. amyloliquefaciens isolated from a red alga, Hypnea valentiae, was active against MRSA and Vibrio haemolyticus [96[94][95],97], similar to 34, produced by B. velezensis of Laurencia papillosa [103][101]. Worthy of mention is 40 (MIC value: 0.78 µg/mL), another active compound from B. amyloliquefaciens of Hypnea valentiae, with a broad spectrum activity against pathogenic bacteria [98][96]. Table 1 lists the MIC values of other compounds and extracts of macroalgae Bacillota. Unfortunately, as seen from the table, some extracts with MIC values lower than 100 µg/mL were not further simplified to isolate their bioactive chemical constituents.5.2. Other Pharmacological Properties of Marine Algae Bacillota
Other biological properties, such as cytotoxicity, anti-inflammatory, antioxidant, antidiabetic, anti-hypercholesterolemic and anti-hyperglycemic, were exclusively determined for extracts/fractions of the macroalgae Bacillota. For example, the only reported in vitro antifungal assay was recorded for a volatile fraction of B. amyloliquefaciens isolated from Zonaria tournefortii [84][82]. Another example of biological activities of the macroalgae Bacillota extract is that of an acetone extract of a Bacillus species isolated from a brown alga, Sargassum muticum. The acetone extract displayed a good in vitro cytotoxic effect (IC50 value of 5.5 µg/mL) against colon cancer cells [93][91], but it was not purified further to pure compounds.References
- Amer, N.F.; Luzzatto Knaan, T. Natural Products of Marine Origin for the Treatment of Colorectal and Pancreatic Cancers: Mechanisms and Potential. Int. J. Mol. Sci. 2022, 23, 8048.
- Lever, J.; Brkljača, R.; Kraft, G.; Urban, S. Natural Products of Marine Macroalgae from South Eastern Australia, with Emphasis on the Port Phillip Bay and Heads Regions of Victoria. Mar. Drugs 2020, 18, 142.
- Zhou, Q.; Hotta, K.; Deng, Y.; Yuan, R.; Quan, S.; Chen, X. Advances in Biosynthesis of Natural Products from Marine Microorganisms. Microorganisms 2021, 9, 2551.
- Wu, L.; Ye, K.; Jiang, S.; Zhou, G. Marine Power on Cancer: Drugs, Lead Compounds, and Mechanisms. Mar. Drugs 2021, 19, 488.
- Luzzatto-Knaan, T.; Garg, N.; Wang, M.; Glukhov, E.; Peng, Y.; Ackermann, G.; Amir, A.; Duggan, B.M.; Ryazanov, S.; Gerwick, L.; et al. Digitizing Mass Spectrometry Data to Explore the Chemical Diversity and Distribution of Marine Cyanobacteria and Algae. eLife 2017, 6, e24214.
- Paulus, C.; Rebets, Y.; Tokovenko, B.; Nadmid, S.; Terekhova, L.P.; Myronovskyi, M.; Zotchev, S.B.; Rückert, C.; Braig, S.; Zahler, S.; et al. New Natural Products Identified by Combined Genomics-Metabolomics Profiling of Marine Streptomyces sp. MP131-18. Sci. Rep. 2017, 7, 42382.
- Altmann, K.H. Drugs from the Oceans: Marine Natural Products as Leads for Drug Discovery. Chimia 2017, 71, 646–651.
- Karthikeyan, A.; Joseph, A.; Nair, B.G. Promising Bioactive Compounds from the Marine Environment and Their Potential Effects on Various Diseases. J. Genet. Eng. Biotechnol. 2022, 20, 14.
- Liu, Q.A.; Zheng, J.J.; Gu, Y.C.; Wang, C.Y.; Shao, C.L. The Chemistry and Bioactivity of Macrolides from Marine Microorganisms. In Studies in Natural Products Chemistry; Elsevier B.V.: Amsterdam, The Netherlands, 2015; Volume 44, pp. 353–401. ISBN 9780444634603.
- Giddings, L.; Newman, D.J. Bioactive Compounds from Marine Extremophiles; SpringerBriefs in Microbiology; Springer International Publishing: Cham, Switzerland, 2015; ISBN 978-3-319-14360-6.
- Haque, N.; Parveen, S.; Tang, T.; Wei, J.; Huang, Z. Marine Natural Products in Clinical Use. Mar. Drugs 2022, 20, 528.
- Matulja, D.; Vranješević, F.; Kolympadi Markovic, M.; Pavelić, S.K.; Marković, D. Anticancer Activities of Marine-Derived Phenolic Compounds and Their Derivatives. Molecules 2022, 27, 1449.
- Bibi, F.; Naseer, M.I.; Azhar, E.I. Assessing the Diversity of Bacterial Communities from Marine Sponges and Their Bioactive Compounds. Saudi J. Biol. Sci. 2021, 28, 2747–2754.
