Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Camila Xu and Version 1 by Gang Wang.
Perovskite solar cells have garnered significant attention as the next-generation photovoltaic devices. After more than a decade of dedicated research, commercializing these cells is now on the horizon. One of the primary focuses for developers aiming at large-scale industrial production is cost reduction.
- perovskite solar cells
- industrialization
- low cost
- room temperature
- continuous production
1. Introduction
In recent years, perovskite solar cells (PSCs) have captured extensive attention from both the academic and industrial sectors. Thanks to the advantages of high light absorption coefficients [1[1][2],2], long carrier transport distances [3,4][3][4] and low exciton binding energies [5,6][5][6] exhibited by perovskite materials, the conversion efficiency of PSCs has steadily improved over the past decade [7[7][8][9][10],8,9,10], with laboratory-certified efficiencies reaching as high as 26.1% for small-scale devices [11]. The rapid development of high-efficiency perovskite solar cells positions them as the most promising next-generation photovoltaic devices. However, the commercialization of PSCs continues to encounter various challenges, encompassing low efficiency in large-area cells, long-term stability concerns, the toxicity of heavy metal lead, and production cost considerations [12,13,14,15][12][13][14][15]. These factors collectively underscore critical research areas in the field of PSCs.
The production cost is a crucial factor to consider in the commercialization of perovskite solar cells, making the effective reduction in production costs a significant focus of research in the field. The production cost of PSCs is closely tied to the PSCs fabrication process. Currently, there are several production methods for PSCs, including solution process, thermal evaporation, and vapor-assisted process [16,17,18,19,20][16][17][18][19][20]. Among these, thermal evaporation requires a high-vacuum environment, imposing stringent equipment requirements. The vapor-assisted process typically demands extended reaction times to obtain perovskite films. In comparison to the aforementioned production processes, the solution process exhibits lower equipment requirements and a simpler preparation procedure, making it the most widely used method for PSCs production [14,18,20,21,22][14][18][20][21][22]. The solution process offers the flexibility to produce PSCs with various specifications, utilizing techniques such as spin-coating, blade-coating, spray-coating, roll-to-roll, and printing [14,23,24,25,26][14][23][24][25][26]. Blade-coating, spray-coating, roll-to-roll, and printing are particularly suitable for large-scale perovskite solar cell production. These methods have shown great potential for cost-effective manufacturing of large-area PSCs.
Although the current solution-based production process provides significant cost advantages, there is still room for further cost reduction. Enhancing the solution-based perovskite solar cell production process by streamlining manufacturing steps could greatly advance the production and application of low-cost perovskite solar cells. As shown in Figure 1, the conventional solution-based fabrication process can typically be broken down into three key steps: (1) preparation of precursor solutions, (2) deposition of films onto substrates, and (3) thermal annealing to facilitate crystal transformation and attain high-quality perovskite films. Within these steps, thermal annealing plays a pivotal role in achieving high-efficiency PSCs production. Nevertheless, the thermal annealing process introduces specific challenges in the development of PSCs. Depending on the specific perovskite compositions and solvent used, the annealing temperature, duration, and environmental conditions for PSCs should vary considerably. For example, the typical annealing temperature for PSCs currently falls within the range of 80–150 °C, with annealing times typically spanning from 10 to 60 min. In the case of high-efficiency two-step PSCs based on the FAI, film deposition occurs in an inert environment, followed by annealing in conditions with approximately 30% humidity, which adds complexity to the production process. These variations in annealing temperature, duration, and environmental conditions not only complicate the fabrication process but also result in increased energy consumption, extended production times, and elevated production costs.
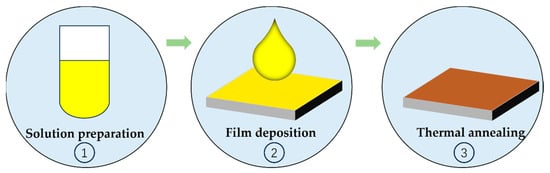
Figure 1.
The schematic of the perovskite film fabrication process.
To mitigate the adverse impacts of thermal annealing and further streamline the production of PSCs, researchers have successfully devised room-temperature PSCs (RT-PSCs, fabricating perovskite films at room temperature) production techniques [27,28,29,30,31,32,33][27][28][29][30][31][32][33]. These methods aim to simplify the manufacture of solution-based PSCs, resulting in reduced energy consumption, shorter production times, and decreased production costs. RT-PSCs offer the dual advantage of simplifying the manufacturing process and mitigating the detrimental effects of thermal annealing on perovskite film quality.
