Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Lindsay Dong and Version 1 by Mohammadreza Naeimirad.
The growing awareness of environmental issues and the pursuit of sustainable materials have sparked a substantial surge in research focused on biodegradable materials, including fibers. Within a spectrum of fabrication techniques, melt-spinning has emerged as an eco-friendly and scalable method for making fibers from biodegradable plastics (preferably bio-based), intended for various applications.
- bio-based polymers
- fiber melt-spinning
- microplastics
- biodegradation
- sustainability
- mechanical properties
1. Introduction
Nowadays, plastic products have become an indispensable part of our daily lives, possessing a wide range of properties that vary from flexibility to rigidity, permeability to impermeability, and hydrophilicity to hydrophobicity. These properties are determined by the composition of the polymer’s repeating units, commonly referred to as the chemical backbone. Plastics play a crucial role in promoting sustainability on various fronts. For instance, the utilization of lightweight plastic materials enhances fuel efficiency in automobiles and aircraft; plastic insulators contribute to energy conservation; and plastic food packaging extends the shelf-life of products, thereby reducing food waste [1]. The remarkable success and continuous expansion of the plastic industry can be attributed to its affordability, durability, favorable strength-to-weight ratios, and the convenience it brings to our everyday lives [2]. Moreover, the plastic industry serves as a significant source of employment, with over 1.6 million individuals employed within the sector across the European Union, resulting in a turnover of 360 billion Euros in 2018 [3]. However, the extensive production and accumulation of plastics have led to severe environmental issues. The annual global production of plastic exceeds 380 million tons (excluding 110 million tons of fibers and also thermoset resins and rubbers), increasing at a rate of 4% each year [4]. Consequently, between 1950 and 2015, a staggering 6300 million tons of plastic waste were generated [5]. This implies that the production of 500 million tons of plastics is obtained from approximately 4–8% of global oil consumption—1000 million tons of oil (500 million tons of C/H in the product and 500 million tons of energy required for the production) [6]. In 2050, this will be threefold, and plastic production will go over 1 billion tons [7,8][7][8].
Various end-of-life scenarios exist for plastic materials, including mechanical recycling [13][9], chemical recycling (hydrolysis, glycolysis, methanolysis, thermolysis, and pyrolysis) [14][10], biological recycling [15][11], composting, biodegradation, incineration (accounting for 40% in the EU), and landfilling (representing 27% in the EU) [3]. However, landfilling and incineration of conventional plastics present several environmental concerns [16][12]. Incineration, while reducing the need for landfilling and enabling energy recovery, must comply with environmental regulations outlined in the EU Hazardous Waste Incineration Directive 2000/76/EC [17][13]. In Europe, for instance, approximately 25.8 million tons of post-consumer plastic waste are generated annually, with 30% being recycled and 40% destined for incineration [18][14]. Plastic products can contribute to litter, leading to the release of micro- and nanoplastics (MNPs) [19,20,21][15][16][17] into the oceans and aquaculture [22][18], then posing potential risks to human health when they enter the lungs [23][19] or bloodstream [24][20].
Arikan and Özsoy [30][21] highlighted several environmental and economic issues resulting from the widespread use of plastics, such as landfill problems, plastic accumulation in oceans, incineration, non-degradability, durability, and economic concerns related to crude oil competition and energy security. Therefore, it is crucial to minimize the environmental impacts of plastics by transitioning to circular and sustainable plastic systems [31][22]. Spierling et al. [32][23] suggested that shifting around 66% (approximately 220 million tons based on 2017 estimates) of plastic production to bio-based materials could potentially save 241–316 million tons of CO2-equivalents annually. Consequently, the development of bio-based and biodegradable plastics has emerged as a topic of interest for creating future materials capable of replacing conventional plastics in various sustainable applications.
Historically, non-fiber plastics have been dominated by polyethylene (PE; 36.4%), polypropylene (PP; 21%), and polyvinyl chloride (PVC; 12%) since 1950. In contrast, the fiber market is largely dominated by polyethylene terephthalate (PET; 60%). In 2015, the largest plastic volumes in global commercial sectors were in packaging (35.9%), construction (16.0%), textiles (14.5%), and consumer goods (10.3%) [5]. Furthermore, global fiber production reached 111 million metric tons, having doubled over the past 20 years [34][24]. Figure 1 illustrates the distribution of plastics in the different sectors and their respective plastic waste management approaches thus far.
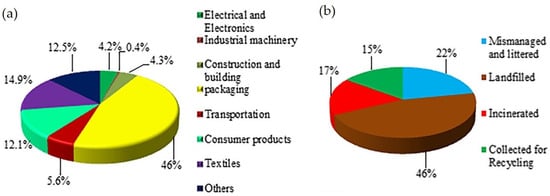

Figure 1. (a) Plastic consumption in various sectors; and (b) Plastic waste management in the world [35][25]. Reprinted with permission from the publisher.
The aforementioned pie charts show that textiles account for 14.9% of global plastic production, with a significant portion of PET (70% of 80 million tons produced annually) being used for fiber and yarn production. However, only 15% of plastic products are collected for recycling. The small size and high aspect ratio of single fibers released from textiles, including microplastics and microfibers, make them particularly hazardous. Detecting and efficiently collecting these microplastics, especially microfibers, from the oceans is not possible. Floating microplastics in coastal areas can reach counts of 103 to 104 per m3. This lack of efficient removal methods is concerning because these floating microplastics accumulate pollutants and transport them through ocean currents. Persistent organic pollutants (POPs) bind to the surface of plastic debris in the marine environment [36][26]. Systematic studies [37,38,39,40][27][28][29][30] indicate that microfibers released from textiles, particularly polyester (PET), pose a significant concern. The release of microfibers during washing can range from 210 to 72,000 microplastic fibers per gram of textile, with median fiber lengths ranging from 165 to 841 μm. So far, more than 20,000 fibers per day were carried downstream by the river, while up to 213,200 fibers per square kilometer were detected drifting on the ocean’s surface [41][31]. These microfibers contribute to marine contamination, often referred to as “plastic soup” with press releases highlighting their contribution of about 35% reported by Statista [42][32], Reuters [43][33], The Guardian [44][34], the European Environment Agency [45[35][36],46], and elsewhere [22,47,48,49][18][37][38][39].
Polylactic acid (PLA), polyhydroxyalkanoates (PHAs), thermoplastic starch (TPS), polybutylene adipate terephthalate (PBAT), polybutylene succinate (PBS), and polycaprolactone (PCL) are typically considered biodegradable thermoplastics that are produced on an industrial scale [7]. However, their commodity applications are limited by their poor physical properties and a low glass transition temperature (Tg) and melting temperature (Tm)—for the majority of biodegradable polymers with lower crystallinity—for the replacement of fossil-based counterparts, such as PET.
All in all, bio-based alternatives to petroleum-derived polymers can mitigate climate change, while biodegradable polymers are needed to tackle the issues caused by their extensive use and improper disposal [57][40]. The textile industry has also embraced the adoption of bio-based and biodegradable polymers, with the textile market projected to grow at a CAGR of 12% over the next decade [31][22]. The clothing sector is expected to play a dominant role in this market. Therefore, biodegradable plastics are the best option when the product or litter is unintentionally fate-in-nature. Aerobic or anaerobic digestion and composting are the routes of final utilization [58][41].
Numerous review articles have been published about bio-based and biodegradable polymers [7,30[7][21][42][43][44][45][46][47][48][49],59,60,61,62,63,64,65,66], making biocomposites [31,67,68][22][50][51], fibrous products [54,69,70,71][52][53][54][55] biomedical applications [69[53][54][56],70,72], and their biodegradation [63,73][46][57]. Rosenboom et al. [1] from the Langer group reviewed the advantages and challenges of bio-based plastics in transitioning towards a circular economy, emphasizing the lower carbon footprint and favorable material properties of bio-based plastics compared to fossil-based plastics. They also highlighted the importance of essential regulations and financial incentives to scale up bio-based and biodegradable plastics from niche polymers to large-scale market applications with a truly sustainable impact.
Meanwhile, melt-spinning is a widely used, sustainable, and cost-effective method for producing man-made fibers and filament yarns from thermoplastic polymers. The process involves feeding polymer pellets or chips into a single screw extruder, where they are melted and pressurized. In some cases, masterbatches can be added through a side extruder for specific applications such as dope-dyed yarns [54,74][52][58]. A melt pump ensures a consistent throughput rate. The spin pack, which includes polymer filtering and distribution components as well as the spinneret, is responsible for forming the filaments with the desired characteristics such as number and cross-section. It is crucial to design the extrusion and spinning lines in a way that prevents melt stagnation, which can lead to polymer degradation and intermittent discharge. After leaving the spinneret, the extruded strands are either directed into a quenching chamber or a water bath to solidify. The filaments undergo cooling and are then subjected to drawing to induce or enhance crystallinity, either online or offline, using several godets (cold or hot). Heating the godets or guiding the filaments over hot plates or through stretching ovens can enhance their drawability. Finally, the filaments are wound onto bobbins using a winder or collected to be cut into staple fibers [54][52].
