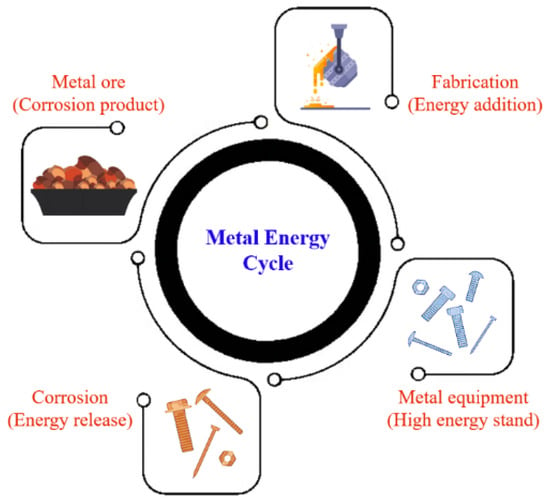
Polythionic acid (PTA) corrosion is a significant challenge in the refinery industry, leading to equipment degradation, safety risks, and costly maintenance.
- polythionic acid
- corrosion
- corrosion mechanism
1. Introduction
2. Polythionic Acids (PTAs)
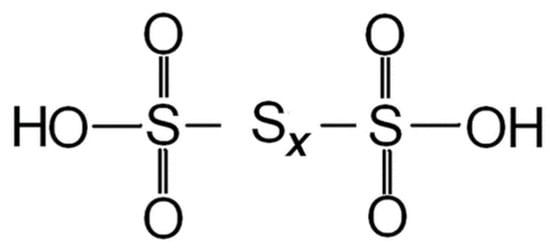
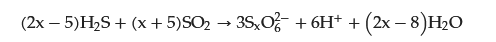
2.1. Factors Affecting PTA
2.2. Polythionates Measurement
Polythionates are challenging to analyze accurately due to their decomposing tendency, particularly in solution. Furthermore, individual polythionate species exhibit similar chemical and physical properties, making their characterization complex. A spectrophotometric method was proposed by Nietzel and De Sesa [21][18] for determining low concentrations of tetrathionate (S4O2−6 ) ions. This method involves the stoichiometric conversion of S4O2−6 to thiocyanate through a reaction with cyanide in an alkaline medium. The excess ferric chloride then forms a red ferric thiocyanate complex. However, this method is unsuitable for measuring higher polythionate concentrations (mainly pentathionate and hexathionate) due to the decomposition experienced by these species.3. PTA in the Refinery
PTA formation in refineries is commonly observed in units exposed to sulfur-containing compounds like H2S and SO2, particularly under corrosive conditions involving the presence of O2, H2O, high temperatures, and low pH [25][19]. Units such as crude distillation, amine systems, and sour water strippers are particularly susceptible to PTA formation due to the high concentrations of sulfur compounds [26][20]. The desulfurization processes employed in refineries, including oxidation-extraction desulfurization (OEDS), oxidative desulfurization (ODS), hydrodesulfurization (HDS), adsorptive desulfurization, and bio-desulfurization (BDS), can also contribute to the generation of polythionates as sulfur compounds are converted and transformed during these processes [26][20]. Operating at high temperatures and pressures, refinery processes can lead to sensitization and reduced ductility in construction materials due to the presence of S and other impurities. The reactions of sulfur impurities with H2O and O2 result in the formation of H2S and SO2, which further react to form complex compounds such as S4O2−6 , polythionates, and polythionic acid [36][21]. PTA formation primarily occurs in refinery equipment through the reaction of O2 and H2O with sulfide corrosion products that accumulate on the internal surfaces of the equipment (Figure 43). Moisture, often present from vessel washing or steaming during shutdowns, and oxygen from the air that enters when the vessel is opened contribute to the PTA formation process [37][22].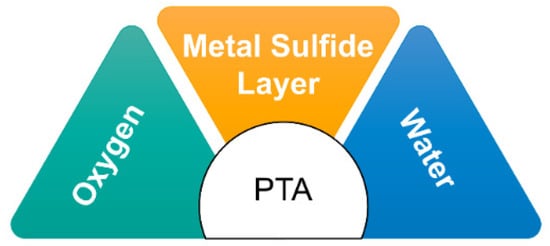
3.1. PTA Corrosion in Refiney
3.2. PTA Corrosion Mechanism
PTA corrosion in refineries occurs in environments containing sulfur-containing compounds such as H2S and SO2. The mechanism of PTA corrosion involves several stages, including the formation of PTA and polythionates, the attack on metal surfaces, and the acceleration of corrosion. When sulfur-containing compounds in refining processes come into contact with H2O, they undergo a series of chemical reactions and form H2SO4, which can further be oxidized (in the presence of O2) to form PTA. The presence of PTA can lead to localized corrosion of metal surfaces by attacking the protective oxide layers on metal surfaces and initiating corrosion. When carbon steel is exposed to SxO2−6, it undergoes immediate attack, releasing hydrogen gas (H2) and forming Fe2⁺. These ferrous ions react with polythionates to create a protective layer of ferrous sulfate (FeSO4) on the metal surface (Equations (2)–(4)). This protective layer is a barrier, safeguarding the metal from further attack.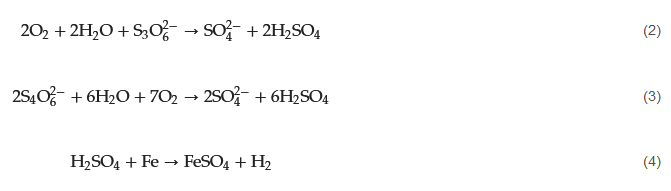