2. Room-Temperature PSCs
The quality of perovskite films plays a crucial role in determining the efficiency of solar cells. Achieving high-quality perovskite films without thermal annealing is a crucial factor in the development of RT-PSCs. Currently, researchers primarily employ techniques such as vacuum deposition, ultrasonic vibration, vacuum assistance, and solvent engineering to fabricate high-quality room-temperature perovskite films [27,30,31,34,35][27][30][31][34][35]. While methods such as vacuum deposition, ultrasonic vibration, and vacuum assistance eliminate the requirement for thermal annealing, they often rely on additional auxiliary equipment or processes to promote the crystallization of perovskite films. In contrast, solvent engineering-based RT-PSCs typically do not necessitate supplementary equipment during the fabrication process, making them more cost-effective and technologically straightforward. Consequently, they are better suited for the development of low-cost PSCs. Table 1 presents the most recent research findings related to RT-PSCs using the partial solution method.Table 1.
Summary of RT-PSCs based on solution process.
| Device Structure | Solvent | Room-Temperature Process | Performance | References |
|---|---|---|---|---|
| ITO/PEDOT:PSS/MAPbI3/PCBM/Ca/Al | DMF | Doping | 9.32% | [33] |
| ITO/TiO2/MAPbI3/Spiro-OMeTAD/Ag | DMF | Vapor-assisted | 15.6% | [36] |
| FTO/TiO2/MAPbI3/Spiro-OMeTAD/Ag | NMP | Antisolvent immersing | 15.2%. | [37] |
| FTO/c-TiO2/m-TiO2/FA0.5MA0.5PbI3/Spiro-OMeTAD/Ag | DMF | Doping | 17.9% | [32] |
| ITO/PEDOT:PSS/MAPbI3/PCBM/PDPIO/Al | DMF | Vapor-assisted | 16.4% | [38] |
| FTO/c-TiO2/m-TiO2/MAPbI3/Spiro-OMeTAD/Au | DMAc/NMP | Antisolvent rinsing | 17.09% | [39] |
| FTO/c-TiO2/m-TiO2/FA0.8MA0.1Cs0.1PbI3/Spiro-OMeTAD/Ag | DMF/DMSO | Ligand exchange | 18.1% | [40] |
| ITO/NiOx/MAPbI3/C60/Bis-C60/Ag | DMF | Ligand exchange | 17.1% | [41] |
| FTO/c-TiO2/m-TiO2/MAPbI3/Spiro-OMeTAD/MnO3/Ag | TME | Novel solvent | 20.02% | [42] |
| ITO/SnO2/(FAPbI3)1(MAPbBr3)0.05/BABr-PEAI/Spiro-OMeTAD/Ag | DMF/DMSO | Doping | 19.09% | [43] |
| FTO/c-TiOx/m-TiOx/MAPb(I1 − xClx)3/Spiro-OMeTAD/Au | MA/EA/ACN | Novel solvent | 22.32% | [44] |
| ITO/NiOx/MAPb(I1 − xClx)3/PCBM/BCP/Ag | MA/EA/ACN | Novel solvent | 23.07% | [44] |
2.1. Antisolvent Engineering for RT-PSCs
The typical polar aprotic solvents used for processing perovskites, such as N,N-dimethylformamide (DMF), dimethyl sulfoxide (DMSO), and N-methylpyrrolidone (NMP), can establish strong coordination bonds with Pb atoms. This results in the formation of a stable intermediate phase structure within the perovskite solution [25,45][25][45]. Typically, the removal of residual solvents from the film through