Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Uche Maryann Chukwudulue and Version 2 by Jason Zhu.
The shift from the terrestrial to the marine environment to discover natural products has given rise to novel bioactive compounds, some of which have been approved for human medicine. However, the ocean, which makes up nearly three-quarters of the Earth’s surface, contains macro- and microorganisms whose natural products are yet to be explored. Among these underexplored marine organisms are macroalgae and their symbiotic microbes, such as Bacillota, a phylum of mostly Gram-positive bacteria previously known as Firmicutes. Macroalgae-associated Bacillota often produce chemical compounds that protect them and their hosts from competitive and harmful rivals.
- seaweeds
- macroalgae
- Firmicutes
- Bacillota
- natural products
1. Introduction
Natural products are molecules produced by organisms that have played a vital role in drug discovery [1]. Generally, the terrestrial environment and its organisms are well-studied for bioactive compounds, and this is due to the relative ease of their sampling [2] and culturing in the laboratory [3]. However, the largest ecosystem, the ocean [4], abundantly endowed with chemical and biological resources [5], is less explored [6][7][6,7]. The challenging conditions of the marine environment, for example, high salt concentration, low temperature, high pressure, low nutrients, and varying light intensities [8], spur marine organisms to synthesise uncommon chemical compounds that help them adapt to their environment [9]. Recently, researchers have been moving away from terrestrial to the marine ecosystem for unique therapeutic molecules with high pharmaceutical and biotechnological potentials [10]. However, many more studies are needed to cover the ocean’s vast biodiversity for this purpose.
So far, about twenty marine natural products (MNPs) are approved as pharmaceutical drugs [11], and twelve of them are specifically for cancer treatments [12]. Despite the vast number of microbes in the marine ecosystem, the number of microbial-derived marine drugs is limited [11]. Investigating more marine microbes, especially symbionts, for drug-like compounds is necessary, since the actual producers of many natural products are microorganisms inhabiting their hosts [13]. A notable example is tetrodotoxin, initially isolated from pufferfish and octopus but later discovered as a secondary metabolite of some symbiotic bacteria [14][15][14,15]. Another example is Taxol, previously obtained from a terrestrial plant, the Pacific yew [16], which was found afterwards to be a product of an endophytic fungus of the plant. Another reason to pursue symbiotic marine microorganisms is their ability to produce a greater variety of novel secondary metabolites than their free-living counterparts [17].
Marine macroalgae (seaweeds) are common hosts of diverse bacterial species [18], and algae–bacterial association is usually host-specific [19][20][21][19,20,21]. Host specificity means that some algae accommodate more microbial species than others [22], and similar microbial species colonise the same algae found in different ecological niches [19]. This algal holobiont, which refers to the host algae and its associated microorganisms, is influenced by the type of nutrients the host provides [23]. In addition to the metabolic duties performed by the bacterial symbionts for their hosts, they also protect them from harmful organisms and substances by producing antimicrobial and antifouling agents [18][24][25][18,24,25]. This advantage is due to the chemical interactions between the host and its associates [26] caused by the quest for survival in a competitive environment [27].
Alongside Actinomycetota and Pseudomonadota, Bacillota has been identified as the bacterial phylum most associative to marine organisms, including algae [28][29][30][28,29,30]. It is a ubiquitous phylum of bacteria found in both terrestrial and aquatic environments. Species of Bacillota are found abundantly in different regions of the human body [31][32][33][31,32,33], animals [34], soil [35][36][35,36], plant stems [37] and rhizospheres [38][39][38,39]. In the aquatic habitat, they can exist in water [40] or sediment [41], or associated with higher marine organisms [24][42][43][24,42,43]. This phylum of bacteria comprises mainly Gram-positive species exhibiting diverse phenotypic forms, most thriving at neutral pH [44]. They form endospores that enable them to adapt to many ecological conditions and are easily cultured in laboratories [45].
Bacillota are among the large genome-sized bacteria that use over 9% of their genomes to encode novel biosynthetic gene clusters (BGCs) that produce new bioactive compounds [46][47][46,47]. These bioactive molecules belong to different chemical classes, such as terpenes, polyketides (PKs), non-ribosomal peptides (NRPs), lasso peptides, bacteriocins, thiopeptides, ectoine, and melanin [28]. Though Bacillota dedicate a significant portion of their genome to producing bioactive compounds, most approved microbial drug products, especially antibiotics, are from a different bacterial phylum, Actinomycetota [48][49][48,49]. So far, Bacillota has only a few products on the market.