- Pratheepa, V.; Vasconcelos, V. Microbial Diversity Associated with Tetrodotoxin Production in Marine Organisms. Environ. Toxicol. Pharmacol. 2013, 36, 1046–1054.
- Hwang, D.F.; Arakawa, O.; Saito, T.; Noguchi, T.; Simidu, U.; Tsukamoto, K.; Shida, Y.; Hashimoto, K. Tetrodotoxin-Producing Bacteria from the Blue-Ringed Octopus Octopus maculosus. Mar. Biol. 1989, 100, 327–332.
- Andrea, S.; Gary, S.; Donald, S.; Stierle, A.; Strobel, G.; Stierle, D. Taxol and Taxane Production by Taxomyces andreanae, an Endophytic Fungus of Pacific Yew. Science 1993, 260, 214–216.
- Viju, N.; Punitha, S.M.J.; Satheesh, S. Antibiofilm Activity of Symbiotic Bacillus Species Associated with Marine Gastropods. Ann. Microbiol. 2020, 70, 11.
- Egan, S.; Harder, T.; Burke, C.; Steinberg, P.; Kjelleberg, S.; Thomas, T. The Seaweed Holobiont: Understanding Seaweed-Bacteria Interactions. FEMS Microbiol. Rev. 2013, 37, 462–476.
- Lachnit, T.; Blümel, M.; Imhoff, J.F.; Wahl, M. Specific Epibacterial Communities on Macroalgae: Phylogeny Matters More than Habitat. Aquat. Biol. 2009, 5, 181–186.
- Lachnit, T.; Meske, D.; Wahl, M.; Harder, T.; Schmitz, R. Epibacterial Community Patterns on Marine Macroalgae Are Host-Specific but Temporally Variable. Environ. Microbiol. 2011, 13, 655–665.
- Aires, T.; Serrão, E.A.; Engelen, A.H. Host and Environmental Specificity in Bacterial Communities Associated to Two Highly Invasive Marine Species (Genus Asparagopsis). Front. Microbiol. 2016, 7, 559.
- Goecke, F.; Labes, A.; Wiese, J.; Imhoff, J.F. Review Chemical Interactions between Marine Macroalgae and Bacteria. Mar. Ecol. Prog. Ser. 2010, 409, 267–300.
- Goecke, F.; Thiel, V.; Wiese, J.; Labes, A.; Imhoff, J.F. Algae as an Important Environment for Bacteria—Phylogenetic Relationships among New Bacterial Species Isolated from Algae. Phycologia 2013, 52, 14–24.
- Li, X.; Gao, X.; Zhang, S.; Jiang, Z.; Yang, H.; Liu, X.; Jiang, Q.; Zhang, X. Characterization of a Bacillus velezensis with Antibacterial Activity and Inhibitory Effect on Common Aquatic Pathogens. Aquaculture 2020, 523, 735165.
- Armstrong, E.; Yan, L.; Boyd, K.G.; Wright, P.C.; Burgess, J.G. The Symbiotic Role of Marine Microbes on Living Surfaces. Hydrobiologia 2001, 461, 37–40.
- Alves, C.; Silva, J.; Pinteus, S.; Gaspar, H.; Alpoim, M.C.; Botana, L.M.; Pedrosa, R. From Marine Origin to Therapeutics: The Antitumor Potential of Marine Algae-Derived Compounds. Front. Pharmacol. 2018, 9, 777.
- Egan, S.; Thomas, T.; Kjelleberg, S. Unlocking the Diversity and Biotechnological Potential of Marine Surface Associated Microbial Communities. Curr. Opin. Microbiol. 2008, 11, 219–225.
- Viju, N.; Punitha, S.M.J.; Satheesh, S. An Analysis of Biosynthesis Gene Clusters and Bioactivity of Marine Bacterial Symbionts. Curr. Microbiol. 2021, 78, 2522–2533.
- Dat, T.T.H.; Cuc, N.T.K.; Cuong, P.V.; Smidt, H.; Sipkema, D. Diversity and Antimicrobial Activity of Vietnamese Sponge-Associated Bacteria. Mar. Drugs 2021, 19, 353.
- Kuo, J.; Yang, Y.T.; Lu, M.C.; Wong, T.Y.; Sung, P.J.; Huang, Y. Sen Antimicrobial Activity and Diversity of Bacteria Associated with Taiwanese Marine Sponge Theonella swinhoei. Ann. Microbiol. 2019, 69, 253–265.
- Garg, N.; Luzzatto-Knaan, T.; Melnik, A.V.; Caraballo-Rodríguez, A.M.; Floros, D.J.; Petras, D.; Gregor, R.; Dorrestein, P.C.; Phelan, V.V. Natural Products as Mediators of Disease. Nat. Prod. Rep. 2017, 34, 194–219.