3.3. PTA Stress Corrosion Cracking (PTASCC)
Austenitic stainless alloys are commonly chosen as structural materials due to their favorable mechanical properties and corrosion resistance. However, under certain circumstances, these alloys can experience stress corrosion cracking (SCC) if they have not undergone appropriate fabrication treatments or are exposed to aggressive solution chemistries. SCC refers to the cracking of a material caused by the combined influence of tensile stress and a specific environment. The initiation and propagation of this type of corrosion form are influenced by factors such as sensitized materials (e.g., stainless steel with high carbon content, copper alloys, carbon steels, etc.), the presence of tensile stress (applied, thermal, or residual stress), and specific environmental conditions (e.g., aqueous solutions, moisture, chloride or caustic solutions, high-temperature water, PTA, etc.) [47][29]. Aggressive ions are required to promote SCC for alloys that develop a protective film. In the case of austenitic stainless steel, PTA and other caustic substances and chlorides can disrupt the protective layer. Cracks often originate from corrosion pits and surface imperfections and propagate in a brittle manner. The fracture behavior is not purely mechanical as it is strongly influenced by the corrosive nature of the environment [48][30]. Once a crack initiates in the metal, it can propagate within the individual grains (transgranular) or along the boundaries between grains (intergranular) (Figure 65). The change in fracture direction occurs when the crack encounters a new grain as the different orientations of atoms within each grain make it easier for the crack to change its path rather than continue tearing through the material [49][31].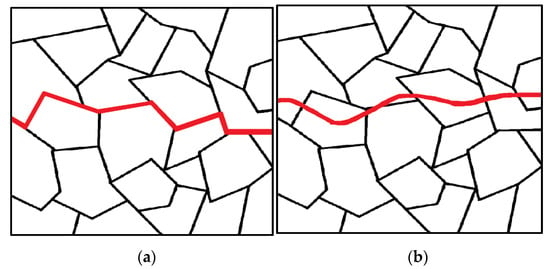
3.4. PTASCC Mechanism
3.5. Detection of PTASCC in Refinery
Detecting PTASCC is most important in preventing risky incidents and ensuring effective maintenance within refinery operations. Regular inspection, non-destructive testing (NDT), and risk-based inspection (RBI) are widely adopted in refineries to identify PTASCC and implement effective prevention and mitigation measures on time. However, each refinery may have specific inspection and detection protocols tailored to industry best practices and regulatory requirements. These practices are essential for safeguarding the integrity of refinery equipment and promoting the safe and efficient operation of the facility. It is worth noting that PTASCC detection is an ongoing process, and a combination of these methods can be employed to ensure comprehensive inspection and continuous monitoring of refinery equipment. Regular inspection and evaluation of equipment and high-risk areas (e.g., piping, vessels, and tanks) by trained inspectors are crucial for reducing corrosion risks and extending the equipment’s lifespan. This proactive approach involves identifying signs of corrosion, thinning, cracking, and pitting, enabling early detection of damage and preventing equipment shutdowns or disruptions to production processes. Visual inspection, the most widely utilized technique, offers several advantages. It is cost-effective, can be conducted while work is in progress, and allows for the early correction of faults. NDT techniques are commonly utilized in refineries for the timely detection of PTASCC, ensuring its prevention and mitigation. These methods offer a cost-effective approach to corrosion detection without causing significant operational disruption. Refineries can detect PTASCC without significantly impacting their operations by utilizing these NDT methods, effectively addressing corrosion-related concerns. NDT techniques commonly include infrared thermography, radiography examination, ultrasonic inspection, and eddy current [58][35].4. Prevention of PTA Corrosion