In human medicine, a deadly toxin (botulinum toxin type A) produced by Clostridium botulinum, commonly found in soil, has been developed into a vital drug (Botox®) [50]. Botox® is widely used to treat health issues ranging from strabismus to spasticity [51][52][51,52]. Studies have also tested its use in treating bruxism [53] and cosmetic procedures [54], and its anticancer properties [55][56][55,56]. Another FDA-approved product of Bacillota is polymyxin B, a chemical compound first obtained from a soil bacterium, Paenibacillus polymyxa [57]. This antibiotic is the last-resort treatment for multidrug-resistant Gram-negative bacterial infections [58]. Other formulated products from Bacillota are nisin (E 234), an antibiotic food additive isolated from a subspecies of Lactobacillus lactis [59][60][59,60], and two enzymes (protease and carbohydrase) from Bacillus subtilis or Bacillus amyloliquefaciens, considered to be GRAS (generally recognised as safe) by the FDA [61].
Some commercially available plant biofungicides from Bacillota in the agricultural industry include SERENADE®, Double Nickel 55TM and other products. These products are formulated from B. velezensis, a member of the operational group Bacillus amyloliquefaciens (OGBa) made up of B. amyloliquefaciens, B. siamensis, B. velezensis and B. nakamurai [62]. In addition, about 50% of approved plant bacterial biocontrol formulations used in different countries are Bacillus species products [63]. Examples include BioPro®, Rhiso Plus®, Biosubtilin, Botrybel®, and NacillusPro™ [64] and also the animal probiotic Ecobiol Plus® from B. amyloliquefaciens, commercially available in Europe for pigs and chickens, with potential use in aquaculture [65].
2. Aquatic Bacillota
A study by da Silva et al., 2013 identified at least a strain of Bacillota in every analysed sample of sea sediment collected from different depths of the South Atlantic Ocean, in contrast to other phyla [66][68], corroborating the easy cultivation of marine Bacillota in the laboratory [67][69]. Also, while assessing the microbial symbionts of sponges and a soft coral obtained from the Red Sea, Refs. [13][68][13,70] found that Bacillota was the most-encountered bacteria phylum, usually synonymous with bioactive secondary metabolites. For instance, some marine Bacillus species produced many novel natural products, ranging from macrolides [69][70][71][71,72,73] to fatty acids [72][74]. A specific example is Bacillus silvesteris from a marine crab, which synthesised two unknown cyclodepsipeptides with very high cytotoxic effects (GI50s: 0.001–0.01 ng/mL) against human cancer cell lines [73][75]. Unfortunately, with these exciting numbers of novel molecules associated with marine Bacillota, only a few studies covered Bacillota from macroalgae; more studies were on Bacillota of corals and sponges.3. Marine Macroalgae, a Good Source of Bioactive Bacillota
Consistently, marine macroalgae have produced various remarkable molecules [2][26][2,26]. Between 1960 and 2012, more than three thousand natural products were identified in different algae types, and they are still receiving attention for their novel bioactive compounds [74][76]. Some examples of algae chemical compounds include two potential anticancer agents, lophocladines B from a red alga [75][77], dieckol from a brown alga [76][78] and Griffithsin from a red alga, currently in phase I clinical trial for HIV prevention [11][77][11,79]. However, algae’s commercial and ecological applications are linked to the chemical communications between them and their associated microbes [18][78][18,80]. To ascertain this assumption, scientists are investigating algae microbial symbionts for their natural products [19][79][80][81][19,81,82,83]. It appears that Bacillota play more beneficial than pathogenic functions in algae [22][79][22,81], by producing chemical compounds that protect algae from fouling and colonising pathogenic microbes.4. Secondary Metabolites of Marine Macroalgae Bacillota and Their Biosynthetic Gene Clusters
Several chemical compounds have been obtained from marine macroalgae-associated Bacillota in laboratories. However, the type of compounds generated by any microorganism in a conventional laboratory can be influenced by the properties of the growth medium [13]. Different classes of compounds would emerge by varying the composition or condition of the culture media. For instance, growing a symbiotic bacterium in a static culture or one consistently shaken at a particular speed affects the types of chemical compounds the bacterium will produce [80][82]. This is a well-known natural-product drug-discovery approach called the one strain many compounds (OSMAC) approach. Table 1 also shows that most bioactive compounds of the marine macroalgae Bacillota belong to the polyketide class, which aligns with the suggestion by Aleti et al., 2015, regarding the prevalence of a polyketide synthase (pks) gene cluster in Bacillota [81][83]. Of the forty-one compounds isolated from the macroalgae Bacillota, thirty-eight are polyketides, while the remaining three belong to the non-ribosomal peptide–polyketide hybrid. The chemical structures of these molecules (1–41) are shown in Figure 1, Figure 2, Figure 3 and Figure 4, and they are grouped according to their chemical classes, including macrolides, esters, furanoterpenoids and amicoumacins. Apart from the forty-one isolated and characterised compounds, there are forty-seven volatile compounds identified in an extract of a B. amyloliquefaciens strain isolated from a brown alga Zonaria tournefortii [82][84], as well as a YbdN protein isolated from B. licheniformis of Fucus serratus [80][82].Table 1.