thermal annealing is required to facilitate the transformation of the crystal structure. However, room-temperature perovskite films often utilize non-thermal processing techniques to facilitate the transition of the intermediate phase and achieve high-quality perovskite films. Antisolvent engineering is a widely employed approach for enhancing the efficiency of PSCs [46]. Moreover, it proves to be an effective technique for the fabrication of RT-PSCs. During the thin film preparation process, the attainment of high-quality room-temperature perovskite films is facilitated by the rinsing or immersion in an antisolvent, as illustrated in Figure 2. The volatile nature of the antisolvent aids in eliminating residual solvents like DMF, DMSO, and NMP from the film, thereby promoting the transformation of the intermediate phase into a perovskite crystal [47,48][47][48]. Since different solvents and antisolvents have distinct properties, the quality of antisolvent room-temperature perovskite films is closely associated with the selection of the perovskite solution and antisolvent [48].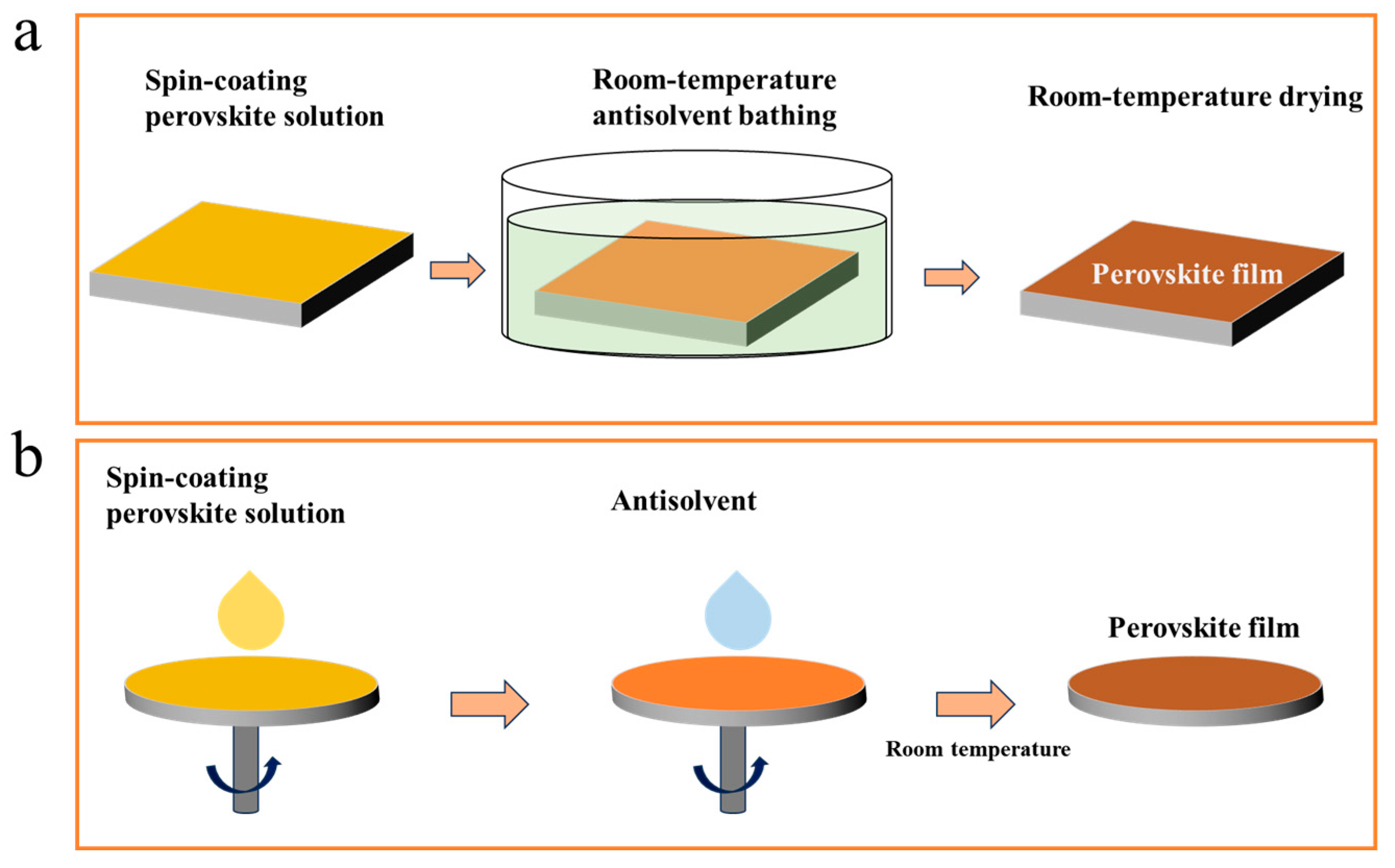
Figure 2.
Schematic of antisolvent immersion (
a
) and rinsing (
b
) for RT-PSC fabrication.
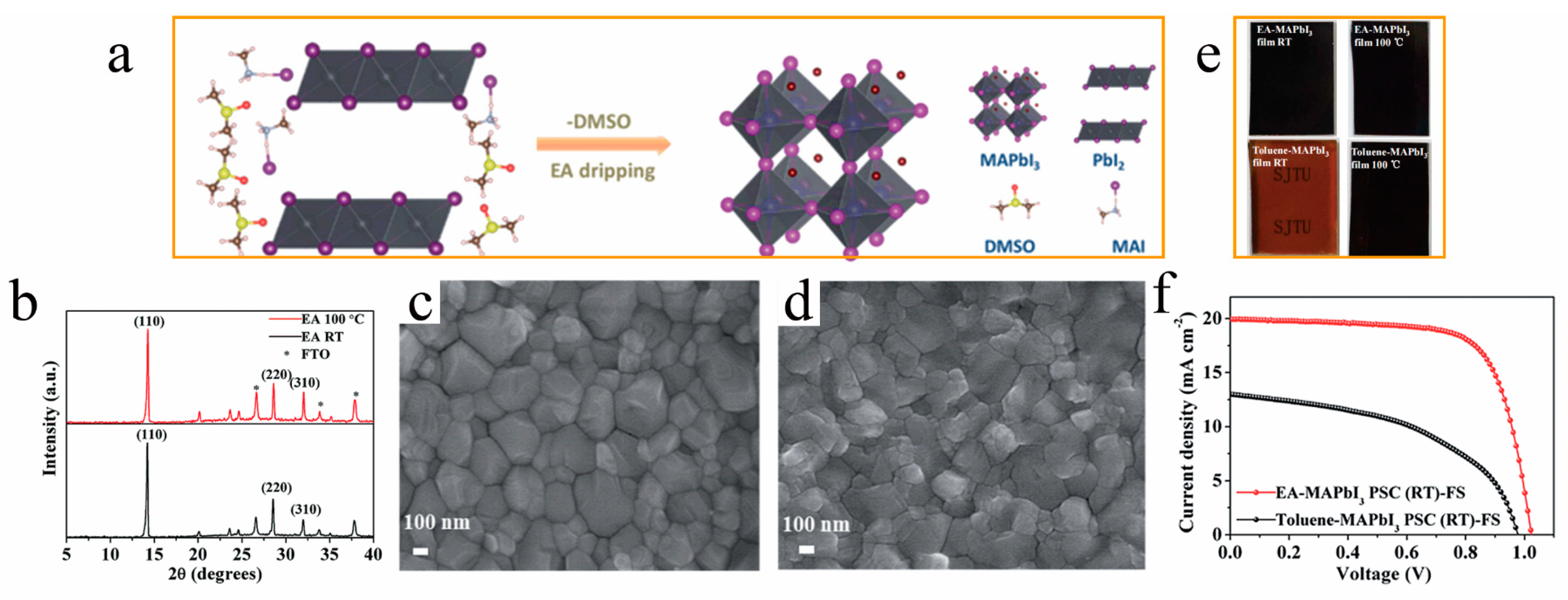