Melt-spinning is considered more economical and sustainable compared to other spinning methods such as dry-spinning and wet-spinning. Dry-spinning and wet-spinning have lower production speeds and involve the use of chemical solvents, which are not environmentally friendly [75][59]. Moreover, these methods can result in surface pores and voids. Therefore, melt-spun fibers have significant potential to replace conventional fiber in various applications where biodegradability is important [31][22].
The physiochemical properties of synthesized biodegradable polymers and their effect on biodegradation are areas of ongoing investigation. Considering the influence of processing parameters on the structure and performance of man-made fibers, along with the end-of-life environment affecting biodegradation routes and rates, designing biodegradable polymers becomes a challenge. Optimizing both physiochemical properties and biodegradability is crucial. A deeper understanding of the relationship between polymer structure and biodegradation will aid in the development of new biodegradable polymers and end products by modifying relevant processing-related properties. This concept contributes to a better comprehension of the polymer’s structure-property relationship [76,77,78][60][61][62].
2. Bio-Based and Biodegradable Plastics
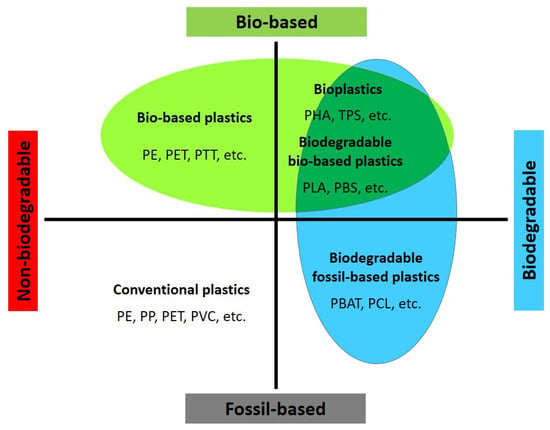
The demand for bio-based polymers and their use in more sustainable products, including fibers and textiles, is driven by both market demand and legislative intentions. When considering sustainability, two main subsectors are relevant: bio-based polymers and biodegradable polymers (some polymers can be both). Bio-based polymers are derived from natural resources such as starch (e.g., thermoplastic starch) or cellulose (e.g., cellulose acetate) and polyhydroxyalkanoates (PHAs) or can be produced from bio-based monomers (e.g., bio-PE, PLA, PBS, PBAT, etc.), while biodegradable polymers are designed to naturally break down and be utilized in the environment through biotic or abiotic processes, regardless of whether they are derived from synthetic or biomass feedstock [53,80][63][64]. Currently, the production of bio-based and biodegradable plastics is around 2.2 million tons per year, and its expansion is considered part of future circular economies, aligning with the United Nations’ Sustainable Development Goals and EU legislation [81][65]. These initiatives aim to shift away from fossil resources, introduce new recycling or degradation pathways, and reduce the use of toxic reagents and solvents in production processes. However, it should be noted that bio-based plastics are not automatically more sustainable than fossil-based plastics. While using renewable resources can help reduce carbon emissions, other factors throughout the life cycle can influence the overall sustainability of the materials [1,53][1][63]. The Langer group has identified five main challenges in the implementation of bio-based plastics, known as the “5Es”: The specific physicochemical, biological, and degradation properties of biodegradable polymers make them attractive for various applications, including fibers and textiles [7]. As environmental concerns related to plastic accumulation and landfill waste continue to grow, extensive research is being conducted to find possible solutions. These polymers can be classified into two main categories based on whether their starting materials are derived from petroleum or non-petroleum sources. According to Figure 42, there are various examples of biodegradable polymers, regardless of whether they are derived from fossil or bio-based sources (considering composting as a human-driven process for utilization). For instance, polylactic acid (PLA) is only compostable, while polyhydroxyalkanoates (PHAs), plasticized thermoplastic starch (TPS), polybutylene succinate (PBS), polycaprolactone (PCL), and polyglycolic acid (PGA) are biodegradable. Meanwhile, polyurethanes are generally less biodegradable due to the strength of the urethane bonds. However, fungi and certain soil bacteria can assist in hydrolyzing the ester groups within polyester-containing polyurethane [83][66]. Additionally, less degradable monomers such as terephthalates can be made more degradable through copolymerization with more hydrolysable, hydrophilic, and less crystalline co-polymers, as observed in poly(butylene-co-adipate terephthalate) (PBAT) [84][67]. The subsequent sections will discuss other biodegradable copolymers, including poly(lactic-co-glycolic acid) (PLGA), poly(butylene succinate-co-butylene adipate) (PBSA), poly(butylene succinate-co-terephthalate) (PBST), polybutylene-co-ethylene succinate-co-adipate (PBEAS), poly(butylene terephthalate-co-succinate-co-adipate) (PBTSA), and poly(isosorbide-co-hexylene) oxalate polyester (PIHO).
Figure 42. Classification of different plastics. Adapted from [6,64,80].
2.1. Biodegradable Thermoplastic Polymers
2.1.1. PLA
PLA (polylactic acid) is an aliphatic polyester and one of the most competitively priced bio-based plastics, with a production capacity exceeding 250,000 tons per year. It is typically produced through the ring-opening polymerization of lactide (the cyclic dimer of lactic acid). PLAs can be made from monomers with two isomers of lactic acid (L-lactic acid) or (D-lactic acid) which will result in PLLA, PDLA, or a copolymer (PLDLA). In general, D-content will reduce crystallinity. PLA has high crystallinity, which contributes to its favorable properties for various applications. However, it is only compostable within 6 months at temperatures higher than its Tg, typically at around 58 °C in industrial composting conditions. At ambient temperature, PLA degrades very slowly in soil and water, with estimates suggesting that complete degradation could take several years depending on the environment [86,87][68][69]. Since its industrial production began in the 1990s, PLA has gradually gained popularity as a commodity thermoplastic, expanding into more diverse applications, including the textile sector, due to its significant drop in price [88][70]. Consequently, PLA has become the most common bio-based and biodegradable (compostable) material used for melt-spun bio-based polymer fibers. Its high crystallinity, better mechanical properties, and lower thermal degradation compared to other biodegradable polymers make it suitable for numerous applications. Melt-spinning of PLA fibers has been employed for a long time, with take-up speeds reaching up to 5000 m/min [89][71]. It is considered the most promising sustainable and biodegradable fiber to replace PET in textile products [75][59]. The development of PLA fiber structure during the melt-spinning process is presented in a chapter by Roungpaisan et al. [90][72].2.1.2. PHAs
PHAs (polyhydroxyalkanoates) are a family of emerging biodegradable aliphatic polyesters with a projected annual market volume of over 100,000 tons in the near future. PHA production can be accomplished through the process of microbial fermentation using bacterial strains [93][73], such as Pseudomonas and Ralstonia, as well as algae. Subsequent extraction of PHAs is performed to isolate the polymer. These microorganisms possess the remarkable ability to store PHA within their cells, reaching levels of up to 80% of their cell volume. A key advantage of the biological PHA production process is its ability to utilize diverse carbon-rich feedstocks, including low-cost food residues and liquefied plastic waste, thereby enhancing circularity [7]. PHAs, composed of (R)-3-hydroxyalkanoic acids, are a class of non-toxic, biodegradable, and biocompatible polyesters that closely resemble petrochemical plastics in their thermoplastic properties [25,94][74][75]. The physical characteristics of PHAs vary due to the diverse compositions of their monomers, enabling them to cater to a wide range of applications. Based on the length of the PHA monomer’s carbon chain, PHAs are classified as short-chain length (scl) PHAs (consisting of 4 or 5 carbons) or medium-chain length (mcl) PHAs (consisting of 6–14 carbons). The properties of PHAs, such as degree of crystallinity, Tm, and Tg, heavily rely on the monomer composition, which, in turn, is influenced by factors such as the organism, growth conditions, and polymer extraction method. While sclPHA exhibits properties closer to conventional plastics such as polypropylene, mclPHA displays more elastomeric properties [7]. Poly-3-hydroxybutyrate (PHB) is the most extensively studied PHA polymer, characterized by its brittle and highly crystalline nature akin to polypropylene. However, to tailor the thermal and mechanical properties of PHAs, copolymerization is often employed [80,95][64][76]. The biodegradation efficiency of PHAs and their biological resources make them highly valuable for replacing traditional plastics [98][77]. In the marine environment, PHAs were found to biodegrade at an average rate of 0.04–0.09 mg/day/cm2, suggesting that it would take approximately 1.5 to 3.5 years for a PHA water bottle to be completely decomposed [99][78]. The mechanical properties of PHAs are influenced by factors such as MW, side chain length, monomer type, and ratio. The interaction of these variables can affect the Tg and Tm, as well as the crystallinity (stiffness/flexibility) of the polymers [96][79]. PHBs, for example, exhibit instability near their melting point of 160–180 °C, with a Tg of 0–5 °C. Thermal degradation occurs around 170 °C, leading to reduced molecular weight [100][80]. The mechanical properties of specific PHA polymers are influenced by the metabolic pathways, enzymes of the bacterial strain, and substrate utilization [70][54]. The superior biocompatibility and biodegradability of PHAs compared to synthetic biodegradable polymers make them highly promising for various fiber applications, particularly in biomedicine [69,70][53][54]. However, purification, high cost, limited mechanical performance, and thermal instability, resulting in molecular weight reduction and a limited processing window, remain significant barriers for PHAs.2.1.3. TPS