Natural products and pharmacological properties of symbiotic Bacillota from marine macroalgae.
| Algae Species | Growth Medium | Bacterial Species | Biosynthetic Gene Cluster | Extract/ Compounds |
Pharmacological Properties | MIC (µg/mL) | References |
|---|---|---|---|---|---|---|---|
| Brown Algae Bacillota | |||||||
| Sargassum wightii | a ZMA * b NA a NA a ZMA |
Bacillus species Bacillus atrophaeus MW821482 |
pks pks nrps Siderophore |
Ethyl acetate extract Ethyl acetate extract |
Antibacterial Antioxidant Antihypertensive Antihypercholesterolamic Anti-inflammatory Anti-hyperglycemic Cytotoxicity Antioxidant Antibacterial Anti-inflammatory Anti-hyperglycemic Antihypertensive Antioxidant Anti-hypercholesterolemic Antibacterial |
6.25–12.5 ⁑ (133–492.04) ⁑ (498.12–735.42) ⁑ (10.21–24.32) ⁑ (5.22–735.45) ⁑ (92.02–759.24) ♯ 29.5 ♯ (133–4167) 6.25–12.5 ⁑ (9.74–788.8) ⁑ (118.1–513.4) ⁑ 713.6 ⁑ (413.2–429.8) ⁑ 22.23 6.25–12.5 |
[83][84][85][86][85,86,87,88] |
| Anthophycus longifolius | a NA ** NA SWA ZMA * NA MA * NA |
Bacillus subtilis MTCC 10403 | pks pks pks |
(1) (35–38) (2) |
Antibacterial Antibacterial Antibacterial |
3.12–50 3.12–25 ND |
[87][88][89][89,90,91] |
| Sargassum myriocystum | MA * NA |
Bacillus subtilis MTCC 10407 | pks | (26 and 27) | Antibacterial | ND | [90][92] |
| Fucus serratus | a TSA DSTA MA NA * CB |
Bacillus licheniformis | ND | YbdN protein | Antibacterial | ND | [80][82] |
| Endarachne binghamiae | MA MB |
Bacillus sp. | ND | Acetone extract | Antibacterial | 188.1–209.7 | [91][93] |
| Sargassum muticum | MA MB |
Bacillus sp. | ND | Acetone extract | Cytotoxicity Antibacterial |
♯ 5.5 174 |
[91][93] |
| Egregia menziesii | MA MB |
Bacillus sp. | ND | Acetone extract | Antibacterial | 203.0–212.3 | [91][93] |
| Padina gymnospora | a NA ** NA SWA ZMA * NA |
Bacillus amyloliquefaciens | pks | (28–31) | Antibacterial | ND | [92][94] |
