Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Catherine Yang and Version 1 by Tommaso A. Dragani.
The proposal by the European Chemicals Agency (ECHA) to ban over 12,000 per- and polyfluoroalkyl substances (PFAS) has sparked a debate about potential consequences for the economy, industry, and the environment. Although some PFAS are known to be harmful, a blanket ban may lead to significant problems in attempting to replace PFAS-based materials for environmental transition, as well as in medical devices and everyday products.
- PFAS
- polyfluoroalkyl
- perfluoroalkyl
- health
- fluoropolymers
- bioremediation
1. Introduction
On 7 February 2023, the European Chemicals Agency (ECHA) published a proposal to ban an entire class of chemicals known as per- and polyfluoroalkyl substances (PFAS) [1]. This proposed ban is unprecedented, and, if approved, would affect more than 12,000 PFAS [2]. Based on the revised OECD definitions, ‘PFASs consist of a fully (per) or partly (poly) fluorinated carbon chain connected to different functional groups.’ [3].
However, PFAS are not a chemical class of similar compounds, but the term includes a wide range of compounds with very different physical, chemical, environmental, and biological properties. Although some PFAS are known to be harmful to both the environment and human health, others are not, and the vast majority have yet to be toxicologically characterized. For example, fluoropolymers do not exhibit the toxicological and environmental properties commonly associated with other PFAS of concern [4].
PFAS are widely used in a variety of industries, including textiles, electronics, food packaging, electric car batteries, various household products, cosmetics, pharmaceuticals, pesticides, and medical device manufacturing. A blanket ban on all substances could have serious economic, industrial, and environmental consequences, and, paradoxically, public health implications [5,6][5][6]. Moreover, replacing PFAS with alternative substances may be prohibitively expensive and even impossible in some cases.