TPS (thermoplastic plasticized starch) is a biodegradable polymer derived from starch, which is typically sourced from corn, potatoes, or other plant materials. It has emerged as an environmentally friendly alternative to petroleum-based plastics. However, when it comes to melt-spinning, TPS faces certain challenges. One significant limitation is its relatively low melt strength and poor processability at high temperatures. TPS tends to degrade and undergo thermal decomposition before it can be effectively melt-spun into fibers. Its narrow processing window and propensity for viscosity reduction and chain scission during melt processing can result in suboptimal mechanical properties and limited control over fiber diameter and properties. Although, with an optimal ratio of the components (amylose and amylopectin), the processability can be improved to some extent, multifilament yarn melt-spinning is far from being achieved.2.1.4. PBS
PBS (polybutylene succinate) is an aliphatic copolyester with longer hydrocarbon repeat units, resulting in a more flexible molecular structure compared to PLA. This gives PBS distinct material properties, such as a low Tg and high elongation at break (>500%), making it more similar to polyolefins. While PBS is typically synthesized from non-renewable feedstock, it can also be produced from renewable sources, resulting in Bio-PBS. For instance, succinic acid, one of the monomers used in PBS production, can be obtained through the fermentation of sugars, while butanediol can be obtained by succinic acid hydrogenation [106][81] or derived from hydrocracking starches and sugars [107][82].2.1.5. PCL
PCL (polycaprolactone) is an aliphatic polyester of synthetic origin that has gained considerable attention in recent times. It is produced by ring-opening polymerization of ε-caprolactone, a cyclic monomer. Catalysts such as stannous octoate are utilized to initiate the polymerization process, and low-molecular-weight alcohols can be employed to control the polymer’s molecular weight. The resulting molecular weight, molecular weight distribution, end group composition, and chemical structure of PCL copolymers can be influenced by various mechanisms such as anionic, cationic, coordination, and radical polymerization. The average molecular weight of PCL samples typically falls within the range of 3000 to 100,000 g/mol, and classification based on molecular weight is common practice. PCL exhibits semi-crystalline characteristics, with a melting point ranging from 59 to 64 °C and a Tg of −60 °C. What makes PCL highly versatile is its ease of modification in terms of physical, chemical, and mechanical properties. This can be achieved through copolymerization or blending with other polymers [111][83].2.1.6. PGA
PGA (polyglycolic acid) is the simplest aliphatic polyester derived from glycolic acid through a ring-opening polymerization process (glycolide). It is well-known for its high strength, excellent biocompatibility, and biodegradability, making it suitable for a wide range of applications. One notable application of PGA is in fiber production. PGA fibers exhibit exceptional tensile strength and find uses in various industries, including medical, textile, and packaging. In the medical field, PGA fibers are commonly used for sutures, providing temporary support during wound healing before naturally degrading over time. The packaging industry utilizes PGA for applications that require high-strength and biodegradable characteristics. PGA fibers offer a unique combination of strength, biocompatibility, and biodegradability, making them an appealing choice for applications that prioritize sustainability and performance. PGA undergoes complete degradation through hydrolysis (both enzymatic and non-enzymatic). This interesting polymer provides a high crystallization rate and content (even higher than PLA) and fast biodegradation (even faster than PCL and PHA) at the same time. PGA provides a fast biodegradation rate (due to hydrolysis), high mechanical properties (due to in-order structure and high crystallization), and biocompatibility for the spun fibers. It is also interesting that PGA is not soluble in most organic solvents, including chloroform, and needs fluorinated solvents such as HFIP. Furthermore, PGA has excellent barrier properties [118][84].2.1.7. PLGA
PLGA (poly(lactic-co-glycolic acid)) is a copolymer of PGA synthesized through ring-opening polymerization of lactide and glycolide cyclic diesters. The copolymerization process allows for the adjustment of the ratio between lactide and glycolide, providing control over the material’s properties, such as crystallization, hydrolysis, biodegradation rate, mechanical strength, and hydrophilicity [119,120][85][86]. PLGA has found wide-ranging applications in the form of fibers. Various techniques, such as electrospinning, melt-spinning, or wet-spinning, can be employed to produce PLGA fibers, resulting in different fiber morphologies and mechanical characteristics. PLGA fibers have been utilized in diverse applications, including drug delivery, tissue engineering, wound dressings, tissue suturing, and biosensing. The versatility and biocompatibility of PLGA make it a promising material for fabricating fibers with tailored properties for a wide range of biomedical and pharmaceutical applications [124][87].2.1.8. PBAT
PBAT (poly(butylene adipate-co-terephthalate)) is a biodegradable copolymer synthesized through the polymerization of the three monomers or from PBA with dimethyl terephthalate (DMT) and 1,4-butanediol (BDO) monomers [125][88]. These copolymerization processes result in a material that combines the flexibility of butylene adipate with the strength and rigidity of butylene terephthalate. PBAT exhibits excellent biodegradability under appropriate conditions. PBAT fibers have gained attention in various applications due to their unique properties. Techniques such as melt-spinning can be used to produce PBAT fibers, allowing for the fabrication of fibers with different morphologies and mechanical characteristics. PBAT applications include fibers, nonwovens, packaging, and hygiene products. The flexibility, biodegradability, and tunable properties of PBAT fibers make them a promising choice for sustainable and eco-friendly fiber applications. PBAT exhibits elastic properties with a low modulus and high recoverability due to its hard-soft segment chemical structure. However, the Tm of PBAT is around 100 °C, which may be a limiting factor for certain applications [126][89].2.1.9. PBSA
PBSA (poly(butylene succinate-co-adipate)) is a copolymer synthesized through the polymerization of succinate and PBA. The specific parameters of the copolymerization process can be adjusted to control the material’s properties, such as mechanical strength, thermal stability, and biodegradability. PBSA can also be used for fiber production. The combination of biodegradability, mechanical performance, and processability makes PBSA fibers an attractive option for sustainable approaches [129][90]. Manufacturers such as Mitsubishi, BASF, and Tianjin GreenBio Materials produce PBSA for various applications.2.1.10. PBST
PBST (poly(butylene succinate-co-terephthalate)) is a versatile biodegradable copolymer that offers unique properties and finds diverse applications in the field of fibers. It is synthesized through the copolymerization of butylene succinate and terephthalate monomers. By combining the desirable traits of polybutylene succinate and terephthalate, PBST exhibits enhanced mechanical strength, thermal stability, and biodegradability. Melt-spun PBST fibers can be produced with different morphologies and mechanical properties, making them suitable for a variety of applications. In industries such as textiles, PBST fibers serve as an eco-friendly alternative, meeting the increasing demand for sustainable materials in today’s world [129][90]. Companies such as Novament, Meredian, Sinopec Yizheng, and Lotte Chemicals are some examples of sources for PBST.2.1.11. PBEAS
PBEAS (poly(butylene-co-ethylene adipate-co-terephthalate)) is a copolymer synthesized through the copolymerization of butylene, ethylene, adipic-, and succinic acid monomers. This combination of monomers results in a copolymer with a balance of flexibility, mechanical strength, and biodegradability. PBEAS fibers have been reported in monofilament and multifilament topologies, although the presence of block copolymers can interfere with the process due to their high elastic behavior. The low melting point of PBEAS can also present a challenge for certain applications. Nevertheless, PBEAS fibers have been considered in some reports for their potential in the green transition of the textile sector [129,130][90][91]. Information on specific manufacturers of PBEAS is limited; however, some examples are Shenzhen Esun Industrial, Tianjin GreenBio Materials, and Zhejiang Hisun Biomaterials.2.1.12. PBTSA
PBTSA (poly(butylene terephthalate-co-succinate-co-adipate)) is a complex copolymer composed of BDO, TA, SA, and AA monomers. It is a less commonly known biodegradable copolymer compared to others, and most of the research on PBTSA is reported in laboratory-scale studies. The ratio of aliphatic units to aromatic units in PBTSA was designed to be around 1:1 in these studies [131][92], with a heat of fusion (ΔHm) of 145 J/g [82][93]. The mole fraction of butylene terephthalate (BT) units in PBTSA terpolymer, as determined by 1H NMR (nuclear magnetic resonance), is reported to be 48 mol% [82][93].2.1.13. PIHO