- Ito, T.; Sekizuka, T.; Kishi, N.; Yamashita, A.; Kuroda, M. Conventional Culture Methods with Commercially Available Media Unveil the Presence of Novel Culturable Bacteria. Gut Microbes 2019, 10, 77–91.
- Novik, G.; Savich, V. Beneficial Microbiota. Probiotics and Pharmaceutical Products in Functional Nutrition and Medicine. Microbes Infect. 2020, 22, 8–18.
- Kang, H.; Kim, H.; Yi, H.; Kim, W.; Yoon, J.; Im, W.; Kim, M.K.; Seong, C.N.; Kim, S.B.; Cha, C.; et al. A Report of 43 Unrecorded Bacterial Species within the Phyla Bacteroidetes and Firmicutes Isolated from Various Sources from Korea in 2019. J. Species Res. 2021, 10, 117–133.
- Jemil, N.; Manresa, A.; Rabanal, F.; Ben Ayed, H.; Hmidet, N.; Nasri, M. Structural Characterization and Identification of Cyclic Lipopeptides Produced by Bacillus methylotrophicus DCS1 Strain. J. Chromatogr. B 2017, 1060, 374–386.
- Zheng, C.J.; Lee, S.; Lee, C.H.; Kim, W.G. Macrolactins O-R, Glycosylated 24-Membered Lactones from Bacillus sp. AH159-1. J. Nat. Prod. 2007, 70, 1632–1635.
- Ngashangva, N.; Mukherjee, P.; Sharma, K.C.; Kalita, M.C.; Indira, S. Analysis of Antimicrobial Peptide Metabolome of Bacterial Endophyte Isolated From Traditionally Used Medicinal Plant Millettia pachycarpa Benth. Front. Microbiol. 2021, 12, 656896.
- Hashmi, I.; Bindschedler, S.; Junier, P. Firmicutes. In Beneficial Microbes in Agro-Ecology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 363–396. ISBN 9780128234143.
- Osei, E.; Kwain, S.; Mawuli, G.T.; Anang, A.K.; Owusu, K.B.A.; Camas, M.; Camas, A.S.; Ohashi, M.; Alexandru-Crivac, C.N.; Deng, H.; et al. Paenidigyamycin A, Potent Antiparasitic Imidazole Alkaloid from the Ghanaian Paenibacillus sp. De2Sh. Mar. Drugs 2019, 17, 9.
- Meene, A.; Herzer, C.; Schlüter, R.; Zayadan, B.; Pukall, R.; Schumann, P.; Schauer, F.; Urich, T.; Mikolasch, A. A Novel Antimicrobial Metabolite Produced by Paenibacillus apiarius Isolated from Brackish Water of Lake Balkhash in Kazakhstan. Microorganisms 2022, 10, 1519.
- Jaruchoktaweechai, C.; Suwanborirux, K.; Tanasupawatt, S.; Kittakoop, P.; Menasveta, P. New Macrolactins from a Marine Bacillus sp. Sc026. J. Nat. Prod. 2000, 63, 984–986.
- Graça, A.P.; Bondoso, J.; Gaspar, H.; Xavier, J.R.; Monteiro, M.C.; de la Cruz, M.; Oves-Costales, D.; Vicente, F.; Lage, O.M. Antimicrobial Activity of Heterotrophic Bacterial Communities from the Marine Sponge Erylus discophorus (Astrophorida, Geodiidae). PLoS ONE 2013, 8, e78992.
- Ou, W.; Yu, G.; Zhang, Y.; Mai, K. Recent Progress in the Understanding of the Gut Microbiota of Marine Fishes. Mar. Life Sci. Technol. 2021, 3, 434–448.
- Schleifer, K.-H. Phylum XIII. Firmicutes Gibbons and Murray 1978, 5 (Firmacutes Gibbons and Murray 1978, 5). In Systematic Bacteriology; Springer New York: New York, NY, USA, 2009; Volume 3, pp. 19–1317. ISBN 978-0-387-68489-5.
- Rodrigues, C.J.C.; de Carvalho, C.C.C.R. Cultivating Marine Bacteria under Laboratory Conditions: Overcoming the “Unculturable” Dogma. Front. Bioeng. Biotechnol. 2022, 10, 964589.
- Baltz, R.H. Gifted Microbes for Genome Mining and Natural Product Discovery. J. Ind. Microbiol. Biotechnol. 2017, 44, 573–588.
- Baltz, R.H. Genome Mining for Drug Discovery: Progress at the Front End. J. Ind. Microbiol. Biotechnol. 2021, 48, kuab044.
- Van Der Heul, H.U.; Bilyk, B.L.; McDowall, K.J.; Seipke, R.F.; Van Wezel, G.P. Regulation of Antibiotic Production in Actinobacteria: New Perspectives from the Post-Genomic Era. Nat. Prod. Rep. 2018, 35, 575–604.
- Robertsen, H.L.; Musiol-Kroll, E.M. Actinomycete-Derived Polyketides as a Source of Antibiotics and Lead Structures for the Development of New Antimicrobial Drugs. Antibiotics 2019, 8, 157.