Figure 3. (a) Schematic diagram of the instant-crystallization mechanism for room-temperature films and (b) X-ray diffraction patterns of perovskite films prepared by dripping ethyl acetate (EA) with 100 °C annealing and without annealing (RT); scanning electron micrographs (SEM) of (c) thermally annealed (d) and room-temperature perovskite films preparing with EA, (e) MAPbI3 perovskite films prepared with toluene and EA at room temperature and annealing at 100 °C for 10 min, and (f) J−V curves of RT-PSCs prepared with EA and toluene, without annealing [49].
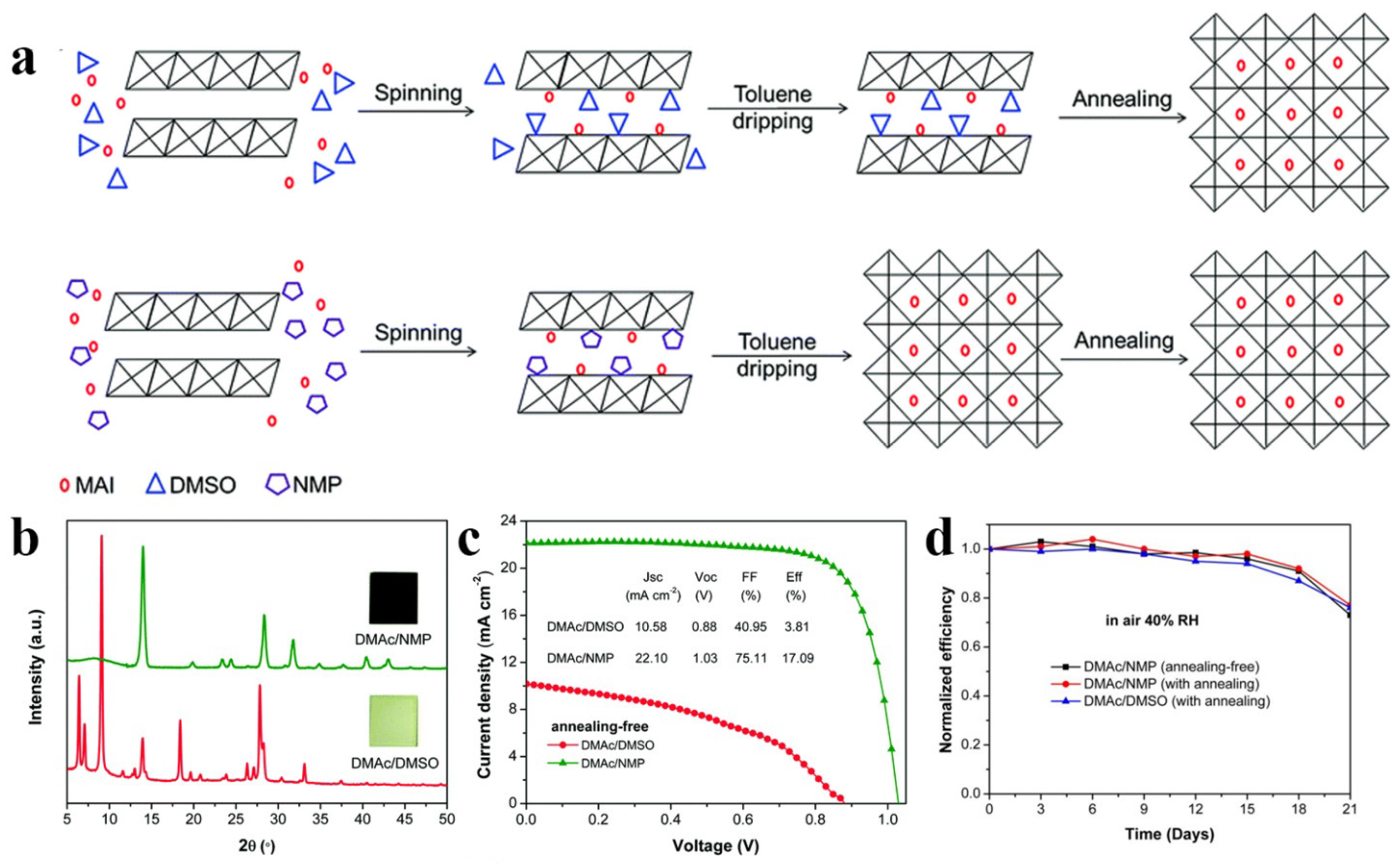
Figure 4. (a) The schematic illustrations of perovskite formed from DMAc/DMSO and DMAc/NMP cosolvents, respectively. (b) XRD patterns of RT perovskite films made from DMAc/DMSO and DMAc/NMP, and the insets show the corresponding photographs. (c) J−V curves of RT-PSCs. (d) Long-term stability of RT-PSCs and annealing PSCs.
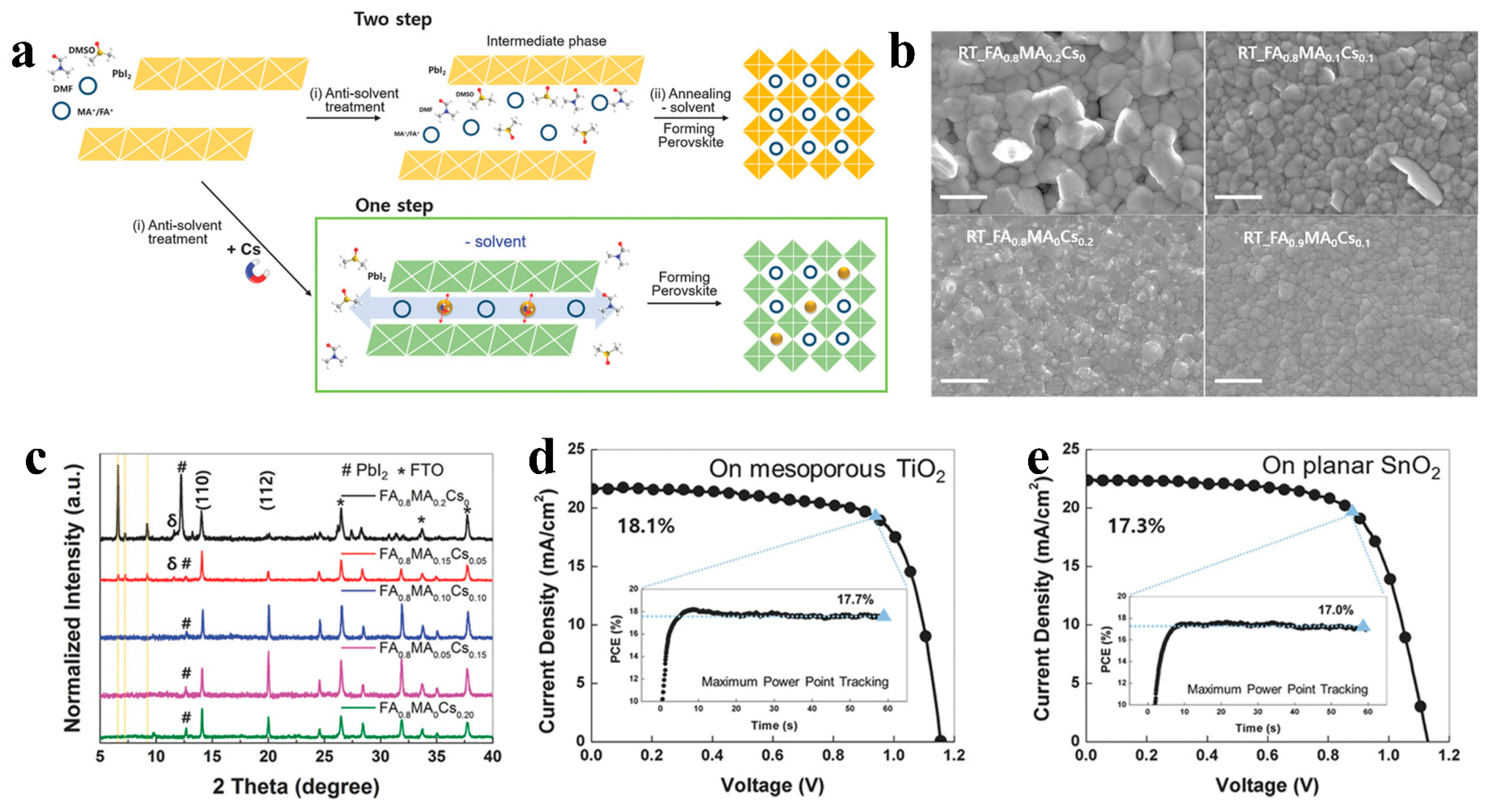
Figure 5. (a) Illustration of procedures with Cs and without Cs for crystalline perovskite film formation by antisolvent processing. (b) SEM images of FA0.8MA0.2 − xCsx films for various Cs contents and FA0.9MA0Cs0.1 at room temperature. (c) XRD analysis for FA0.8MA0.2 − xCsx films of varying Cs content x without annealing (δ represents the intermediate phase). J−V curve and maximum power point tracking of the champion FA0.8MA0.1Cs0.1 device without annealing on (d) mesoporous TiO2 and (e) planar SnO2.