| Zonaria tournefortii | d LB | Bacillus amyloliquefaciens S13 | ND | Volatile compounds | Antimicrobial | 64–>500 | [82][84] |
| Red algae Bacillota | |||||||
| Hypnea valentiae | a ZMA * MBSA |
Bacillus amyloliquefaciens MB6 (MTCC 12716) | pks pks-nrps ND |
(3–5) and (6–8) (39–41) Ethyl acetate extract |
Antibacterial Antibacterial Antibacterial Anti-inflammatory Anti-hypercholesterolemic Antidiabetic Antioxidant Antibacterial |
0.38–5.00 ¶ (−9.06)–(−10.13) ¶ (−11.33)–(−13.61) 0.78–3.12 3.125–12.5 ⁑ (6.06–675.36) ⁑ 17.30 ⁑ (84.00–639.54) ⁑ (136.78–278.19) 6.25–12.50 |
[93][94][95[98][95,96][,9796][,9897],99,100] |
| Kappaphycus alvarezii | a ZMA * MBSA |
Bacillus amyloliquefaciens MTCC 12713 | pks pks |
(9–12) (22–25) |
Antibacterial Antibacterial |
‡ 2–9 × 10−3 1.56–6.25 ¶ (−9.06)–(−12.61) |
[99][100][101,102] |
| Laurencia papillosa | a NA c ZMA * NA a ZMA a NA * NA a NA ** NA SWA ZMA * NA |
Bacillus velezensis MBTDLP1 MTCC 13048 Bacillus velezensis MBTDLP1 Bacillus amyloliquefaciens |
pks ND pks |
(34) Ethyl acetate extract (32 and 33) |
Antibacterial Antibacterial Anti-inflammatory Cytotoxicity Antidiabetic Antioxidant Antibacterial |
0.38 7.5–15 ♯ 17 ♯ (32.3–200) ♯ (120–420) ♯ (107–4127) ND |
[101][102][103][103,104,105] |
| Laurencia pacifica | MA MB |
Bacillus sp. | ND | Acetone extract | Antibacterial | 288.1 | [91][93] |
| Centroceras clavulatum | MA MB |
Bacillus sp. | ND | Acetone extract | Antibacterial | 217.1 | [91][93] |
| Schizymenia dubyi | MB | Bacillus sp. PP19-H3 | pks | (13–21) | Antibacterial | 10–>100 | [71][73] |
| Green Algae Bacillota | |||||||
| Codium fragile | MA MB |
Bacillus sp. | ND | Acetone extract | Antibacterial | 196 | [91][93] |
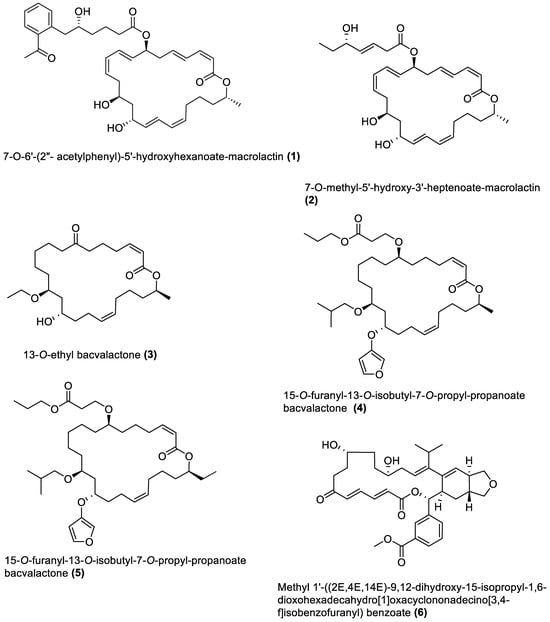
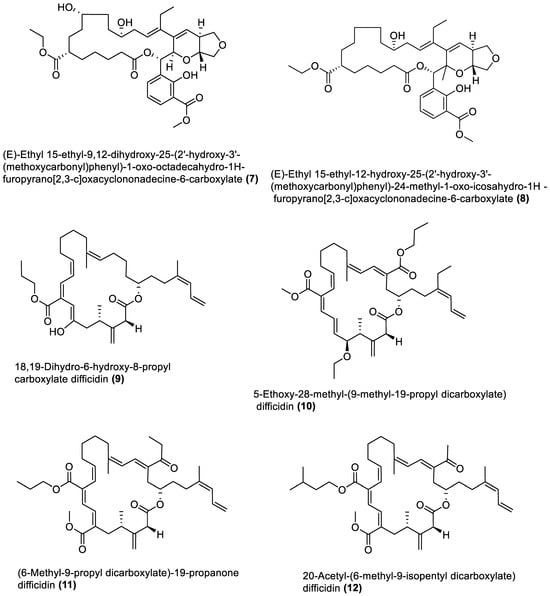
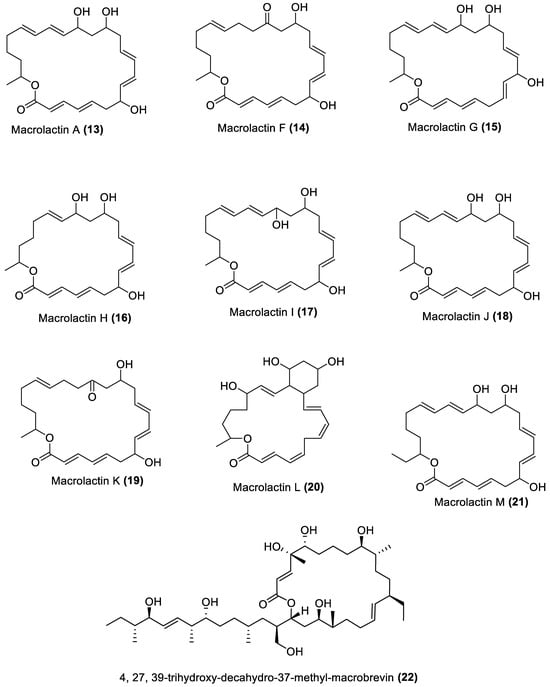
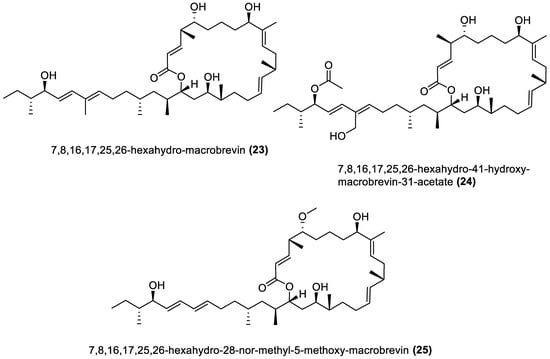
Figure 1.