- Hallett, M. One Man’s Poison-Clinical Applications of Botulinum Toxin. N. Engl. J. Med. 1999, 341, 118–120.
- Srivastava, S.; Kharbanda, S.; Pal, U.; Shah, V. Applications of Botulinum Toxin in Dentistry: A Comprehensive Review. Natl. J. Maxillofac. Surg. 2015, 6, 152–159.
- Orsini, M.; Leite, M.A.A.; Chung, T.M.; Bocca, W.; de Souza, J.A.; de Souza, O.G.; Moreira, R.P.; Bastos, V.H.; Teixeira, S.; Oliveira, A.B.; et al. Botulinum Neurotoxin Type A in Neurology: Update. Neurol. Int. 2015, 7, 31–35.
- Chen, Y.; Tsai, C.-H.; Bae, T.H.; Huang, C.-Y.; Chen, C.; Kang, Y.-N.; Chiu, W.-K. Effectiveness of Botulinum Toxin Injection on Bruxism: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Aesthetic Plast. Surg. 2023, 47, 775–790.
- Hong, S.O. Cosmetic Treatment Using Botulinum Toxin in the Oral and Maxillofacial Area: A Narrative Review of Esthetic Techniques. Toxins 2023, 15, 82.
- Karpiński, T.; Adamczak, A. Anticancer Activity of Bacterial Proteins and Peptides. Pharmaceutics 2018, 10, 54.
- Bandala, C.; Perez-Santos, J.L.M.; Lara-Padilla, E.; Lopez, M.G.D.; Anaya-Ruiz, M. Effect of Botulinum Toxin A on Proliferation and Apoptosis in the T47D Breast Cancer Cell Line. Asian Pac. J. Cancer Prev. 2013, 14, 891–894.
- Trimble, M.J.; Mlynárčik, P.; Kolář, M.; Hancock, R.E.W. Polymyxin: Alternative Mechanisms of Action and Resistance. Cold Spring Harb. Perspect. Med. 2016, 6, a025288.
- Avedissian, S.N.; Liu, J.; Rhodes, N.J.; Lee, A.; Pais, G.M.; Hauser, A.R.; Scheetz, M.H. A Review of the Clinical Pharmacokinetics of Polymyxin B. Antibiotics 2019, 8, 31.
- Caulier, S.; Nannan, C.; Gillis, A.; Licciardi, F.; Bragard, C.; Mahillon, J. Overview of the Antimicrobial Compounds Produced by Members of the Bacillus subtilis Group. Front. Microbiol. 2019, 10, 302.
- Klaenhammer, T.R. Bacteriocins of Lactic Acid Bacteria. Biochimie 1988, 70, 337–349.
- Sewalt, V.; Shanahan, D.; Gregg, L.; La Marta, J.; Carillo, R. The Generally sRecognised as Safe (GRAS) Process for Industrial Microbial Enzymes. Ind. Biotechnol. 2016, 12, 295–302.
- Ngalimat, M.S.; Yahaya, R.S.R.; Baharudin, M.M.A.A.; Yaminudin, S.M.; Karim, M.; Ahmad, S.A.; Sabri, S. A Review on the Biotechnological Applications of the Operational Group Bacillus amyloliquefaciens. Microorganisms 2021, 9, 614.
- Olishevska, S.; Nickzad, A.; Déziel, E. Bacillus and Paenibacillus Secreted Polyketides and Peptides Involved in Controlling Human and Plant Pathogens. Appl. Microbiol. Biotechnol. 2019, 103, 1189–1215.
- Cawoy, H.; Bettiol, W.; Fickers, P.; Ongena, M. Bacillus-Based Biological Control of Plant Diseases. In Pesticides in the Modern World-Pesticides Use and Management; IntechOpen: London, UK, 2011; Volume 1849, pp. 273–302. ISBN 9789533074597.
- Llario, F.; Falco, S.; Sebastiá-Frasquet, M.T.; Escrivá, J.; Rodilla, M.; Poersch, L.H. The Role of Bacillus amyloliquefaciens on Litopenaeus vannamei During the Maturation of a Biofloc System. J. Mar. Sci. Eng. 2019, 7, 228.
- da Silva, M.A.C.; Cavalett, A.; Spinner, A.; Rosa, D.C.; Jasper, R.B.; Quecine, M.C.; Bonatelli, M.L.; Pizzirani-Kleiner, A.; Corção, G.; de Souza Lima, A.O. Phylogenetic Identification of Marine Bacteria Isolated from Deep-Sea Sediments of the Eastern South Atlantic Ocean. Springerplus 2013, 2, 127.
- Stincone, P.; Brandelli, A. Marine Bacteria as Source of Antimicrobial Compounds. Crit. Rev. Biotechnol. 2020, 40, 306–319.