4.1. Material Selection
Highly alloyed materials are required for effective resistance to different types of corrosion, such as general and PTA corrosion. These materials must have high chromium and nickel content to resist corrosion. Additionally, stabilization with titanium or niobium is necessary to resist intergranular sensitivity and reduce PTA corrosion. Austenitic stainless steel (ASS) is an excellent choice for PTA corrosion. ASS contains high levels of nickel and chromium, which allows the formation of a very thin (1–3 nm) chromium oxide/hydroxide-rich passive film, giving it excellent corrosion resistance [61][36]. Thus, selecting the appropriate grade of ASS prevents PTA corrosion as the material’s microstructure significantly influences its susceptibility to corrosion. Notably, types 321, 347, and 347LN have shown high resistance against PTA corrosion [62][37]. On the other hand, materials that are not resistant to PTASCC include some sensitized alloys that are susceptible to the corrosive effects of PTA. This proneness can occur when certain alloys are exposed to specific environmental conditions. The materials composed of austenitic stainless steels, high-nickel alloys, and iron–nickel–chromium alloys are open to attack by PTA. These acids act as cathodic depolarizers, facilitating metal dissolution at chromium-depleted grain boundaries through cathodic reduction [9]. It was observed that Undeformed AISI 304, sensitized at 500 °C for 24 h, exhibited a ductile fracture in the PTA solution due to its limited chromium-depleted zone, reducing PTASCC susceptibility [63][38]. Cold rolling at 20% and 40% before sensitization (at 500 °C for 24 h) made stainless steel prone to PTASCC, which is attributed to severe chromium depletion. Deformation beyond 40% prevented PTASCC despite higher sensitization levels. Only 20% and 40% deformation induced sufficient chromium depletion along grain boundaries for crack propagation. Deformation greater than 40% did not induce this effect, even with a higher degree of sensitization (at 60% deformation).4.2. Nitrogen Purging
This method involves purging the equipment by displacing the oxygen present in the environment with nitrogen, leading to the generation of an inert environment during shutdown and preventing PTA formation. Moreover, this method can eliminate existing PTA from the metal surface, decreasing maintenance requirements. Additionally, nitrogen’s non-toxicity and non-flammability certify the operational safety and environmental friendliness of this approach. The method is applicable during start-ups, shutdowns, and maintenance processes, proving particularly beneficial for preserving catalysts. Ensuring that the nitrogen used is dry and free of O2 is crucial as commercial nitrogen often contains around 1000 ppm of O2. When steam is employed for purging, steam injection should be halted before the metal temperature drops to 72 °C (130 °F).4.3. Alkaline Washing
The standard method for protecting sensitized stainless steel involves either preventing the formation of PTAs or neutralizing them. To neutralize PTA, washing the equipment with a weak soda ash solution (1–5%) before exposing it to air is recommended. It is essential to soak the equipment for at least 2 h to ensure effective neutralization. Simply spraying the equipment with a soda ash solution is insufficient to prevent PTA formation. If deposits or sludge are present, the solution should be circulated vigorously for at least 2 h [66][39]. Using a soda ash solution for neutralizing acids should consider the formation of a Na2CO3 film that can further neutralize acids. It is advisable to assess the influence of alkaline chemicals on catalysts before employing a soda ash wash. Equipment should be hydrojetted with a soda ash solution and reinstalled with the residual soda ash film on surfaces.4.4. Amide Solutions