It is likely that the ban will not be fully implemented for reasons of economic and social sustainability, but the current proposal, if not quickly withdrawn, will lead to a hasty search for substitutes that may perform worse than PFAS, be more expensive, and most likely be less characterized toxicologically.
It is difficult for material chemists to imagine possibly replacing all PFAS, within a few years, with alternative non-fluorinated compounds that have the same chemical and physical properties as the products they are intended to replace.
It should also be noted that the proposed ban on the entire class of PFAS is being proposed in the absence of scientific evidence to prove that the end products made with PFAS are harmful. This is particularly true for fluoropolymers and perfluoropolyether oils, which are used in a wide range of industries and applications, including the automotive, aerospace, chemical, and nuclear industries, and electronics, medical devices, and green-economy initiatives [7,8][7][8]. These materials have not been linked to any adverse effects on humans but have rather improved quality of life and well-being.
2. PFAS Are Not a Small Group of Chemicals with Similar Properties
A recent paper on PFAS terminology, developed within the Organization for Economic Cooperation and Development (OECD), highlights the tremendous heterogeneity in the chemical structures of different PFAS (Figure 1) and the need to revise PFAS terminology [17][9]. The term PFAS, which is commonly used to define the entire chemical class, is too general and likely to cause further confusion. The most studied PFAS that pose toxicological risks to humans and the environment are non-polymeric perfluoroalkyl carboxylic acids (PFCA), e.g., perfluorooctanoic acid, PFOA, and perfluorosulfonic acids (PFSA), e.g., perfluorooctane sulfonic acid and PFOS.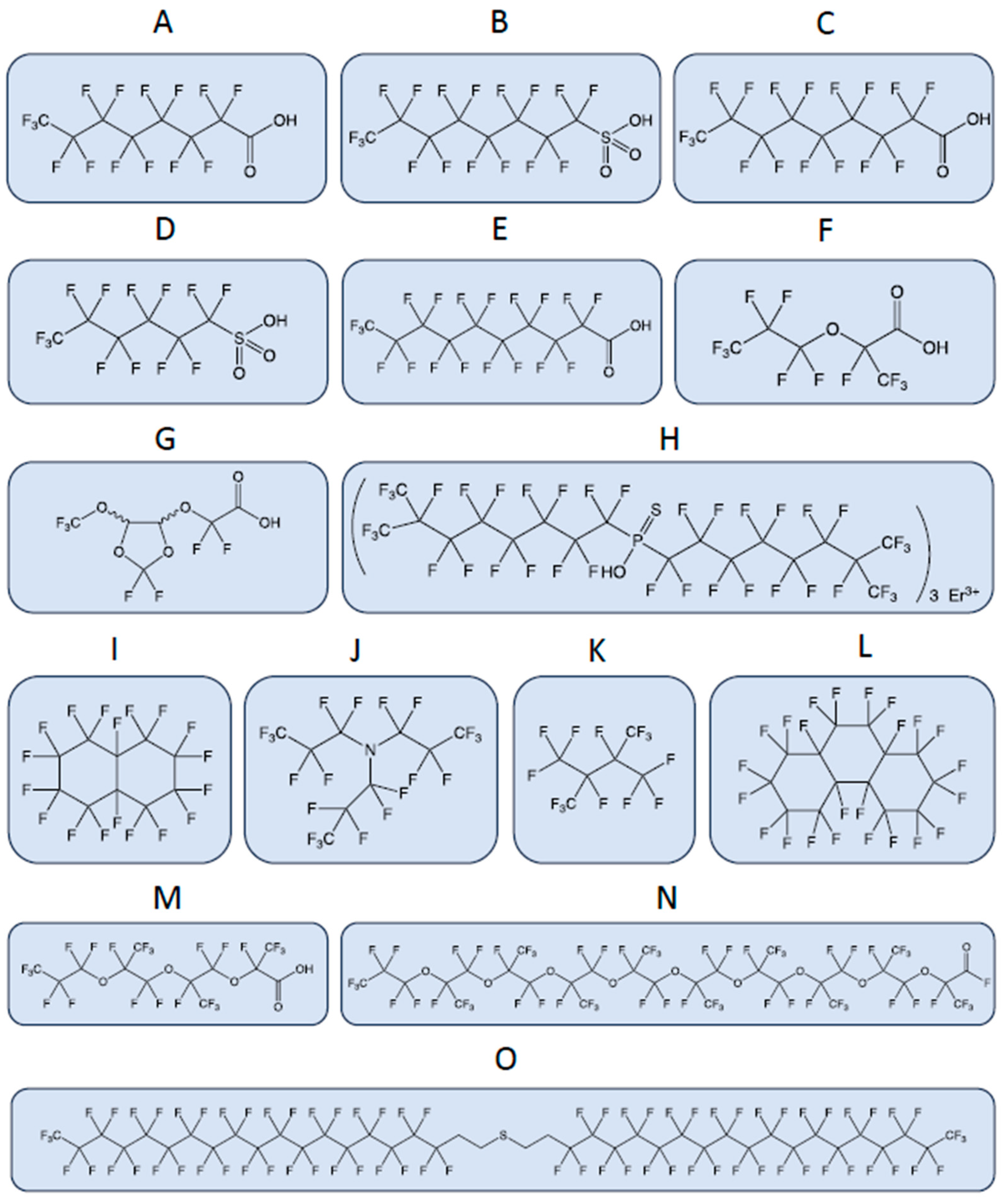

Figure 1.