PISOX, or PIHO (polyisosorbide-co-1,6-hexanediol oxalate), is a biodegradable copolymer derived from isosorbide and 1,6-hexanediol, with oxalate linking the two diols. Isosorbide is a biobased compound derived from sorbitol (hydrogenated glucose). PISOX exhibits properties such as good thermal stability, mechanical strength, and biodegradability, making it suitable for various applications. The presence of oxalate linkages in the polymer structure enables its biodegradation by enzymatic and non-enzymatic hydrolysis, which can occur in various environmental conditions.2.2. Biodegradation
Biodegradable plastics undergo biodegradation in three steps: disintegration, depolymerization, and assimilation and mineralization by microorganisms. Abiotic factors such as heat, light, mechanical stress, and moisture directly impact the first two steps and indirectly affect the third step. Biotic factors, such as microorganisms (type and quantity), through their enzymatic actions, can directly influence all three steps. Biodegradable condensation polymers break down into oligomers and monomers, which microorganisms utilize as substrates for metabolism and growth [108,120][86][94]. Hydrolysis is the rate-limiting step for polyester biodegradation, and microbial activity inhibition also plays a significant role [63,120][46][86]. Depolymerization, specifically the hydrolysis of ester bonds at room temperature in soil, is a crucial prerequisite for mineralization, which involves the microbial utilization of polymer carbon. Physical processes aid depolymerization by facilitating fragmentation and reducing particle size. Amorphization of crystalline structures in semi-crystalline plastics through processes such as micronization or extrusion can indeed increase their susceptibility to enzymatic degradation. Hydrolysis is faster in accessible amorphous regions of the polymer, particularly aliphatic esters, and can be enhanced by microbial enzymes, acids, or bases [132][95]. Photodegradation, induced by UV light or oxygen, breaks tertiary and aromatic C–C bonds, resulting in brittle and discolored materials. This process can be further enhanced by incorporating metallic catalysts into the polymer. Oxo-degradation, triggered by metals, leads to fragmentation into microplastics and incomplete digestion, which is why it has been restricted [1]. It is worth mentioning that biodegradation is a surface action. The rate of biodegradation is highly dependent on the chemical structure of the polymer, stabilizing additives, product geometry (particularly size and surface area), surrounding conditions (e.g., the presence of water and oxygen), and the availability of microorganisms. Complete biodegradation or mineralization in a specific environment is influenced by characteristics such as crystallinity, as well as the presence of additives such as plasticizers and environmental factors such as temperature, moisture, pH, and the presence of suitable microorganisms [133][96]. Certifications and labels are used to identify biodegradable materials, often based on industrial standards discussed elsewhere [134][97], like EN 13432 [135][98] or ASTM D6400 [136][99].2.3. Bio-Based and Biodegradable Plastics Market
While bio-based and biodegradable plastics remain a niche market with only 2.22 million tons in 2022 (0.5% of total plastic production), there is a movement towards their wider deployment. The global biodegradable plastics market is projected to reach USD6.73 billion by 2025, up from USD3.02 billion in 2018 [140][100]. Meanwhile, PHAs and PLA are reported to be the main contributors to the growth of bio-based biodegradable plastics [4]. They have a share of 3.9% and 20.7% of the bio-based plastic market, respectively. However, bio-based plastics are associated with disadvantages such as high cost, uneconomic feasibility, brittleness, low thermal properties and instabilities, and hydrophilic nature [36][26].3. Melt-Spun Biodegradable Fibers
3.1. Monocomponent Filaments and Fibers
Using only one polymer without compounding or additive through a plant with one extruder or piston-cylinder setup is the first approach for making thermoplastic fibers. Numerous studies have focused on investigating the impact of processing conditions on the properties of melt-spun biodegradable fibers [54][52]. For example, Schick et al. [87][69] compared PBS, PBAT, and TPS with PP as a reference material through melt-spinning, achieving benchmark tenacities of over 500 mN/tex for PP, while PBS and PBAT exhibited only 100 mN/tex. This study revealed higher crystallinity for PBS yarns (70%) compared to PBAT (14%). Meanwhile, TPS was found to be unsuitable for yarn production. By optimizing the process, the tenacity of home-compostable bio-based polymers can potentially be improved, making them suitable for applications that require moderate mechanical properties. Yuan et al. [153][101] utilized a micro-extruder with a 1 mm diameter die to produce monofilaments from PLLA, using three different grades with varying molecular weights. Subsequently, hot drawing was performed. The findings indicated a significant decrease in the viscosity-average molecular weight of PLLA, ranging from 13.1% to 19.5% during pulverization and from 39.0% to 69.0% during melt extrusion. The final PLLA fibers, with diameters ranging from 110 µm to 160 µm, exhibited tensile strengths between 300 MPa and 600 MPa. Nishimura et al. [154][102] achieved a PLLA fiber with a tensile strength of 810 MPa through melt-spinning, coupled with two steps of drawing at a draw ratio of 18 in hot water. Even after the fiber was exposed to the environment for 1 year, the surface remained smooth, and the tensile strength did not experience a significant decline. Hydrolysis tests demonstrated that the fiber was not susceptible to non-enzymatic hydrolysis after 1 month of immersion in a buffer solution at 37 °C, but rapid hydrolysis occurred above 60 °C, highlighting the effectiveness of surpassing the Tg. SEM observations revealed a regular pattern of cracks oriented vertically to the fiber axis, indicating the development of a highly ordered structure aligned with the fiber axis. PHAs remain a fascinating option for producing biodegradable bio-based fibers. PHB and PHBV were introduced for fiber spinning by Liu et al. [157][103]. Qing et al. [158,159][104][105] conducted melt-spinning experiments with PHBH (containing 5.4 mol% hydroxyhexanoate) at various speeds ranging from 500 to 4000 m/min. They discovered that reducing the processing temperature (below the melting point of PHB, which is 170 °C) and increasing the take-up speed led to improved crystallization, primarily due to the orientation of α-crystals and the formation of β-crystals. It is widely recognized that the presence of a crystalline structure, particularly the planar-zigzag conformation of PHAs, enhances the mechanical properties of the fibers [160][106]. Moreover, the modification of biodegradable polymers through co-polymerization or blending (considering miscibility-structure-property relationships for the best compatibilization) can be explored to facilitate melt-spinning or enhance the performance of the compound [161][107]. While composite and blend fibers will be discussed separately, bio-copolymers have shown promising results thus far. Miyao et al. [165][108] utilized a Liquid Isothermal Bath during the melt-spinning process to enhance the mechanical properties of PHBH fibers containing 5.4 mol% comonomer. By incorporating a liquid bath with temperatures ranging from 30 to 100 °C at a depth of 20 cm and 1 m from the spinneret, they successfully increased the strength of the fibers from 100 MPa to 176 MPa. Similarly, Tanaka et al. [166][109] achieved high-strength fibers with a tensile strength exceeding 1 GPa and a modulus of 8 GPa using PHBV (8% HV content). Notably, they achieved these exceptional results without the use of nucleation agents or other additives. The fibers were directly quenched in ice water to obtain an amorphous state, followed by a 24 h immersion in ice water to induce small crystal nuclei during isothermal crystallization. The fibers were then subjected to drawing at room temperature and annealing subsequently. It is worth mentioning that this procedure is time-consuming and costly. Meanwhile, different bio-based and biodegradable polymers such as PLA, PHBH, and PEF (poly(ethylene 2,5-furan dicarboxylate)) are considered by Kikutani [171][110] for the replacement of PET fibers. They could reach an even take-up speed of 6000 m/min. PGA is also a high-crystalline polymer with a high biodegradation rate (due to hydrolysis). This makes it interesting for investigation by melt-spinning in homopolymer or modified copolymer schemes. Yang et al. [172][111] could make PGA yarns with 645 mN/tex tenacity via melt-spinning as-spun yarns at 30 m/min speed and drawing five times afterward. Hot water shrinkage measurement is used by them to assess internal stress. They asserted that reducing the winding speed during the spinning process resulted in decreased internal stress within the as-spun fibers, ultimately leading to an increased drawing ratio. Consequently, the drawn fibers exhibited notably superior mechanical properties. Meanwhile, they reported a lower orientation increment via increasing the windup rates (due to processing close to the melting point, high viscosity, and fast crystallization, preventing the molecular flow), which is in contrast with the processing of conventional synthetic fibers in the literature by Ziabicki or others [173,174][112][113].3.2. Blend and Composite Fibers