2.2. Vapor-Assisted for RT-PSCs
Researchers have found that exposing unannealed films to a specific humidity level can result in the formation of a more active metastable phase, MAPbI3·H2O. This transitional intermediate phase readily transforms into high-quality perovskite crystals as moisture evaporates [36]. Therefore, subjecting the film to an appropriate humidity environment can leverage water vapor to aid in film crystallization and facilitate crystal structure transformation, ultimately leading to high-quality room-temperature perovskite films. Dubey et al. [52] observed that when films are exposed to the atmosphere, DMSO from MAI-PbI2-DMSO dissolves into the ambient moisture and escapes with water evaporation, prompting the conversion of the intermediate phase into perovskite crystals. After 5 h of exposure to air, they obtained nanorod-shaped perovskite films, achieving a room-temperature cell efficiency of 16.83%. Wang et al. [38] report a water-vapor annealing (WVA) method at room temperature for the formation of high-crystallinity and void-free MAPbI3 perovskite films (as illustrated in Figure 6a). Figure 6b illustrates the mechanisms of crystal growth processes in a perovskite film: (1) Water molecules are absorbed on the perovskite surface, mediating strong hydrogen bonding between methylammonium cations and the Pb-I cage. (2) Hydrolysis of perovskite into PbI2 and MAI by water. (3) Restraining MAI from reacting with nearby PbI2 to reform perovskite after water evaporates. They conducted a comprehensive study on the influence of environmental humidity and exposure duration on the quality of room-temperature perovskite films and determined that exposing the film for 60 min under relative humidity conditions ranging from 36% to 43% resulted in the highest crystalline quality, as depicted in Figure 6c–h. Ultimately, they achieved room-temperature perovskite solar cells with a higher conversion efficiency (16.39%) compared to thermally annealed ones (12.88%), as shown in Figure 6i.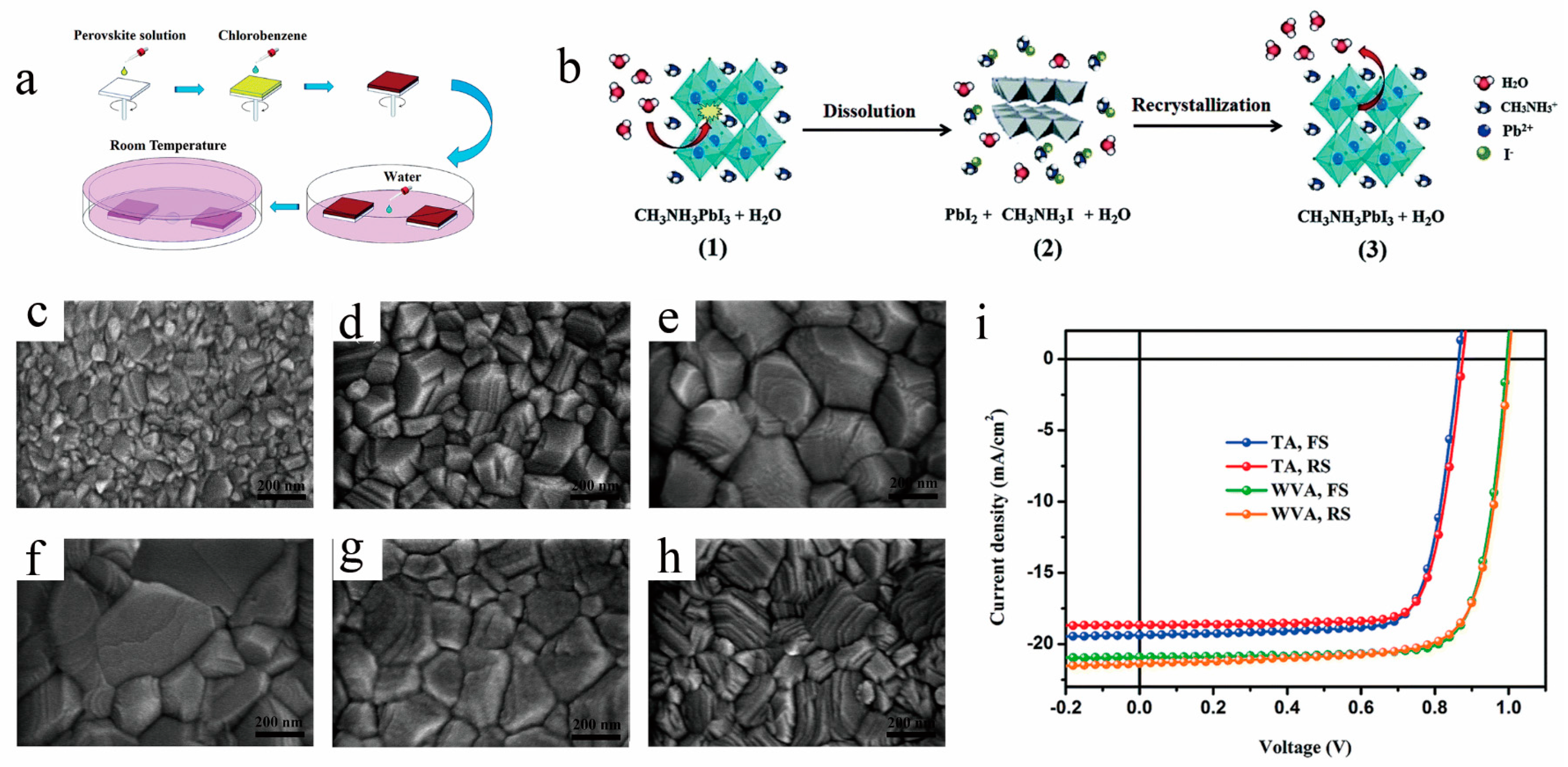