A few examples highlight the huge heterogeneity of chemical structures of PFAS, which poses challenges for their regulation. The physical and chemical properties of PFASs vary widely depending on their chain length, branching, and functional groups. These properties affect their environmental fate and transport, bioaccumulation potential, and toxicity. The bonds between the atoms are represented by lines. CAS, Chemical Abstracts Service Registry Number, is a numeric identifier assigned by the Chemical Abstracts Service (CAS) division of the American Chemical Society (ACS). (
A
) Perfluorooctanoic acid (PFOA), CAS: 335-67-1; (
B
) perfluorooctanesulfonic acid (PFOS), CAS: 1763-23-1; (
C
) perfluorononanoic acid (PFNA), CAS: 375-95-1; (
D
) perfluorohexanesulfonic acid (PFHxS), CAS: 355-46-4; (
E
) perfluorodecanoic acid (PFDA), CAS: 335-76-2; (
F
) 2,3,3,3-Tetrafluoro-2-(heptafluoropropoxy)propanoic acid (GenX or HFPO-DA), CAS: 13252-13-6; (
G
) 2,2-difluoro-2-{[2,2,4,5-tetrafluoro-5-(trifluoromethoxy)-1,3-dioxolan-4-yl]oxy}acetic acid (C6O4), CAS: 682-238-0; (
H
) P,P-Bis(perfluoro-7-methyloctyl) phosphinothioic acid erbium(3+) salt (3:1), CAS: 500776-89-6; (
I
) perfluorodecalin, CAS: 306-94-5; (
J
) perfluorotripropylamine, CAS: 338-83-0; (
K
) perfluoro-2,3-dimethylbutane, CAS: 354-96-1; (
L
) perfluoroperhydrophenanthrene, CAS: 306-91-2; (
M
) perfluoro-(2,5,8-trimethyl-3,6,9-trioxadodecanoic)acid, CAS: 65294-16-8; (
N
) dotriacontafluorononakis(trifluoromethyl)nonaoxatriacontanoyl fluoride, CAS: 65150-88-1; (
O) bis 18:2 fluorotelomer thioether.
) bis 18:2 fluorotelomer thioether.
3. Bioaccumulation of PFAS: The Role of Chain Length
Bioaccumulation or biopersistence refers to the accumulation of a substance in an organism over time. Obviously, the bioavailability of a substance is a prerequisite for bioaccumulation. PFCAs and PFSAs, but not all PFAS, exhibit high stability and their lipophilicity depends on the length of the alkylic chain, resulting in their accumulation in several tissues [56][20]. Moreover, they can bind to human serum albumin and other transporters in the blood [57][21]. These properties make PFCA and PFSA potentially able to bioaccumulate in humans and animals. The bioaccumulation of PFCAs and PFSAs is, indeed, influenced by their chemical structure. Compounds with longer carbon chains, such as PFOA and PFOS, are the most persistent in the environment and can accumulate in living organisms [26,58,59][18][22][23]. By contrast, short-chain PFAS are less likely to bioaccumulate [59,60,61,62][23][24][25][26]. In fact, although short-chain PFAS have been detected in aquatic systems, their concentrations are generally lower than those of long-chain PFAS [63][27]. In particular, long-chain PFAS are more likely to accumulate in the brain than short-chain PFAS due to their ability to cross brain barriers [64][28]. A study analyzing PFAS profiles in drinking water and biological samples from airport workers exposed to contaminated groundwater found that ‘historical’ PFAS accounted for 50% of the total PFAS in drinking water and 90% in serum. Branched PFOS isomers had shorter half-lives than linear PFOS isomers, with half-lives generally decreasing with decreasing chain length [65][29]. Fluoropolymers, on the other hand, do not pose a bioaccumulation risk because their high molecular weight prevents their absorption by the body, and thus their bioavailability [66][30].4. Fluoropolymers Are a Separate Class from Smaller PFAS Molecules
Although they fall into the PFAS category, fluoropolymers are a distinct class of chemical compounds characterized by much larger molecular sizes (typical molecular weights > 100,000 Da) and more complex structures than the smaller PFAS molecules. Fluoropolymers consist of long carbon chains with multiple repeating units and fluorine atoms, occasionally accompanied by branching or cross-linking between polymer chains. Compared to small PFAS molecules, the larger size and often complex structures of fluoropolymers likely limit their uptake by living organisms, thereby reducing their likelihood of bioaccumulation. In addition, the large size of fluoropolymers results in their lower solubility in water, further limiting their mobility and potential for dispersion in the environment [66,67][30][31]. In fact, fluoropolymers can be classified as low-risk polymers (PLCs), as they meet all the requirements for this classification [68,69][32][33]. Overall, size, structure, and water solubility play a key role in determining the biological fate and potential damage of fluorinated substances [66][30].Size Limits for Small-Molecule Biological Activity