- Bibi, F.; Yasir, M.; Al-Sofyani, A.; Naseer, M.I.; Azhar, E.I. Antimicrobial Activity of Bacteria from Marine Sponge Suberea mollis and Bioactive Metabolites of Vibrio sp. EA348. Saudi J. Biol. Sci. 2020, 27, 1139–1147.
- Mondol, M.A.M.; Kim, J.H.; Lee, H.-S.; Lee, Y.-J.; Shin, H.J. Macrolactin W, a New Antibacterial Macrolide from a Marine Bacillus sp. Bioorg. Med. Chem. Lett. 2011, 21, 3832–3835.
- Mondol, M.A.M.; Tareq, F.S.; Kim, J.H.; Lee, M.A.; Lee, H.S.; Lee, Y.J.; Lee, J.S.; Shin, H.J. Cyclic Ether-Containing Macrolactins, Antimicrobial 24-Membered Isomeric Macrolactones from a Marine Bacillus sp. J. Nat. Prod. 2011, 74, 2582–2587.
- Nagao, T.; Adachi, K.; Sakai, M.; Nishijima, M.; Sano, H. Novel Macrolactins as Antibiotic Lactones from a Marine Bacterium. J. Antibiot. 2001, 54, 333–339.
- Mondol, M.A.M.; Tareq, F.S.; Kim, J.H.; Lee, M.A.; Lee, H.S.; Lee, J.S.; Lee, Y.J.; Shin, H.J. New Antimicrobial Compounds from a Marine-Derived Bacillus sp. J. Antibiot. 2013, 66, 89–95.
- Pettit, G.R.; Knight, J.C.; Herald, D.L.; Pettit, R.K.; Hogan, F.; Mukku, V.J.; Hamblin, J.S.; Dodson, M.J.; Chapuis, J.C. Antineoplastic Agents. 570. Isolation and Structure Elucidation of Bacillistatins 1 and 2 from a Marine Bacillus silvestris. J. Nat. Prod. 2009, 72, 366–371.
- Leal, M.C.; Munro, M.H.G.; Blunt, J.W.; Puga, J.; Jesus, B.; Calado, R.; Rosa, R.; Madeira, C. Biogeography and Biodiscovery Hotspots of Macroalgal Marine Natural Products. Nat. Prod. Rep. 2013, 30, 1380–1390.
- Gross, H.; Goeger, D.E.; Hills, P.; Mooberry, S.L.; Ballantine, D.L.; Murray, T.F.; Valeriote, F.A.; Gerwick, W.H. Lophocladines, Bioactive Alkaloids from the Red Alga Lophocladia sp. J. Nat. Prod. 2006, 69, 640–644.
- Park, S.J.; Jeon, Y.J. Dieckol from Ecklonia Cava Suppresses the Migration and Invasion of HT1080 Cells by Inhibiting the Focal Adhesion Kinase Pathway Downstream of Rac1-ROS Signaling. Mol. Cells 2012, 33, 141–149.
- Lee, C. Griffithsin, a Highly Potent Broad-Spectrum Antiviral Lectin from Red Algae: From Discovery to Clinical Application. Mar. Drugs 2019, 17, 567.
- Ren, C.G.; Liu, Z.Y.; Wang, X.L.; Qin, S. The Seaweed Holobiont: From Microecology to Biotechnological Applications. Microb. Biotechnol. 2022, 15, 738–754.
- Kanagasabhapathy, M.; Sasaki, H.; Nagata, S. Phylogenetic Identification of Epibiotic Bacteria Possessing Antimicrobial Activities Isolated from Red Algal Species of Japan. World J. Microbiol. Biotechnol. 2008, 24, 2315–2321.
- Jamal, M.T.; Morris, P.C.; Hansen, R.; Jamieson, D.J.; Burgess, J.G.; Austin, B. Recovery and Characterization of a 30.7-KDa Protein from Bacillus licheniformis Associated with Inhibitory Activity against Methicillin-Resistant Staphylococcus aureus, Vancomycin-Resistant Enterococci, and Listeria monocytogenes. Mar. Biotechnol. 2006, 8, 587–592.
- Aleti, G.; Sessitsch, A.; Brader, G. Genome Mining: Prediction of Lipopeptides and Polyketides from Bacillus and Related Firmicutes. Comput. Struct. Biotechnol. J. 2015, 13, 192–203.
- Hamiche, S.; Badis, A.; Jouadi, B.; Bouzidi, N.; Daghbouche, Y.; Utczás, M.; Mondello, L.; El Hattab, M. Identification of Antimicrobial Volatile Compounds Produced by the Marine Bacterium Bacillus amyloliquefaciens Strain S13 Newly Isolated from Brown Alga Zonaria tournefortii. J. Essent. Oil Res. 2019, 31, 203–210.