An alternative approach to washing and neutralizing with an aqueous alkali solution addresses the challenges posed by stress-corrosion cracking due to repulsion by sulfide-containing fluids on the equipment’s surface. Additionally, residual aqueous alkali solution in certain areas can lead to corrosion, making the procedure complex. Instead, washing the equipment with amide solutions prevents the formation of PTA when iron sulfide contacts mineral oil, effectively safeguarding against stress-corrosion cracking of austenitic stainless steel. This technique ensures adequate protection of metal surfaces from PTA-induced corrosion, providing increased durability and dependability for metal equipment exposed to sulfide-containing fluids by leveraging the unique properties of amides and their derivatives to prevent stress-corrosion cracking [67][40].4.5. Dry Air
Eliminating moisture is vital for suppressing corrosion rates in atmospheric conditions. The dry air method effectively limits the risk of polythionic acid (PTA) corrosion by preventing free water formation, a crucial component in PTA production. The dry air method effectively mitigates the risk of PTA corrosion by reducing moisture in the environment to a level where surface wetness cannot form. This preventative technique is essential when metal surfaces are susceptible to corrosion or exposed to sulfur-containing components. Utilizing dry (dehumidified) air offers a cost-effective means to prevent free water formation and reduce the risk of PTASCC. When handling non-regenerable catalysts, which may be pyrophoric, it is essential to keep them moist or isolated from oxygen.5. Conclusions
Future investigation in PTA corrosion prevention could explore several promising directions. One avenue is the development of smart coatings and protective materials tailored to resist PTA corrosion under specific environmental conditions. Investigating advanced monitoring technologies, such as sensors and non-destructive testing methods, could enable real-time corrosion detection and intervention. Exploring innovative alloy compositions with enhanced resistance to PTA corrosion is another area of interest. Additionally, delving into eco-friendly inhibitors and coatings aligns with sustainability goals in the industry. These avenues collectively offer exciting opportunities to enhance PTA corrosion prevention measures in the oil and gas sector.
References
- Popoola, L.T.; Grema, A.S.; Latinwo, G.K.; Gutti, B.; Balogun, A.S. Corrosion problems during oil and gas production and its mitigation. Int. J. Ind. Chem. 2013, 4, 1–15.
- Vedavyasan, C.V. Corrosion. In Encyclopedia of Membranes; Drioli, E., Giorno, L., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 1–9.
- Fernandes, J.S.; Montemor, F. Corrosion. In Materials for Construction and Civil Engineering: Science, Processing, and Design; Gonçalves, M.C., Margarido, F., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 679–716.
- Khoma, M.S.; Korniy, S.A.; Vynar, V.A.; Datsko, B.M.; Maksishko, Y.Y.; Dykha, O.V.; Bukliv, R.L. Influence of hydrogen sulfide on the carbon-dioxide corrosion and the mechanical characteristics of high-strength pipe steel. Mater. Sci. 2022, 57, 805–812.
- Virtanen, S. Electrochemical theory|Corrosion. In Encyclopedia of Electrochemical Power Sources; Garche, J., Ed.; Elsevier: Amsterdam, The Netherlands, 2009; pp. 56–63.
- Letardi, P. Electrochemical impedance measurements in the conservation of metals. In Radiation in Art and Archeometry; Creagh, D.C., Bradley, D.A., Eds.; Elsevier Science B.V.: Amsterdam, The Netherlands, 2000; pp. 15–39.