Macrolides from marine macroalgae Bacillota.
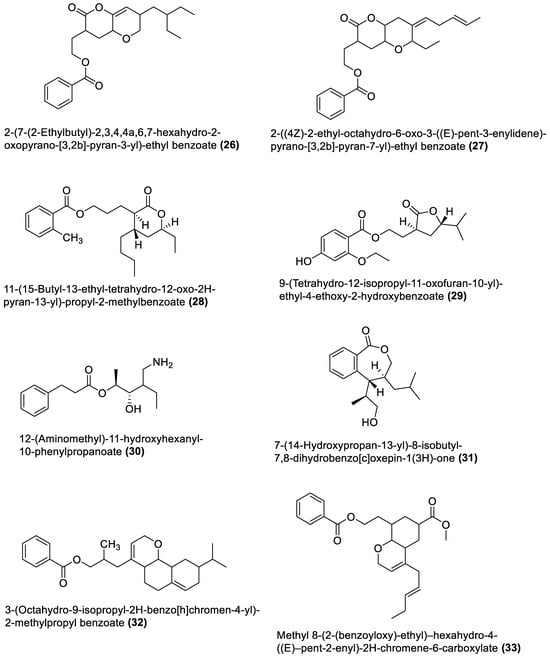
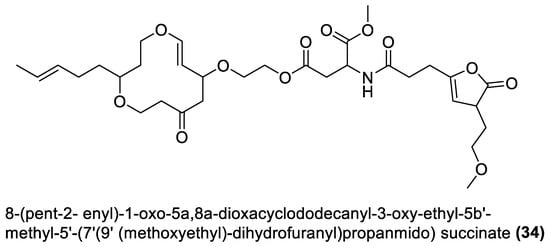
Figure 2.
Esters from marine macroalgae Bacillota.
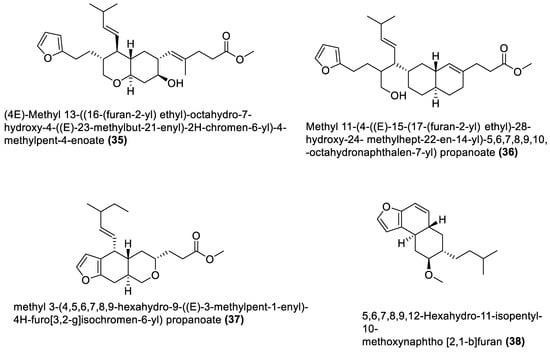
Figure 3.
Furanoterpenoids from marine macroalgae Bacillota.
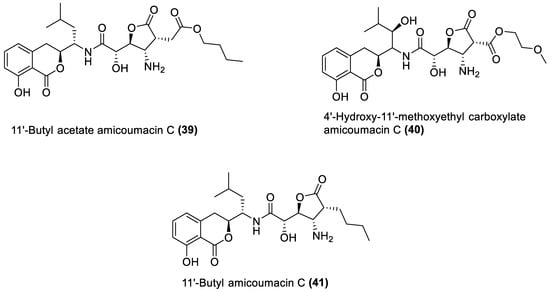
Figure 4.
Amicoumacins from marine macroalgae Bacillota.