- Asharaf, S.; Chakraborty, K.; Chakraborty, R.D. Seaweed-Associated Heterotrophic Bacteria: Are They Future Novel Sources of Antimicrobial Agents against Drug-Resistant Pathogens? Arch. Microbiol. 2022, 204, 232.
- Asharaf, S.; Chakraborty, K. Seaweed-Associated Heterotrophic Bacillus altitudinis MTCC13046: A Promising Marine Bacterium for Use against Human Hepatocellular Adenocarcinoma. Arch. Microbiol. 2023, 205, 10.
- Chakraborty, K.; Varghese, C.; Asharaf, S.; Chakraborty, R.D. Antibiotic-Active Heterotrophic Firmicutes Sheltered in Seaweeds: Can They Add New Dimensions to Future Antimicrobial Agents? Arch. Microbiol. 2022, 204, 183.
- Varghese, C.; Chakraborty, K.; Asharaf, S. Pharmacological Potential of Seaweed-Associated Heterotrophic Bacterium Bacillus atrophaeus. Arch. Microbiol. 2023, 205, 6.
- Chakraborty, K.; Thilakan, B.; Kizhakkekalam, V.K. Antibacterial Aryl-Crowned Polyketide from Bacillus subtilis Associated with Seaweed Anthophycus longifolius. J. Appl. Microbiol. 2018, 124, 108–125.
- Chakraborty, K.; Thilakan, B.; Raola, V.K. Antimicrobial Polyketide Furanoterpenoids from Seaweed-Associated Heterotrophic Bacterium Bacillus subtilis MTCC 10403. Phytochemistry 2017, 142, 112–125.
- Chakraborty, K.; Thilakan, B.; Raola, V.K. Polyketide Family of Novel Antibacterial 7-O-Methyl-5′-Hydroxy-3′-Heptenoate-Macrolactin from Seaweed-Associated Bacillus subtilis MTCC 10403. J. Agric. Food Chem. 2014, 62, 12194–12208.
- Chakraborty, K.; Thilakan, B.; Chakraborty, R.D.; Raola, V.K.; Joy, M. O-Heterocyclic Derivatives with Antibacterial Properties from Marine Bacterium Bacillus subtilis Associated with Seaweed, Sargassum myriocystum. Appl. Microbiol. Biotechnol. 2017, 101, 569–583.
- Villarreal-Gómez, L.J.; Soria-Mercado, I.E.; Guerra-Rivas, G.; Ayala-Sánchez, N.E. Antibacterial and Anticancer Activity of Seaweeds and Bacteria Associated with Their Surface. Rev. Biol. Mar. Oceanogr. 2010, 45, 267–275.
- Chakraborty, K.; Thilakan, B.; Raola, V.K. Previously Undescribed Antibacterial Polyketides from Heterotrophic Bacillus amyloliquefaciens Associated with Seaweed Padina gymnospora. Appl. Biochem. Biotechnol. 2018, 184, 716–732.
- Chakraborty, K.; Kizhakkekalam, V.K.; Joy, M.; Chakraborty, R.D. Moving Away from Traditional Antibiotic Treatment: Can Macrocyclic Lactones from Marine Macroalga-Associated Heterotroph Be the Alternatives? Appl. Microbiol. Biotechnol. 2020, 104, 7117–7130.
- Chakraborty, K.; Kizhakkekalam, V.K.; Joy, M.; Chakraborty, R.D. A Leap Forward towards Unraveling Newer Anti-Infective Agents from an Unconventional Source: A Draft Genome Sequence Illuminating the Future Promise of Marine Heterotrophic Bacillus sp. Against Drug-Resistant Pathogens. Mar. Biotechnol. 2021, 23, 790–808.
- Kizhakkekalam, V.K.; Chakraborty, K.; Joy, M. Oxygenated Elansolid-Type of Polyketide Spanned Macrolides from a Marine Heterotrophic Bacillus as Prospective Antimicrobial Agents against Multidrug-Resistant Pathogens. Int. J. Antimicrob. Agents 2020, 55, 105892.
- Chakraborty, K.; Kizhakkekalam, V.K.; Joy, M.; Chakraborty, R.D. Novel Amylomacins from Seaweed-Associated Bacillus amyloliquefaciens as Prospective Antimicrobial Leads Attenuating Resistant Bacteria. World J. Microbiol. Biotechnol. 2021, 37, 200.
- Kizhakkekalam, V.K.; Chakraborty, K. Marine Macroalgae-Associated Heterotrophic Firmicutes and Gamma-Proteobacteria: Prospective Anti-Infective Agents against Multidrug Resistant Pathogens. Arch. Microbiol. 2020, 202, 905–920.
- Kizhakkekalam, V.K.; Chakraborty, K. Pharmacological Properties of Marine Macroalgae-Associated Heterotrophic Bacteria. Arch. Microbiol. 2019, 201, 505–518.