Polymers can be modified through blending with other polymers or by compounding with additives, enabling improvements in processing, mechanical properties, specific functionality, and biodegradation behavior [31,201][22][114]. However, when blending polymers to enhance mechanical properties, it is important to consider the potential impact on biodegradation behavior. While blending PLA with PCL can lead to improved mechanical properties and biodegradability of PLA under home composting conditions [202][115], it may also result in a decrease in the biodegradation potential of PCL. Hence, the final properties of blend fibers depend on the nature of the blend components and their interfacial compatibility [31,201][22][114]. Furthermore, Furuhashi et al. [203][116] blended PLLA and PDLA and investigated the influence of drawing temperature on the crystallization of PLA blend fibers. So far, thanks to annealing and hot drawing, fibers mainly consisting of the stereocomplex crystal phase with a higher melting point have been achieved. Their findings revealed that PLA fibers drawn at 90 °C exhibited higher crystallinity and mechanical properties (520 MPa strength and 8.5 GPA modulus). However, higher drawing temperatures resulted in a decrease in the intensity of crystal reflections, indicating reduced crystal orientation. Padee et al. [204][117] employed a twin-screw extruder to blend PLA and poly(trimethylene terephthalate) PTT at various PTT contents (0–50 wt%) for fiber melt-spinning. The spinning of PLA/PTT blend fibers posed challenges due to the differing melting characteristics of PLA and PTT. However, successful spinning of PLA/PTT blend fibers was achieved at a 10 wt% PTT content with a barrel temperature of 250 °C, making them suitable for textile applications. Jompang et al. [205][118] and Panichsombat et al. [206][119] investigated the melt spinnability of PLA/PBS blends at different ratios (90:10, 80:20, 70:30, 60:40, and 50:50). The spinnability was found to depend directly on the miscibility of the blends. At a 90:10 PLA/PBS ratio, the blend exhibited miscibility and could be processed into fibers. However, spinning became difficult when the PBS content exceeded 10 wt% due to the immiscibility of the blend matrices. After the production of hollow fibers from PHB [211][120], a blend of PCL in PHB (70/30 wt%) was created through dissolution and precipitation from the solvent to enhance the mechanical properties, particularly the flexibility of the melt-spun hollow fibers (Figure 7g). It was demonstrated that the blend obtained from precipitated material exhibited good processability, while granulate material or powder mixtures were not suitable for spinning due to phase separation phenomena [211][120]. Additionally, Huang et al. [202][115] performed the blending of PCL with PLA using electron-induced reactive processing, followed by fiber production through piston spinning. The modified PLA/PCL blends exhibited improved melt strength and elastic behavior, attributed to the formation of long chain branches and enhanced chain entanglements. The PLA/PCL blends treated with 12.5 kGy demonstrated 2.4-fold higher impact toughness compared to neat PLA, indicating superior interfacial adhesion and chain interactions between the two phases. However, fibers spun from irradiated blends showed similar initial modulus but reduced tenacity compared to fibers spun from non-irradiated blends. Using previous research [220][121] as a reference, Takasaki et al. [221][122] conducted melt-spinning of racemate polylactide (r-PLA, an equal blend of PLLA and PDLA) at high speeds of up to 7500 m/min to study the formation of stereocomplex crystals. Stereocomplex crystals have a higher melting temperature than homocrystals. The amount of stereocomplex crystals was higher under spinning conditions involving higher take-up velocity, a lower throughput rate, and a lower extrusion temperature. The high winding speeds applied higher tensile stress to the spinning line, leading to orientation-induced crystallization. Additionally, annealing improved the fibers’ mechanical properties and thermal stability. These findings contradict the reported β to α retransformation observed elsewhere [166][109].3.3. Bicomponent Filament Yarns and Staple Fibers
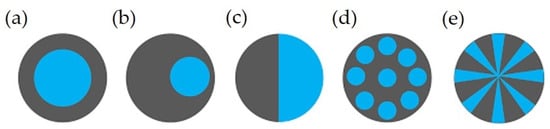
In addition to blending or compounding, two or more different polymers can make a single fiber with a distinguished boundary between them, known as a multicomponent with the desired configuration(s). Bicomponent fibers are a common example of multicomponent fibers comprising two co-extruded polymers forming a single fiber with one or more interfaces. The aim of this approach is to use/modify the properties of two polymers or to use the functionality of additives for specific purposes. Some more details can be found elsewhere [142,248,249][123][124][125]. Figure 63 shows typical examples of different cross-sections, which include the core/heath (C/S) (concentric or eccentric), side-by-side (S/S), islands-in-the-sea (I/S), and segmented-pie configuration [54,248,250,251][52][124][126][127].
Figure 63. Different cross sections of bicomponent fibers: (a) core/sheath (concentric), (b) core/sheath (eccentric), (c), side-by-side, (d) islands-in-the-sea, and (e) Segmented pie [31][22]. Reprinted with permission.
The Advanced Fibers group at Empa (Switzerland) has performed several studies on the melt-spinning of bicomponent fibers from bio-based and biodegradable polymers, particularly PHAs. They reported that PHB and PHBV exhibit the crystallization rates required for extrusion processes [252][128]. They produced mono- and bicomponent filaments from PHBV (Enmat Y1000) and PLLA (Natureworks 6200D). According to their findings, PHB with a molecular weight of approximately 500,000 Da could not be drawn into fibers, mainly due to its low crystallization rate. This resulted in the formation of large spherulitic structures, leading to brittle fibers with poor mechanical properties. The presence of HV (hydroxyvalerate) in the PHBV copolymer could be controlled during biosynthesis and resulted in a reduction in crystallinity. However, it was not possible to produce pure PHBV fibers due to winding issues caused by the stickiness of the fiber.
5. Conclusions
Although biodegradable polymers face certain barriers (e.g., thermal degradation, low crystallization rates, and limited melt strength) when it comes to melt-spinning, researchers have demonstrated the ability to achieve significant results by modifying either the co-polymer structure/compound or the spinning process. These advancements include achieving even take-up speeds exceeding 7000 m/min, tensile strength of 1 GPa, modulus of 10 GPa, and 70% crystallinity. Most studies focus on establishing correlations between polymer structure, melt-spinning parameters, and the biodegradation assessment environment, as these factors profoundly affect processing, fiber performance, and biodegradation rates. These interconnections are illustrated schematically. Biodegradable fibers represent a promising solution for textiles and other fibrous products with unclear end-lives, especially in addressing the issue of MP pollution in oceans. Furthermore, copolymers have yielded more promising results compared to homopolymers or blends. This is due to the avoidance of polymer incompatibilities and the preservation of mechanical strength and recyclability/degradability, which tend to become more complex as the number of different materials within a product increases. Additionally, copolymers offer greater flexibility in tailoring performance attributes. However, the selection of the most suitable material should be based on the intended application and the required performance criteria. Moreover, the compilation of studies provides a comprehensive overview of the polymers used in this context, as well as other process details and outcomes, which can serve as a foundation for future research endeavors aimed at bridging existing gaps and facilitating the development of biodegradable fibers for various sectors.References
- Rosenboom, J.-G.; Langer, R.; Traverso, G. Bioplastics for a circular economy. Nat. Rev. Mater. 2022, 7, 117–137.
- Andrady, A.L.; Neal, M.A. Applications and societal benefits of plastics. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1977–1984.
- Plastics Europe. Plastics—The Facts 2019; Plastics Europe: Brussels, Belgium, 2019.
- Available online: https://docs.european-bioplastics.org/publications/market_data/2022/Report_Bioplastics_Market_Data_2022_short_version.pdf (accessed on 15 August 2023).
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782.
- Gruter, G.-J.M. Using carbon above the ground as feedstock to produce our future polymers. Curr. Opin. Green Sustain. Chem. 2022, 40, 100743.
- Narancic, T.; Cerrone, F.; Beagan, N.; O’Connor, K.E. Recent advances in bioplastics: Application and biodegradation. Polymers 2020, 12, 920.
- Defruyt, S. Towards a new plastics economy. Field Actions Sci. Rep. J. Field Actions 2019, 78–81.
- Hong, M.; Chen, E.Y.-X. Chemically recyclable polymers: A circular economy approach to sustainability. Green Chem. 2017, 19, 3692–3706.
- Alberti, C.; Enthaler, S. Depolymerization of End-of-Life Poly (lactide) to Lactide via Zinc-Catalysis. ChemistrySelect 2020, 5, 14759–14763.
- Wei, R.; Breite, D.; Song, C.; Gräsing, D.; Ploss, T.; Hille, P.; Schwerdtfeger, R.; Matysik, J.; Schulze, A.; Zimmermann, W. Biocatalytic degradation efficiency of postconsumer polyethylene terephthalate packaging determined by their polymer microstructures. Adv. Sci. 2019, 6, 1900491.
- Roohi; Zaheer, M.R.; Kuddus, M. PHB (poly-β-hydroxybutyrate) and its enzymatic degradation. Polym. Adv. Technol. 2018, 29, 30–40.
- European Commission. The Waste Incineration Directive. 2000. Available online: http://ec.europa.eu/environment/archives/air/stationary/wid/legislation.htm (accessed on 15 August 2023).
- European Commission. A European Strategy for Plastics in A Circular Economy; European Commission: Brussels, Belgium, 2018.
- Miri, S.; Saini, R.; Davoodi, S.M.; Pulicharla, R.; Brar, S.K.; Magdouli, S. Biodegradation of microplastics: Better late than never. Chemosphere 2022, 286, 131670.