- Shein, A. Corrosion-electrochemical behavior of iron family silicides in various electrolytes. Prot. Met. Phys. Chem. 2010, 46, 479–488.
- Li, X.; Zhang, L.; Khan, F.; Han, Z. A data-driven corrosion prediction model to support digitization of subsea operations. Process Saf. Environ. Prot. 2021, 153, 413–421.
- Groysman, A. Corrosion problems and solutions in oil, gas, refining and petrochemical industry. Koroze Ochr. Mater. 2016, 61, 100–117.
- Spatolisano, E.; Pellegrini, L.A.; Gelosa, S.; Broglia, F.; Bonoldi, L.; de Angelis, A.R.; Moscotti, D.G.; Nali, M. Polythionic acids in the wackenroder reaction. ACS Omega 2021, 6, 26140–26149.
- Spatolisano, E.; Pellegrini, L.A.; Bonoldi, L.; de Angelis, A.R.; Moscotti, D.G.; Nali, M. Kinetic modelling of polythionic acids in Wackenroder reaction. Chem. Eng. Sci. 2022, 250, 117403.
- Zhang, H.; Jeffrey, M.I. A kinetic study of rearrangement and degradation reactions of tetrathionate and trithionate in near-neutral solutions. Inorg. Chem. 2010, 49, 10273–10282.
- Varga, D.; Horváth, A.K. Kinetics and mechanism of the decomposition of tetrathionate ion in alkaline medium. Inorg. Chem. 2007, 46, 7654–7661.
- Ji, C.; Yan, X.; Pan, C.; Lv, F.; Gao, Q. The key heterolysis selectivity of divalent sulfur–sulfur bonds for a unified mechanistic scheme in the thiosulfatolysis and sulfitolysis of the pentathionate ion. Eur. J. Inorg. Chem. 2016, 2016, 5497–5503.
- Xu, H.; Zhou, S.; Zhu, Y.; Xu, W.; Xiong, X.; Tan, H. Experimental study on the effect of H2S and SO2 on high temperature corrosion of 12Cr1MoV. Chin. J. Chem. Eng. 2019, 27, 1956–1964.
- Ji, C.; Yan, X.; Horváth, A.K.; Pan, C.; Zhao, Y.; Gao, Q. Comprehensive simultaneous kinetic study of sulfitolysis and thiosulfatolysis of tetrathionate ion: Unravelling the unique pH dependence of thiosulfatolysis. J. Phys. Chem. A. 2015, 119, 1238–1245.
- Steudel, R.; Göbel, T.; Holdt, G. The molecular nature of the hydrophilic sulfur prepared from aqueous sulfide and sulfite (selmi sulfur sol). Z. Naturforsch. B. 1989, 44, 526–530.
- Nietzel, O.A.; DeSesa, M.A. Specrophotometric determination of tetrathionate. Anal. Chem. 1955, 27, 1839–1841.
- Τawancy, H. Failure of hydrocracker heat exchanger tubes in an oil refinery by polythionic acid-stress corrosion cracking. Eng. Fail. Anal. 2009, 16, 2091–2097.
- Rajasuriyan, S.; Mohd Zaid, H.F.; Majid, M.F.; Ramli, R.M.; Jumbri, K.; Lim, J.W.; Mohamad, M.; Show, P.L.; Yuliarto, B. Oxidative extractive desulfurization system for fuel oil using acidic eutectic-based ionic liquid. Processes 2021, 9, 1050.
- Muhsin, W.; Zhang, J. Modelling and optimal operation of a crude oil hydrotreating process with atmospheric distillation unit utilising stacked neural networks. In Computer Aided Chemical Engineering; Espuña, A., Graells, M., Puigjaner, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 2479–2484.
- Baylor, V.; Keiser, J. Corrosion and stress corrosion cracking in coal liquefaction processes. J. Mater. Energy Syst. 1980, 2, 12–27.
- Speight, J.G. Chapter 2-Materials of Construction for Refinery Units. In Oil and Gas Corrosion Prevention; Speight, J.G., Ed.; Gulf Professional Publishing: Boston, DC, USA, 2014; pp. 3–37.