Size plays, along with charge and structure, a critical role in determining the penetration of molecules across cell membranes. In the development of new drugs, 500 Da is often quoted as the maximum molecular weight parameter. However, it has been observed that molecules with higher molecular weights are also capable of being absorbed, and the limits of oral bioavailability appear to extend to about MW ≤ 1000 Da [70,71,72][34][35][36]. Thus, data accumulated from extensive investigations of various pharmacological and non-pharmacological substances indicate that molecules with molecular weights above 1000 Da have very little, if any, ability to diffuse across cell membranes and, as a result, are not bioavailable when taken orally. Therefore, substances with molecular weights greater than 1000 Da, such as fluoropolymers, which generally have molecular weights much greater than 1000 Da, have negligible bioavailability, resulting in limited potential toxicity and bioaccumulation.5. Bioremediation of PFAS: Challenges and Opportunities
Recent advances in PFAS degradation via thermal and non-thermal methods have been recently reviewed. Along with physicochemical techniques [27][19], bioremediation appears to be a successful solution for PFAS removal from the environment [73,74][37][38]. Bioremediation is a process that utilizes the metabolic capabilities of microorganisms to degrade and detoxify contaminants. The microbial degradation of PFAS is emerging as a promising approach for the remediation of contaminated waters and sites. For example, Acidimicrobium sp. strain A6 is capable of defluorinating PFOA and PFOS through a reaction in which iron is reduced and ammonium or hydrogen are used as electron donors; this reaction leads to the formation of shorter-chain perfluorinated products and acetate [75][39]. Another study investigated the role of carbon–carbon double bonds in the biodegradation of unsaturated PFAS, showing that α,β unsaturation is critical for anaerobic reductive defluorination and highlighting the enhanced degradability of unsaturated fluorinated carboxylic acids with α/β-trifluoromethyl branches [76][40]. Several microbial enzymes, including esterases, hydrolases, oxidases, reductases, and dehalogenases, play key roles in PFAS biodegradation, and advances in enzyme engineering and biocatalysis offer the potential for the development of efficient and sustainable PFAS bioremediation strategies [77,78][41][42]. However, the diversity of PFAS structures poses a challenge for bioremediation. Long-chain PFCAs and PFSAs may be more resistant to biodegradation than their short-chain counterparts. Despite these challenges, bioremediation offers several advantages over other remediation methods, such as chemical treatment and incineration. Bioremediation has very low costs and is environmentally friendly because it does not require expensive equipment and does not produce harmful byproducts [79][43]. More research is needed to determine the feasibility of bioremediation as an effective strategy for PFAS remediation and to optimize the degradation of PFAS with different chemical structures. Should PFAS bioremediation techniques demonstrate their effectiveness, the depiction in media and the emphasis in regulatory proposals that currently categorize PFAS contaminants as ‘forever chemicals’ to underscore their environmental risk may need to be reevaluated.References
- ECHA. Annex XV Restriction Report: Proposal for a Restriction. Available online: https://echa.europa.eu/documents/10162/f605d4b5-7c17-7414-8823-b49b9fd43aea (accessed on 21 February 2023).
- EPA. PFAS Master List of PFAS Substances. Available online: https://comptox.epa.gov/dashboard/chemical-lists/pfasmaster (accessed on 21 February 2023).
- OECD. What Are PFASS and What Are They Used for? Available online: https://www.oecd.org/chemicalsafety/portal-perfluorinated-chemicals/aboutpfass/ (accessed on 8 August 2023).