- United Nations. Strengthening Actions for Nature to Achieve the Sustainable Development Goals; United Nations: New York, NY, USA, 2022.
- Tian, L.; van Putten, R.J.; Gruter, G.J.M. Plastic pollution. The role of (bio) degradable plastics and other solutions. In Biodegradable Polymers in the Circular Plastics Economy; Wiley-VCH: Weinheim, Germany, 2022; pp. 59–81.
- Iheanacho, S.; Ogbu, M.; Bhuyan, M.S.; Ogunji, J. Microplastic pollution: An emerging contaminant in aquaculture. Aquac. Fish. 2023, 8, 603–616.
- Amato-Lourenço, L.F.; Carvalho-Oliveira, R.; Júnior, G.R.; dos Santos Galvão, L.; Ando, R.A.; Mauad, T. Presence of airborne microplastics in human lung tissue. J. Hazard. Mater. 2021, 416, 126124.
- Leslie, H.A.; Van Velzen, M.J.; Brandsma, S.H.; Vethaak, A.D.; Garcia-Vallejo, J.J.; Lamoree, M.H. Discovery and quantification of plastic particle pollution in human blood. Environ. Int. 2022, 163, 107199.
- Arikan, E.B.; Ozsoy, H.D. A review: Investigation of bioplastics. J. Civ. Eng. Arch. 2015, 9, 188–192.
- Motloung, M.P.; Mofokeng, T.G.; Mokhena, T.C.; Ray, S.S. Recent advances on melt-spun fibers from biodegradable polymers and their composites. Int. Polym. Process. 2022, 37, 523–540.
- Spierling, S.; Knüpffer, E.; Behnsen, H.; Mudersbach, M.; Krieg, H.; Springer, S.; Albrecht, S.; Herrmann, C.; Endres, H.-J. Bio-based plastics-A review of environmental, social and economic impact assessments. J. Clean. Prod. 2018, 185, 476–491.
- Chen, X.; Memon, H.A.; Wang, Y.; Marriam, I.; Tebyetekerwa, M. Circular Economy and sustainability of the clothing and textile Industry. Mater. Circ. Econ. 2021, 3, 12.
- Sharma, V.; Sehgal, R.; Gupta, R. Polyhydroxyalkanoate (PHA): Properties and modifications. Polymer 2021, 212, 123161.
- Acharjee, S.A.; Bharali, P.; Gogoi, B.; Sorhie, V.; Walling, B. Alemtoshi PHA-based bioplastic: A potential alternative to address microplastic pollution. Water Air Soil Pollut. 2023, 234, 21.
- Cai, Y.; Yang, T.; Mitrano, D.M.; Heuberger, M.; Hufenus, R.; Nowack, B. Systematic study of microplastic fiber release from 12 different polyester textiles during washing. Environ. Sci. Technol. 2020, 54, 4847–4855.
- Cai, Y.; Lin, J.; Gimeno, S.; Begnaud, F.; Nowack, B. Country-Specific Environmental Risks of Fragrance Encapsulates Used in Laundry Care Products. Environ. Toxicol. Chem. 2022, 41, 905–916.
- Cai, Y.; Mitrano, D.M.; Hufenus, R.; Nowack, B. Formation of fiber fragments during abrasion of polyester textiles. Environ. Sci. Technol. 2021, 55, 8001–8009.
- Patti, A.; Acierno, D. Towards the sustainability of the plastic industry through biopolymers: Properties and potential applications to the textiles World. Polymers 2022, 14, 692.
- De Haan, W.P.; Quintana, R.; Vilas, C.; Cózar, A.; Canals, M.; Uviedo, O.; Sanchez-Vidal, A. The dark side of artificial greening: Plastic turfs as widespread pollutants of aquatic environments. Environ. Pollut. 2023, 334, 122094.
- Armstrong, M. Where the Ocean’s Microplastics Come From, 2022. Available online: https://www.statista.com/chart/17957/where-the-oceans-microplastics-come-from/ (accessed on 15 August 2023).
- Stanvay, D. Plastic entering oceans could nearly triple by 2040 if left unchecked—Research. Reuters, 8 March 2023.
- Michael, C. Plastic in the depths: How pollution took over our oceans. The Guardian, 25 July 2022.
- Manshoven, S.; Smeets, A.; Malarciuc, C.; Tenhunen-Lunkka, A.; Mortensen, L.F. Microplastic Pollution from Textile Consumption in Europe; VTT: Espoo, Finland, 2022.
- Microplastics from Textiles: Towards a Circular Economy for Textiles in Europe; European Environment Agency: Copenhagen, Denmark, 2022.
- Cesa, F.S.; Turra, A.; Baruque-Ramos, J. Synthetic fibers as microplastics in the marine environment: A review from textile perspective with a focus on domestic washings. Sci. Total Environ. 2017, 598, 1116–1129.
- Habib, R.Z.; Thiemann, T. Microplastic in the marine environment of the Red Sea—A short review. Egypt. J. Aquat. Res. 2022, 48, 383–388.
- MacArthur, E. Beyond Plastic Waste; American Association for the Advancement of Science: Washington, DC, USA, 2017; Volume 358, p. 843.
- Narayanan, M.; Kandasamy, S.; Kumarasamy, S.; Gnanavel, K.; Ranganathan, M.; Kandasamy, G. Screening of polyhydroxybutyrate producing indigenous bacteria from polluted lake soil. Heliyon 2020, 6, e05381.
- Narancic, T.; Verstichel, S.; Reddy Chaganti, S.; Morales-Gamez, L.; Kenny, S.T.; De Wilde, B.; Babu Padamati, R.; O’Connor, K.E. Biodegradable plastic blends create new possibilities for end-of-life management of plastics but they are not a panacea for plastic pollution. Environ. Sci. Technol. 2018, 52, 10441–10452.
- Nakajima, H.; Dijkstra, P.; Loos, K. The recent developments in biobased polymers toward general and engineering applications: Polymers that are upgraded from biodegradable polymers, analogous to petroleum-derived polymers, and newly developed. Polymers 2017, 9, 523.
- Mecking, S. Nature or petrochemistry?—Biologically degradable materials. Angew. Chem. Int. Ed. 2004, 43, 1078–1085.
- Rai, P.; Mehrotra, S.; Priya, S.; Gnansounou, E.; Sharma, S.K. Recent advances in the sustainable design and applications of biodegradable polymers. Bioresour. Technol. 2021, 325, 124739.
- Leja, K.; Lewandowicz, G. Polymer biodegradation and biodegradable polymers—A review. Pol. J. Environ. Stud. 2010, 19, 255–266.
- Polman, E.M.; Gruter, G.-J.M.; Parsons, J.R.; Tietema, A. Comparison of the aerobic biodegradation of biopolymers and the corresponding bioplastics: A review. Sci. Total Environ. 2021, 753, 141953.
- Nanda, S.; Patra, B.R.; Patel, R.; Bakos, J.; Dalai, A.K. Innovations in applications and prospects of bioplastics and biopolymers: A review. Environ. Chem. Lett. 2022, 20, 379–395.
- Emadian, S.M.; Onay, T.T.; Demirel, B. Biodegradation of bioplastics in natural environments. Waste Manag. 2017, 59, 526–536.
- Chen, S.; Wu, Z.; Chu, C.; Ni, Y.; Neisiany, R.E.; You, Z. Biodegradable elastomers and gels for elastic electronics. Adv. Sci. 2022, 9, 2105146.
- Vinod, A.; Sanjay, M.; Suchart, S.; Jyotishkumar, P. Renewable and sustainable biobased materials: An assessment on biofibers, biofilms, biopolymers and biocomposites. J. Clean. Prod. 2020, 258, 120978.
- Ganesh, S.; Lakshmanan Saraswathy, J.; Raghunathan, V.; Sivalingam, C. Extraction and characterization chemical treated and untreated lycium ferocissimum fiber for epoxy composites. J. Nat. Fibers 2022, 19, 6509–6520.
- Hufenus, R.; Yan, Y.; Dauner, M.; Kikutani, T. Melt-spun fibers for textile applications. Materials 2020, 13, 4298.
- Chang, C.; Ginn, B.; Livingston, N.K.; Yao, Z.; Slavin, B.; King, M.W.; Chung, S.; Mao, H.-Q. Medical fibers and biotextiles. In Biomaterials Science; Academic Press: Cambridge, MA, USA, 2020; pp. 575–600.
- Kopf, S.; Åkesson, D.; Skrifvars, M. Textile Fiber Production of Biopolymers–A Review of Spinning Techniques for Polyhydroxyalkanoates in Biomedical Applications. Polym. Rev. 2023, 63, 200–245.
- Pivsa-Art, S.; Srisawat, N.; Narongchai, O.; Pavasupree, S.; Pivsa-Art, W. Preparation of knitting socks from poly (lactic acid) and poly (PHBV) blends for textile industrials. Energy Procedia 2011, 9, 589–597.