- Dorofeeva, T.I.; Fedorischeva, M.V.; Gubaidulina, T.A.; Sergeev, O.V.; Sungatulin, A.R.; Sergeev, V.P. Investigation of corrosion properties and composition of the surface formed on AISI 321 stainless steel by ion implantation. Metals 2023, 13, 1468.
- Zatkalíková, V.; Uhríčik, M.; Markovičová, L.; Kuchariková, L. Corrosion behavior of sensitized AISI 304 stainless steel in acid chloride solution. Materials 2022, 15, 8543.
- You, X.; Ning, K.; Bai, D.; Liu, Y.; Zhang, H.; Liu, F. Corrosion behavior of high-nitrogen steel hybrid welded joints fabricated by hybrid laser–arc welding. Materials 2023, 16, 3617.
- Davíðsdóttir, S.; Gunnarsson, B.G.; Kristjánsson, K.B.; Ledésert, B.A.; Ólafsson, D.I. Study of corrosion resistance properties of heat exchanger metals in two different geothermal environments. Geosciences 2021, 11, 498.
- Swaminathan, J.; Singh, R.; Gunjan, M.K.; Mahato, B. Sensitization induced stress corrosion failure of AISI 347 stainless steel fractionator furnace tubes. Eng. Fail. Anal. 2011, 18, 2211–2221.
- Alireza, K. Stress corrosion cracking behavior of materials. In Engineering Failure Analysis; Kary, T., Ed.; IntechOpen: Rijeka, Croatia, 2020; pp. 55–76.
- Marrow, T.J.; Babout, L.; Jivkov, A.P.; Wood, P.; Engelberg, D.; Stevens, N.; Withers, P.J.; Newman, R.C. Three dimensional observations and modelling of intergranular stress corrosion cracking in austenitic stainless steel. J. Nucl. Mater. 2006, 352, 62–74.
- Was, G.S.; Allen, T.R. Chapter 6-Corrosion issues in current and next-generation nuclear reactors. In Structural Alloys for Nuclear Energy Applications; Odette, G.R., Zinkle, S.J., Eds.; Elsevier: Boston, DC, USA, 2019; pp. 211–246.
- Yonezu, A.; Kusano, R.; Chen, X. On the mechanism of intergranular stress corrosion cracking of sensitized stainless steel in tetrathionate solution. J. Mater. Sci. 2013, 48, 2447–2453.
- Singh, P.M.; Ige, O.; Mahmood, J. Stress corrosion cracking of 304L stainless steel in sodium sulfide containing caustic solutions. J. Corros. Sci. Eng. 2003, 59, 843–850.
- Khalifeh, A.R.; Banaraki, A.D.; Daneshmanesh, H.; Paydar, M.H. Stress corrosion cracking of a circulation water heater tubesheet. Eng. Fail. Anal. 2017, 78, 55–66.
- Cao, Q.; Pojtanabuntoeng, T.; Esmaily, M.; Thomas, S.; Brameld, M.; Amer, A.; Birbilis, N. A review of corrosion under insulation: A critical issue in the oil and gas industry. Metals 2022, 12, 561.
- Borgioli, F. The corrosion behavior in different environments of austenitic stainless steels subjected to thermochemical surface treatments at low temperatures: An overview. Metals 2023, 13, 776.
- Bradley, S.A.; Mucek, M.W.; Anada, H.; Osuki, T. Alloy for resistance to polythionic acid stress corrosion cracking for hydroprocessing applications. Mater. Des. 2016, 110, 296–303.
- Singh, R. Influence of cold rolling on sensitization and intergranular stress corrosion cracking of AISI 304 aged at 500 °C. J. Mater. Process. Technol. 2008, 206, 286–293.
- Lobley, G.R. Stress corrosion cracking: Cases in refinery equipment. In Environment-Induced Cracking of Materials; Shipilov, S.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 401–410.
- Zomorodian, A.; Behnood, A. Review of corrosion inhibitors in reinforced concrete: Conventional and green materials. Buildings 2023, 13, 1170.