- Ameduri, B. Fluoropolymers: A Special Class of per- and Polyfluoroalkyl Substances (PFASs) Essential for Our Daily Life. J. Fluor. Chem. 2023, 267, 110117.
- Chetty, R.; Stepner, M.; Abraham, S.; Lin, S.; Scuderi, B.; Turner, N.; Bergeron, A.; Cutler, D. The Association Between Income and Life Expectancy in the United States, 2001–2014. JAMA 2016, 315, 1750–1766.
- Saito, M.; Kondo, N.; Oshio, T.; Tabuchi, T.; Kondo, K. Relative Deprivation, Poverty, and Mortality in Japanese Older Adults: A Six-Year Follow-Up of the JAGES Cohort Survey. Int. J. Environ. Res. Public Health 2019, 16, 182.
- Lv, J.; Cheng, Y. Fluoropolymers in Biomedical Applications: State-of-the-Art and Future Perspectives. Chem. Soc. Rev. 2021, 50, 5435–5467.
- Wang, M.; Tsuda, M.; Deguchi, S.; Higuchi, Y.; So, K.; Torisawa, Y.-S.; Takayama, K.; Yamashita, F. Application of Perfluoropolyether Elastomers in Microfluidic Drug Metabolism Assays. Int. J. Pharm. 2022, 627, 122253.
- Wang, Z.; Buser, A.M.; Cousins, I.T.; Demattio, S.; Drost, W.; Johansson, O.; Ohno, K.; Patlewicz, G.; Richard, A.M.; Walker, G.W.; et al. A New OECD Definition for Per- and Polyfluoroalkyl Substances. Environ. Sci. Technol. 2021, 55, 15575–15578.
- Polishchuk, P. Interpretation of Quantitative Structure-Activity Relationship Models: Past, Present, and Future. J. Chem. Inf. Model. 2017, 57, 2618–2639.
- Brusseau, M.L.; Van Glubt, S. The Influence of Molecular Structure on PFAS Adsorption at Air-Water Interfaces in Electrolyte Solutions. Chemosphere 2021, 281, 130829.
- Lampic, A.; Parnis, J.M. Property Estimation of Per- and Polyfluoroalkyl Substances: A Comparative Assessment of Estimation Methods. Environ. Toxicol. Chem. 2020, 39, 775–786.
- Zhang, M.; Yamada, K.; Bourguet, S.; Guelfo, J.; Suuberg, E.M. Vapor Pressure of Nine Perfluoroalkyl Substances (PFASs) Determined Using the Knudsen Effusion Method. J. Chem. Eng. Data 2020, 65, 2332–2342.
- Schindler, B.J.; Buchanan, J.H.; Mahle, J.J.; Peterson, G.W.; Glover, T.G. Ambient Temperature Vapor Pressure and Adsorption Capacity for (Perfluorooctyl) Ethylene, 3-(Perfluorobutyl)Propanol, Perfluorohexanoic Acid, Ethyl Perfluorooctanoate, and Perfluoro-3,6-Dioxaheptanoic Acid. J. Chem. Eng. Data 2013, 58, 1806–1812.
- Barton, C.A.; Botelho, M.A.; Kaiser, M.A. Solid Vapor Pressure and Enthalpy of Sublimation for Ammonium Perfluorooctanoate. J. Chem. Eng. Data 2009, 54, 752–755.
- Kirsch, P. Modern Fluoroorganic Chemistry: Synthesis, Reactivity, Applications, 2nd ed.; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2013.
- Xing, Y.; Li, Q.; Chen, X.; Huang, B.; Ji, L.; Zhang, Q.; Fu, X.; Li, T.; Wang, J. PFASs in Soil: How They Threaten Human Health through Multiple Pathways and Whether They Are Receiving Adequate Concern. J. Agric. Food Chem. 2023, 71, 1259–1275.