- Alaswad, S.O.; Mahmoud, A.S.; Arunachalam, P. Recent Advances in Biodegradable Polymers and Their Biological Applications: A Brief Review. Polymers 2022, 14, 4924.
- Nair, N.; Sekhar, V.; Nampoothiri, K.; Pandey, A. Biodegradation of biopolymers. In Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2017; pp. 739–755.
- Narayan, R.; Balakrishnan, S.; Kubik, D.A.; Gencer, M.A. Biodegradable Polymer Masterbatch, and a Composition Derived Therefrom Having Improved Physical Properties. U.S. Patent US8008373B2, 30 August 2011.
- Yang, Y.; Zhang, M.; Ju, Z.; Tam, P.Y.; Hua, T.; Younas, M.W.; Kamrul, H.; Hu, H. Poly (lactic acid) fibers, yarns and fabrics: Manufacturing, properties and applications. Text. Res. J. 2021, 91, 1641–1669.
- Van den Oever, M.; Molenveld, K.; van der Zee, M.; Bos, H. Bio-Based and Biodegradable Plastics: Facts and Figures: Focus on Food Packaging in the Netherlands; Wageningen Food & Biobased Research: Wageningen, The Netherlands, 2017.
- Van der Zee, M. 1. Methods for evaluating the biodegradability of environmentally degradable polymers. In Handbook of Biodegradable Polymers; De Gruyter: Berlin, Germany, 2020; pp. 1–22.
- Van der Zee, M.; Stoutjesdijk, J.; Van der Heijden, P.; De Wit, D. Structure-biodegradation relationships of polymeric materials. 1. Effect of degree of oxidation on biodegradability of carbohydrate polymers. J. Environ. Polym. Degrad. 1995, 3, 235–242.
- Science Advice for Policy by European Academies. Biodegradability of Plastics in the Open Environment; Evidence Review Report No. 8; SAPEA: Brussels, Belgium, 2020; pp. 52–57.
- Tavanaie, M.A. Engineered biodegradable melt-spun fibers. In Engineered Polymeric Fibrous Materials; Elsevier: Amsterdam, The Netherlands, 2021; pp. 191–232.
- Available online: https://docs.european-bioplastics.org/publications/EUBP_Facts_and_figures.pdf (accessed on 20 August 2023).
- Espinosa, M.J.C.; Blanco, A.C.; Schmidgall, T.; Atanasoff-Kardjalieff, A.K.; Kappelmeyer, U.; Tischler, D.; Pieper, D.H.; Heipieper, H.J.; Eberlein, C. Toward biorecycling: Isolation of a soil bacterium that grows on a polyurethane oligomer and monomer. Front. Microbiol. 2020, 11, 404.
- Terzopoulou, Z.; Tsanaktsis, V.; Bikiaris, D.N.; Exarhopoulos, S.; Papageorgiou, D.G.; Papageorgiou, G.Z. Biobased poly (ethylene furanoate-co-ethylene succinate) copolyesters: Solid state structure, melting point depression and biodegradability. RSC Adv. 2016, 6, 84003–84015.
- Gruter, G.J.M.; Lange, J.P. Tutorial on Polymers—Manufacture, Properties, and Applications. In Biodegradable Polymers in the Circular Plastics Economy; Wiley-VCH: Weinheim, Germany, 2022; pp. 83–111.
- Schick, S.; Groten, R.; Seide, G.H. Performance Spectrum of Home-Compostable Biopolymer Fibers Compared to a Petrochemical Alternative. Polymers 2023, 15, 1372.
- Zhang, H.; Bai, H.; Liu, Z.; Zhang, Q.; Fu, Q. Toward high-performance poly (L-lactide) fibers via tailoring crystallization with the aid of fibrillar nucleating agent. ACS Sustain. Chem. Eng. 2016, 4, 3939–3947.
- Mezghani, K.; Spruiell, J. High speed melt spinning of poly (L-lactic acid) filaments. J. Polym. Sci. Part B Polym. Phys. 1998, 36, 1005–1012.
- Roungpaisan, N.; Takasaki, M.; Takarada, W.; Kikutani, T. Mechanism of fiber structure development in melt spinning of PLA. In Poly (Lactic Acid) Synthesis, Structures, Properties, Processing, Applications, and End of Life; John Wiley & Sons: Hoboken, NJ, USA, 2022; pp. 425–438.
- Yang, J.; Liu, X.; Zhao, J.; Pu, X.; Shen, Z.; Xu, W.; Liu, Y. The Structural Evolution of β-to-α Phase Transition in the Annealing Process of Poly (3-hydroxybutyrate-co-3-hydroxyvalerate). Polymers 2023, 15, 1921.
- Center for International Environmental Law (CIEL). Plastic & Climate. The Hidden Costs of a Plastic Planet. 2020. Available online: https://www.ciel.org/plasticandclimate/ (accessed on 15 August 2023).
- Xu, J.; Guo, B.H. Poly (butylene succinate) and its copolymers: Research, development and industrialization. Biotechnol. J. 2010, 5, 1149–1163.
- Chen, Y.; Tan, L.; Chen, L.; Yang, Y.; Wang, X. Study on biodegradable aromatic/aliphatic copolyesters. Braz. J. Chem. Eng. 2008, 25, 321–335.
- Robledo-Ortíz, J.R.; González-López, M.E.; Martín del Campo, A.S.; Pérez-Fonseca, A.A. Lignocellulosic materials as reinforcement of polyhydroxybutyrate and its copolymer with hydroxyvalerate: A review. J. Polym. Environ. 2021, 29, 1350–1364.
- Dilkes-Hoffman, L.S.; Lant, P.A.; Laycock, B.; Pratt, S. The rate of biodegradation of PHA bioplastics in the marine environment: A meta-study. Mar. Pollut. Bull. 2019, 142, 15–24.
- Guimarães, T.C.; Araújo, E.S.; Hernández-Macedo, M.L.; López, J.A. Polyhydroxyalkanoates: Biosynthesis from alternative carbon sources and analytic methods: A short review. J. Polym. Environ. 2022, 30, 2669–2684.
- Van der Walle, G.A.M.; De Koning, G.J.M.; Weusthuis, R.A.; Eggink, G. Properties, modifications and applications of biopolyesters. Biopolyesters 2001, 71, 263–291.
- Heisig, C.; Diedenhoven, J.; Jensen, C.; Gehrke, H.; Turek, T. Selective hydrogenation of biomass-derived succinic acid: Reaction network and kinetics. Chem. Eng. Technol. 2020, 43, 484–492.
- Varghese, S.A.; Pulikkalparambil, H.; Rangappa, S.M.; Siengchin, S.; Parameswaranpillai, J. Novel biodegradable polymer films based on poly (3-hydroxybutyrate-co-3-hydroxyvalerate) and Ceiba pentandra natural fibers for packaging applications. Food Packag. Shelf Life 2020, 25, 100538.
- Azimi, B.; Nourpanah, P.; Rabiee, M.; Arbab, S. Poly (∊-caprolactone) fiber: An overview. J. Eng. Fibers Fabr. 2014, 9, 155892501400900309.
- Saigusa, K.; Saijo, H.; Yamazaki, M.; Takarada, W.; Kikutani, T. Influence of carboxylic acid content and polymerization catalyst on hydrolytic degradation behavior of Poly (glycolic acid) fibers. Polym. Degrad. Stab. 2020, 172, 109054.
- Murcia Valderrama, M.A.; van Putten, R.-J.; Gruter, G.-J.M. PLGA barrier materials from CO2. The influence of lactide co-monomer on glycolic acid polyesters. ACS Appl. Polym. Mater. 2020, 2, 2706–2718.
- Wang, Y.; Murcia Valderrama, M.A.; van Putten, R.-J.; Davey, C.J.; Tietema, A.; Parsons, J.R.; Wang, B.; Gruter, G.-J.M. Biodegradation and non-enzymatic hydrolysis of poly (lactic-co-glycolic acid)(PLGA12/88 and PLGA6/94). Polymers 2021, 14, 15.
- Bansode, S.H.; Khare, P.V.; Mahanwar, P.A. Synthesis of PLGA and Its Fabrication for the Tissue Engineering by Electro and Melt Spinning.
- Xu, W. In A study on the synthesis, modification and current market status of PBAT. E3S Web Conf. 2023, 385, 04007.
- Shi, X.; Ito, H.; Kikutani, T. Structure development and properties of high-speed melt spun poly (butylene terephthalate)/poly (butylene adipate-co-terephthalate) bicomponent fibers. Polymer 2006, 47, 611–616.
- Baidurah, S. Methods of analyses for biodegradable polymers: A review. Polymers 2022, 14, 4928.
- Kim, J.; Park, S.; Bang, J.; Jin, H.J.; Kwak, H.W. Biodegradation in Composting Conditions of PBEAS Monofilaments for the Sustainable End-Use of Fishing Nets. Glob. Chall. 2023, 7, 2300020.