- Chakraborty, K.; Kizhakkekalam, V.K.; Joy, M.; Dhara, S. Difficidin Class of Polyketide Antibiotics from Marine Macroalga-Associated Bacillus as Promising Antibacterial Agents. Appl. Microbiol. Biotechnol. 2021, 105, 6395–6408.
- Chakraborty, K.; Kizhakkekalam, V.K.; Joy, M. Polyketide-Derived Macrobrevins from Marine Macroalga-Associated Bacillus amyloliquefaciens as Promising Antibacterial Agents against Pathogens Causing Nosocomial Infections. Phytochemistry 2022, 193, 112983.
- Chakraborty, K.; Francis, A.; Chakraborty, R.D.; Asharaf, S.; Kizhakkekalam, V.K.; Paulose, S.K. Marine Macroalga-Associated Heterotrophic Bacillus velezensis: A Novel Antimicrobial Agent with Siderophore Mode of Action against Drug-Resistant Nosocomial Pathogens. Arch. Microbiol. 2021, 203, 5561–5575.
- Francis, A.; Chakraborty, K. Marine Macroalga—Associated Heterotroph Bacillus velezensis as Prospective Therapeutic Agent. Arch. Microbiol. 2021, 203, 1671–1682.
- Chakraborty, K.; Thilakan, B.; Raola, V.K.; Joy, M. Antibacterial Polyketides from Bacillus amyloliquefaciens Associated with Edible Red Seaweed Laurenciae papillosa. Food Chem. 2017, 218, 427–434.
- Gaillard, T.; Dormoi, J.; Madamet, M.; Pradines, B. Macrolides and Associated Antibiotics Based on Similar Mechanism of Action like Lincosamides in Malaria. Malar. J. 2016, 15, 85.
- Das, R.; Rauf, A.; Mitra, S.; Emran, T.B.; Hossain, M.J.; Khan, Z.; Naz, S.; Ahmad, B.; Meyyazhagan, A.; Pushparaj, K.; et al. Therapeutic Potential of Marine Macrolides: An Overview from 1990 to 2022. Chem. Biol. Interact. 2022, 365, 110072.
- Dinos, G.P. The Macrolide Antibiotic Renaissance. Br. J. Pharmacol. 2017, 174, 2967–2983.
- Wang, B.; Wang, L.; Li, Y.; Liu, Y. Heterocyclic Terpenes: Linear Furano- and Pyrroloterpenoids. RSC Adv. 2014, 4, 12216–12234.
- Peterson, L.A. Reactive Metabolites in the Biotransformation of Molecules Containing a Furan Ring. Chem. Res. Toxicol. 2013, 26, 6–25.
- Li, H.; Peng, Y.; Zheng, J. Metabolic Activation and Toxicities of Furanoterpenoids; Elsevier: Amsterdam, The Netherlands, 2016; Volume 10, ISBN 9780128047002.
- Fouche, G.; Nieuwenhuizen, N.; Maharaj, V.; van Rooyen, S.; Harding, N.; Nthambeleni, R.; Jayakumar, J.; Kirstein, F.; Emedi, B.; Meoni, P. Investigation of In Vitro and In Vivo Anti-Asthmatic Properties of Siphonochilus aethiopicus. J. Ethnopharmacol. 2011, 133, 843–849.
- Lategan, C.A.; Campbell, W.E.; Seaman, T.; Smith, P.J. The Bioactivity of Novel Furanoterpenoids Isolated from Siphonochilus aethiopicus. J. Ethnopharmacol. 2009, 121, 92–97.
- Park, H.; Perez, C.; Perry, E.; Crawford, J. Activating and Attenuating the Amicoumacin Antibiotics. Molecules 2016, 21, 824.
- Brinkmann, C.; Kearns, P.; Evans-Illidge, E.; Kurtböke, D. Diversity and Bioactivity of Marine Bacteria Associated with the Sponges Candidaspongia flabellata and Rhopaloeides odorabile from the Great Barrier Reef in Australia. Diversity 2017, 9, 39.
- Ali, A.I.-B.; El Bour, M.; Ktari, L.; Bolhuis, H.; Ahmed, M.; Boudabbous, A.; Stal, L.J. Jania rubens-Associated Bacteria: Molecular Identification and Antimicrobial Activity. J. Appl. Phycol. 2012, 24, 525–534.
- Chen, X.H.; Koumoutsi, A.; Scholz, R.; Eisenreich, A.; Schneider, K.; Heinemeyer, I.; Morgenstern, B.; Voss, B.; Hess, W.R.; Reva, O.; et al. Comparative Analysis of the Complete Genome Sequence of the Plant Growth–Promoting Bacterium Bacillus amyloliquefaciens FZB42. Nat. Biotechnol. 2007, 25, 1007–1014.
- Albakosh, M.A.; Naidoo, R.K.; Kirby, B.; Bauer, R. Identification of Epiphytic Bacterial Communities Associated with the Brown Alga Splachnidium rugosum. J. Appl. Phycol. 2016, 28, 1891–1901.