- Ahrens, L.; Bundschuh, M. Fate and Effects of Poly- and Perfluoroalkyl Substances in the Aquatic Environment: A Review. Environ. Toxicol. Chem. 2014, 33, 1921–1929.
- Trang, B.; Li, Y.; Xue, X.-S.; Ateia, M.; Houk, K.N.; Dichtel, W.R. Low-Temperature Mineralization of Perfluorocarboxylic Acids. Science 2022, 377, 839–845.
- Greaves, A.K.; Letcher, R.J.; Sonne, C.; Dietz, R.; Born, E.W. Tissue-Specific Concentrations and Patterns of Perfluoroalkyl Carboxylates and Sulfonates in East Greenland Polar Bears. Environ. Sci. Technol. 2012, 46, 11575–11583.
- Crisalli, A.M.; Cai, A.; Cho, B.P. Probing the Interactions of Perfluorocarboxylic Acids of Various Chain Lengths with Human Serum Albumin: Calorimetric and Spectroscopic Investigations. Chem. Res. Toxicol. 2023, 36, 703–713.
- Hsu, J.-Y.; Hsu, J.-F.; Ho, H.-H.; Chiang, C.-F.; Liao, P.-C. Background Levels of Persistent Organic Pollutants in Humans from Taiwan: Perfluorooctane Sulfonate and Perfluorooctanoic Acid. Chemosphere 2013, 93, 532–537.
- Xu, B.; Qiu, W.; Du, J.; Wan, Z.; Zhou, J.L.; Chen, H.; Liu, R.; Magnuson, J.T.; Zheng, C. Translocation, Bioaccumulation, and Distribution of Perfluoroalkyl and Polyfluoroalkyl Substances (PFASs) in Plants. iScience 2022, 25, 104061.
- Liang, X.; Yang, X.; Jiao, W.; Zhou, J.; Zhu, L. Simulation Modelling the Structure Related Bioaccumulation and Biomagnification of Per- and Polyfluoroalkyl Substances in Aquatic Food Web. Sci. Total Environ. 2022, 838 Pt 3, 156397.
- Cheng, W.; Doering, J.A.; LaLone, C.; Ng, C. Integrative Computational Approaches to Inform Relative Bioaccumulation Potential of Per- and Polyfluoroalkyl Substances Across Species. Toxicol. Sci. 2021, 180, 212–223.
- Lewis, A.J.; Yun, X.; Spooner, D.E.; Kurz, M.J.; McKenzie, E.R.; Sales, C.M. Exposure Pathways and Bioaccumulation of Per- and Polyfluoroalkyl Substances in Freshwater Aquatic Ecosystems: Key Considerations. Sci. Total Environ. 2022, 822, 153561.
- Li, F.; Duan, J.; Tian, S.; Ji, H.; Zhu, Y.; Wei, Z.; Zhao, D. Short-Chain per- and Polyfluoroalkyl Substances in Aquatic Systems: Occurrence, Impacts and Treatment. Chem. Eng. J. 2020, 380, 122506.
- Cao, Y.; Ng, C. Absorption, Distribution, and Toxicity of per- and Polyfluoroalkyl Substances (PFAS) in the Brain: A Review. Environ. Sci. Process. Impacts 2021, 23, 1623–1640.
- Xu, Y.; Fletcher, T.; Pineda, D.; Lindh, C.H.; Nilsson, C.; Glynn, A.; Vogs, C.; Norström, K.; Lilja, K.; Jakobsson, K.; et al. Serum Half-Lives for Short- and Long-Chain Perfluoroalkyl Acids after Ceasing Exposure from Drinking Water Contaminated by Firefighting Foam. Environ. Health Perspect. 2020, 128, 77004.