Figure 6. Schematic diagram of (a) the preparation procedure of perovskite films with room-temperature water vapor annealing and (b) the crystal growth processes in a perovskite film. SEM images of the perovskite films treated by water-vapor annealing in the (c) RH% < 10%, (d) RH% = 33–36%, (e) RH% = 33–42%,(f) RH% = 36–43%, (g) RH% = 36–51%, and (h) thermal annealing in air (RH% < 10%); (i) J−V curves for thermal annealing (TA) and WVA PSCs [38].
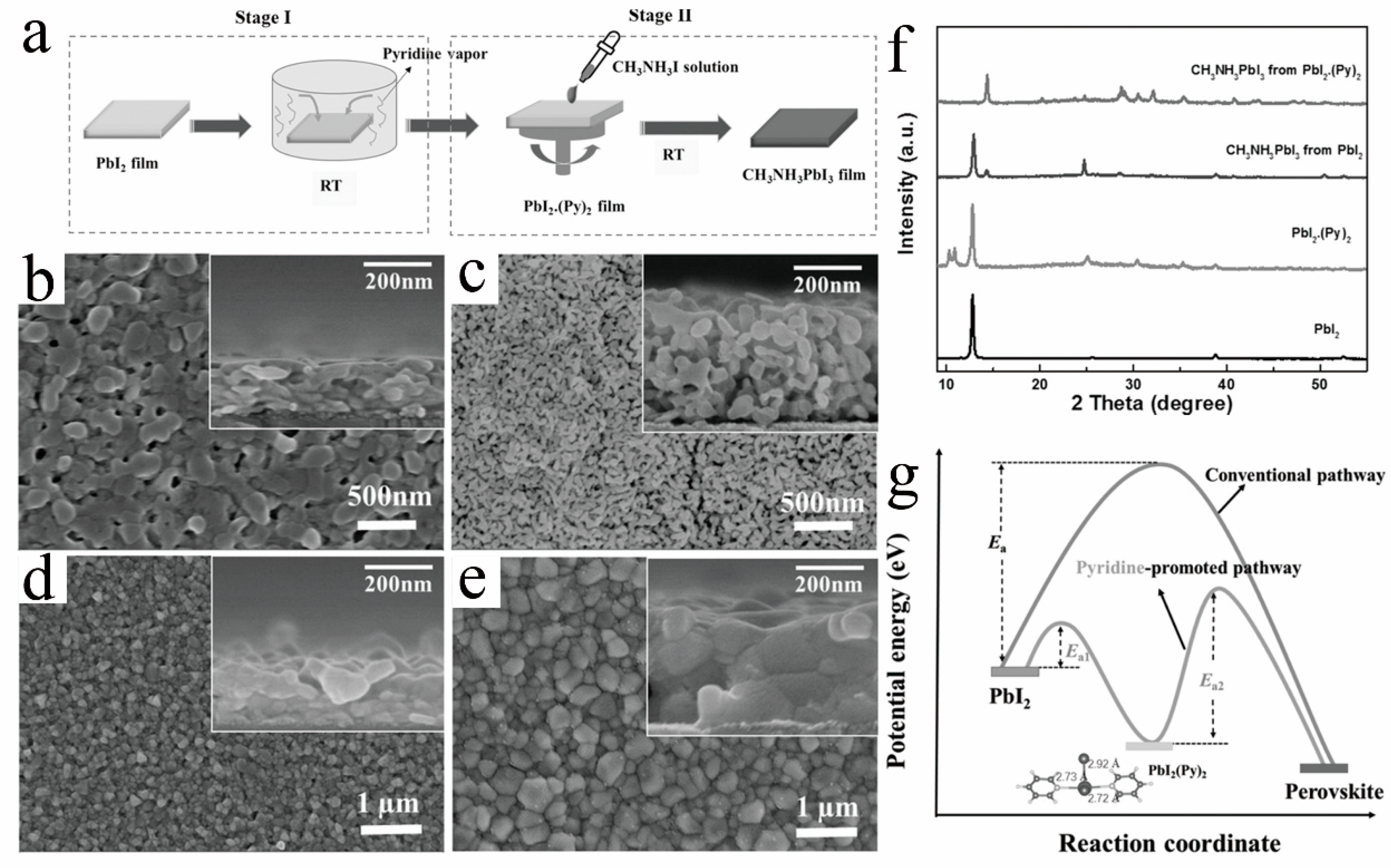
Figure 7. (a) Schematics of the pyridine-promoted formation of MAPbI3 film. Top-view SEM images of (b) pristine PbI2 and (c) PbI2.(Py)2; MAPbI3 films from (d) PbI2 and (e) PbI2.(Py)2 films. (f) XRD of PbI2 film, PbI2.(Py)2 film, and relevant perovskite films. (g) The reaction coordinate diagram of the perovskite formation via the conventional pathway and pyridine-promoted pathway [41].
2.3. Novel Solvent for RT-PSCs
Because traditional solvents like DMF, DMSO, and NMP have high boiling points and form stable intermediate phase structures in the film, it is typically required to employ additional processes to evaporate the remaining solvents in the film and promote the transformation of the crystal structure. This constraint hinders the advancement of RT-PSCs utilizing traditional solvents. To tackle this challenge, researchers have developed novel solvents, such as acetonitrile (ACN), 1,2-dimethoxyethane (DME), tetrahydrofuran (THF), mixtures of methylamine in ethanol (ME) in combination with a secondary solvent (ACN, or THF), 2-methoxyethanol (2-ME), and gamma-valerolactone (GVL), which are better suited for the fabrication of RT-PSCs [28,56,57,58,59,60,61,62][28][56][57][58][59][60][61][62]. These