- Prambauer, M.; Wendeler, C.; Weitzenböck, J.; Burgstaller, C. Biodegradable geotextiles—An overview of existing and potential materials. Geotext. Geomembr. 2019, 47, 48–59.
- Shi, X.; Ito, H.; Kikutani, T. Characterization on mixed-crystal structure and properties of poly (butylene adipate-co-terephthalate) biodegradable fibers. Polymer 2005, 46, 11442–11450.
- Wang, Y.; Davey, C.J.; Van der Maas, K.; Van Putten, R.-J.; Tietema, A.; Parsons, J.R.; Gruter, G.-J.M. Biodegradability of novel high Tg poly (isosorbide-co-1, 6-hexanediol) oxalate polyester in soil and marine environments. Sci. Total Environ. 2022, 815, 152781.
- Austin, H.P.; Allen, M.D.; Donohoe, B.S.; Rorrer, N.A.; Kearns, F.L.; Silveira, R.L.; Pollard, B.C.; Dominick, G.; Duman, R.; El Omari, K. Characterization and engineering of a plastic-degrading aromatic polyesterase. Proc. Natl. Acad. Sci. USA 2018, 115, E4350–E4357.
- Mohee, R.; Unmar, G. Determining biodegradability of plastic materials under controlled and natural composting environments. Waste Manag. 2007, 27, 1486–1493.
- Viera, J.S.; Marques, M.R.; Nazareth, M.C.; Jimenez, P.C.; Sanz-Lázaro, C.; Castro, Í.B. Are biodegradable plastics an environmental rip off? J. Hazard. Mater. 2021, 416, 125957.
- EN 13432; Packaging—Requirements for Packaging Recoverable through Composting and Biodegradation—Test Scheme and Evaluation Criteria for the Final Acceptance of Packaging. Institute for Standardization of Serbia: Belgrade, Serbia, 2000.
- ASTM D6400; Standard Specification for Labeling of Plastics Designed to be Aerobically Composted in Municipal or Industrial Facilities. ATSM International: West Conshohocken, PA, USA, 2021.
- SkyQuest Technology. Biodegradable Plastics Market Size, Share & Trends Analysis, by Type (PLA, Starch Blends, PHA, Biodegradable Polyesters), End Use Industry (Packaging, Consumer Goods, Textile, Agriculture & Horticulture), Region and Forecast Period 2022–2030; SkyQuest Technology: Westford, MA, USA, 2022.
- Yuan, X.; Mak, A.F.; Kwok, K.W.; Yung, B.K.; Yao, K. Characterization of poly (L-lactic acid) fibers produced by melt spinning. J. Appl. Polym. Sci. 2001, 81, 251–260.
- Nishimura, Y.; Takasu, A.; Inai, Y.; Hirabayashi, T. Melt spinning of poly (L-lactic acid) and its biodegradability. J. Appl. Polym. Sci. 2005, 97, 2118–2124.
- Liu, Q.; Zhang, H.; Deng, B.; Zhao, X. Poly (3-hydroxybutyrate) and Poly (3-hydroxybutyrate-co-3-hydroxyvalerate): Structure, Property, and Fiber. Int. J. Polym. Sci. 2014, 2014, 374368.
- Qin, Q.; Takarada, W.; Kikutani, T. Fiber structure formation in melt spinning of bio-based aliphatic co-polyesters. AIP Conf. Proc. 2015, 1664, 080004.
- Qin, Q.; Takarada, W.; Kikutani, T. Fiber structure development of PHBH through stress-induced crystallization in high-speed melt spinning process. J. Fiber Sci. Technol. 2017, 73, 49–60.
- Iwata, T.; Tanaka, T. Manufacturing of PHA as Fibers. In Plastics from Bacteria: Natural Functions and Applications; Springer: Berlin/Heidelberg, Germany, 2010; pp. 257–282.
- Imre, B.; Pukánszky, B. Compatibilization in bio-based and biodegradable polymer blends. Eur. Polym. J. 2013, 49, 1215–1233.
- Miyao, Y.; Takarada, W.; Kikutani, T. In Improvement of mechanical properties of biodegradable PHBH fibers through high-speed melt spinning process equipped with a liquid isothermal bath. AIP Conf. Proc. 2020, 2289, 020038.
- Tanaka, T.; Fujita, M.; Takeuchi, A.; Suzuki, Y.; Uesugi, K.; Ito, K.; Fujisawa, T.; Doi, Y.; Iwata, T. Formation of highly ordered structure in poly high-strength fibers. Macromolecules 2006, 39, 2940–2946.
- Kikutani, T. Fiber Formation through Melt Spinning of Bio-polymers. In Proceedings of the International Conference on Technology and Social Science, Kiryu, Japan, 2–4 December 2020.
- Yang, Q.; Shen, X.; Tan, Z. Investigations of the preparation technology for polyglycolic acid fiber with perfect mechanical performance. J. Appl. Polym. Sci. 2007, 105, 3444–3447.
- Ziabicki, A. Fundamentals of Fibre Formation: The Science of Fibre Spinning and Drawing; John Wiley & Sons: Hoboken, NJ, USA, 1976.
- Fourné, F. Synthetic Fibers: Machines and Equipment, Manufacture, Properties: Handbook for Plant Engineering, Machine Design, and Operation; Hanser Publication: Cincinnati, OH, USA, 1999.
- Niaounakis, M. Biopolymers: Processing and Products; William Andrew: Norwich, NY, USA, 2014.
- Huang, Y.; Brünig, H.; Müller, M.T.; Wießner, S. Melt spinning of PLA/PCL blends modified with electron induced reactive processing. J. Appl. Polym. Sci. 2022, 139, 51902.
- Furuhashi, Y.; Kimura, Y.; Yoshie, N.; Yamane, H. Higher-order structures and mechanical properties of stereocomplex-type poly (lactic acid) melt spun fibers. Polymer 2006, 47, 5965–5972.
- Padee, S.; Thumsorn, S.; On, J.W.; Surin, P.; Apawet, C.; Chaichalermwong, T.; Kaabbuathong, N.; Narongchai, O.; Srisawat, N. Preparation of poly (lactic acid) and poly (trimethylene terephthalate) blend fibers for textile application. Energy Procedia 2013, 34, 534–541.
- Jompang, L.; Thumsorn, S.; On, J.W.; Surin, P.; Apawet, C.; Chaichalermwong, T.; Kaabbuathong, N.; Narongchai, O.; Srisawat, N. Poly (lactic acid) and poly (butylene succinate) blend fibers prepared by melt spinning technique. Energy Procedia 2013, 34, 493–499.
- Panichsombat, K.; Panbangpong, W.; Poompiew, N.; Potiyaraj, P. Biodegradable fibers from poly (lactic acid)/poly (butylene succinate) blends. IOP Conf. Ser. Mater. Sci. Eng. 2019, 600, 012004.
- Hinüber, C.; Häussler, L.; Vogel, R.; Brünig, H.; Heinrich, G.; Werner, C. Hollow fibers made from a poly (3-hydroxybutyrate)/poly-ε-caprolactone blend. Express Polym. Lett. 2011, 5, 643–652.
- Okihara, T.; Tsuji, M.; Kawaguchi, A.; Katayama, K.-I.; Tsuji, H.; Hyon, S.-H.; Ikada, Y. Crystal structure of stereocomplex of poly (L-lactide) and poly (D-lactide). J. Macromol. Sci. Part B Phys. 1991, 30, 119–140.
- Takasaki, M.; Ito, H.; Kikutani, T. Development of stereocomplex crystal of polylactide in high-speed melt spinning and subsequent drawing and annealing processes. J. Macromol. Sci. Part B 2003, 42, 403–420.
- Naeimirad, M.; Zadhoush, A.; Kotek, R.; Esmaeely Neisiany, R.; Nouri Khorasani, S.; Ramakrishna, S. Recent advances in core/shell bicomponent fibers and nanofibers: A review. J. Appl. Polym. Sci. 2018, 135, 46265.
- Hufenus, R.; Yan, Y.; Dauner, M.; Yao, D.; Kikutani, T. Bicomponent fibers. In Handbook of Fibrous Materials; Wiley: Hoboken, NJ, USA, 2020; pp. 281–313.
- Elahi, M.F.; Lu, W.; Guoping, G.; Khan, F. Core-shell fibers for biomedical applications—A review. J. Bioeng. Biomed. Sci 2013, 3, 1–14.
- Hufenus, R. Novel synthetic fibers by multocomponent melt-spinning. In Proceedings of the XII International İzmir Textile and Apparel Symposium, Izmir, Turkey, 28–30 October 2010; pp. 299–307.
- Hufenus, R. Fiber Development by Multicomponent Melt-Spinning; Research Gate: Berlin, Germany, 2011.
- Zinn, M.; Dilettoso, S.; Lischer, S.; Maniura, K.; Milz, S.; Weisse, B.; Hufenus, R. Melt-Spun Fibers From Polyhydroxyalkanoate And Polylactate For Fiber Implant Applications. Eur. Cells Mater. 2010, 20, 12.
More