- Korzeniowski, S.H.; Buck, R.C.; Newkold, R.M.; El Kassmi, A.; Laganis, E.; Matsuoka, Y.; Dinelli, B.; Beauchet, S.; Adamsky, F.; Weilandt, K.; et al. A Critical Review of the Application of Polymer of Low Concern Regulatory Criteria to Fluoropolymers II: Fluoroplastics and Fluoroelastomers. Integr. Environ. Assess. Manag. 2023, 19, 326–354.
- Henry, B.J.; Carlin, J.P.; Hammerschmidt, J.A.; Buck, R.C.; Buxton, L.W.; Fiedler, H.; Seed, J.; Hernandez, O. A Critical Review of the Application of Polymer of Low Concern and Regulatory Criteria to Fluoropolymers. Integr. Environ. Assess. Manag. 2018, 14, 316–334.
- OECD. Chemicals Committee. Data Analysis of the Identification of Correlations between Polymer Characteristics and Potential for Health or Ecotoxicological Concern; Environment Directorate Joint Meeting of the Chemicals Committee and the Working Party on Chemicals, Pesticides and Biotechnology (Env/Jm/Mono(2009)); JT03258707; OECD: Paris, France, 2009; pp. 1–41.
- AICIS. Polymer of Low Concern. Available online: https://www.industrialchemicals.gov.au/glossary/polymer-low-concern (accessed on 25 May 2023).
- Veber, D.F.; Johnson, S.R.; Cheng, H.-Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular Properties That Influence the Oral Bioavailability of Drug Candidates. J. Med. Chem. 2002, 45, 2615–2623.
- Doak, B.C.; Over, B.; Giordanetto, F.; Kihlberg, J. Oral Druggable Space beyond the Rule of 5: Insights from Drugs and Clinical Candidates. Chem. Biol. 2014, 21, 1115–1142.
- Matsson, P.; Kihlberg, J. How Big Is Too Big for Cell Permeability? J. Med. Chem. 2017, 60, 1662–1664.
- Verma, S.; Lee, T.; Sahle-Demessie, E.; Ateia, M.; Nadagouda, M.N. Recent Advances on PFAS Degradation via Thermal and Nonthermal Methods. Chem. Eng. J. Adv. 2022, 13, 100421.
- Zhang, Z.; Sarkar, D.; Biswas, J.K.; Datta, R. Biodegradation of Per- and Polyfluoroalkyl Substances (PFAS): A Review. Bioresour. Technol. 2022, 344 Pt B, 126223.
- Huang, S.; Jaffé, P.R. Defluorination of Perfluorooctanoic Acid (PFOA) and Perfluorooctane Sulfonate (PFOS) by Acidimicrobium Sp. Strain A6. Environ. Sci. Technol. 2019, 53, 11410–11419.
- Yu, Y.; Che, S.; Ren, C.; Jin, B.; Tian, Z.; Liu, J.; Men, Y. Microbial Defluorination of Unsaturated Per- and Polyfluorinated Carboxylic Acids under Anaerobic and Aerobic Conditions: A Structure Specificity Study. Environ. Sci. Technol. 2022, 56, 4894–4904.
- Berhanu, A.; Mutanda, I.; Taolin, J.; Qaria, M.A.; Yang, B.; Zhu, D. A Review of Microbial Degradation of Per- and Polyfluoroalkyl Substances (PFAS): Biotransformation Routes and Enzymes. Sci. Total Environ. 2023, 859 Pt 1, 160010.
- Tang, K.H.D.; Kristanti, R.A. Bioremediation of Perfluorochemicals: Current State and the Way Forward. Bioprocess Biosyst. Eng. 2022, 45, 1093–1109.
- Mishra, B.; Varjani, S.; Agrawal, D.C.; Mandal, S.K.; Ngo, H.H.; Taherzadeh, M.J.; Chang, J.-S.; You, S.; Guo, W. Engineering Biocatalytic Material for the Remediation of Pollutants: A Comprehensive Review. Environ. Technol. Innov. 2020, 20, 101063.
More