- Prieto, M.L.; O’Sullivan, L.; Tan, S.P.; McLoughlin, P.; Hughes, H.; O’Connor, P.M.; Cotter, P.D.; Lawlor, P.G.; Gardiner, G.E. Assessment of the Bacteriocinogenic Potential of Marine Bacteria Reveals Lichenicidin Production by Seaweed-Derived Bacillus spp. Mar. Drugs 2012, 10, 2280–2299.
- Burgess, J.G.; Boyd, K.G.; Armstrong, E.; Jiang, Z.; Yan, L.; Berggren, M.; May, U.; Pisacane, T.; Granmo, Å.; Adams, D.R. The Development of a Marine Natural Product-Based Antifouling Paint. Biofouling 2003, 19, 197–205.
- Thilakan, B.; Chakraborty, K.; Chakraborty, R.D. Antimicrobial Properties of Cultivable Bacteria Associated with Seaweeds in the Gulf of Mannar on the Southeast Coast of India. Can. J. Microbiol. 2016, 62, 668–681.
- Trischman, J.A.; Oeffner, R.E.; De Luna, M.G.; Kazaoka, M. Competitive Induction and Enhancement of Indole and a Diketopiperazine in Marine Bacteria. Mar. Biotechnol. 2004, 6, 215–220.
- Wiese, J.; Thiel, V.; Nagel, K.; Staufenberger, T.; Imhoff, J.F. Diversity of Antibiotic-Active Bacteria Associated with the Brown Alga Laminaria saccharina from the Baltic Sea. Mar. Biotechnol. 2009, 11, 287–300.
- Penesyan, A.; Marshall-Jones, Z.; Holmstrom, C.; Kjelleberg, S.; Egan, S. Antimicrobial Activity Observed among Cultured Marine Epiphytic Bacteria Reflects Their Potential as a Source of New Drugs. FEMS Microbiol. Ecol. 2009, 69, 113–124.
- Kanagasabhapathy, M.; Sasaki, H.; Haldar, S.; Yamasaki, S.; Nagata, S. Antibacterial Activities of Marine Epibiotic Bacteria Isolated from Brown Algae of Japan. Ann. Microbiol. 2006, 56, 167–173.
- Ismail, A.; Ktari, L.; Ahmed, M.; Bolhuis, H.; Boudabbous, A.; Stal, L.J.; Cretoiu, M.S.; El Bour, M. Antimicrobial Activities of Bacteria Associated with the Brown Alga Padina pavonica. Front. Microbiol. 2016, 7, 1072.
- Susilowati, R.; Sabdono, A.; Widowati, I. Isolation and Characterisation of Bacteria Associated with Brown Algae Sargassum spp. from Panjang Island and Their Antibacterial Activities. Procedia Environ. Sci. 2015, 23, 240–246.
- Kumar, V.; Rao, D.; Thomas, T.; Kjelleberg, S.; Egan, S. Antidiatom and Antibacterial Activity of Epiphytic Bacteria Isolated from Ulva lactuca in Tropical Waters. World J. Microbiol. Biotechnol. 2011, 27, 1543–1549.
- Quinn, G.A.; Maloy, A.P.; McClean, S.; Carney, B.; Slater, J.W. Lipopeptide Biosurfactants from Paenibacillus polymyxa Inhibit Single and Mixed Species Biofilms. Biofouling 2012, 28, 1151–1166.
- Suresh, M.; Renugadevi, B.; Brammavidhya, S.; Iyapparaj, P.; Anantharaman, P. Antibacterial Activity of Red Pigment Produced by Halolactibacillus alkaliphilus MSRD1—An Isolate from Seaweed. Appl. Biochem. Biotechnol. 2015, 176, 185–195.
- Karthick, P.; Mohanraju, R. Antimicrobial Potential of Epiphytic Bacteria Associated with Seaweeds of Little Andaman, India. Front. Microbiol. 2018, 9, 611.
- Karthick, P.; Mohanraju, R. Antimicrobial Compounds Produced by Lysinibacillus odysseyi Epiphytic Bacteria Associated with Red Algae. Brazilian J. Microbiol. 2020, 51, 1683–1690.
- Horta, A.; Alves, C.; Pinteus, S.; Lopes, C.; Fino, N.; Silva, J.; Ribeiro, J.; Rodrigues, D.; Francisco, J.; Rodrigues, A.; et al. Identification of Asparagopsis armata-associated Bacteria and Characterization of Their Bioactive Potential. MicrobiologyOpen 2019, 8, e00824.
- Vairagkar, U.; Mirza, Y. Antagonistic Activity of Antimicrobial Metabolites Produced from Seaweed-Associated Bacillus amyloliquefaciens MTCC 10456 Against Malassezia spp. Probiotics Antimicrob. Proteins 2021, 13, 1228–1237.
More