solvents possess properties such as low boiling points, volatility, and the absence of stable intermediate phases. They can rapidly evaporate from the thin film at room temperature, facilitating the formation of perovskite thin films with complete crystal structure transformation. When working with novel solvents, it is common practice to blend multiple solvents. This enhances the processability of the perovskite solution and improves the quality of perovskite films by either increasing solubility or preventing phase separation within the solution. As illustrated in Figure 8a–c, Liu et al. [42] introduced an innovative solvent for RT-PSCs, which is a mixture of ME and THF, abbreviated as TME. TME evaporates rapidly during the preparation process, allowing the MAPbI3 solution to promptly attain supersaturation and generate a multitude of nuclei, resulting in a dense and high-quality film. Furthermore, the TME solvent does not manifest intermediate phase structures, enabling the film to completely transition into a perovskite crystal structure at room temperature. Capitalizing on this, they achieved an impressive 20% conversion efficiency for RT-PSCs without any additional processing (Figure 8d). Additionally, they also realized a remarkable efficiency of 15.6% for large-area (10 cm2) RT-PSCs based on the TME solvent (Figure 8e). Wang et al. dissolved MAPb(I1 − xClx)3 single crystals in a solution containing ME and ACN to create perovskite ink. Utilizing this ink, they achieved isothermal crystallization at room temperature, resulting in the formation of compact and high-quality perovskite films (as depicted in Figure 9a–c). Based on this new type of perovskite ink, the efficiency of n-i-p and p-i-n RT-PSCs reached 22.3% and 23.1%, respectively (as demonstrated in Figure 9d,e), surpassing that of traditional thermal annealing PSCs [44].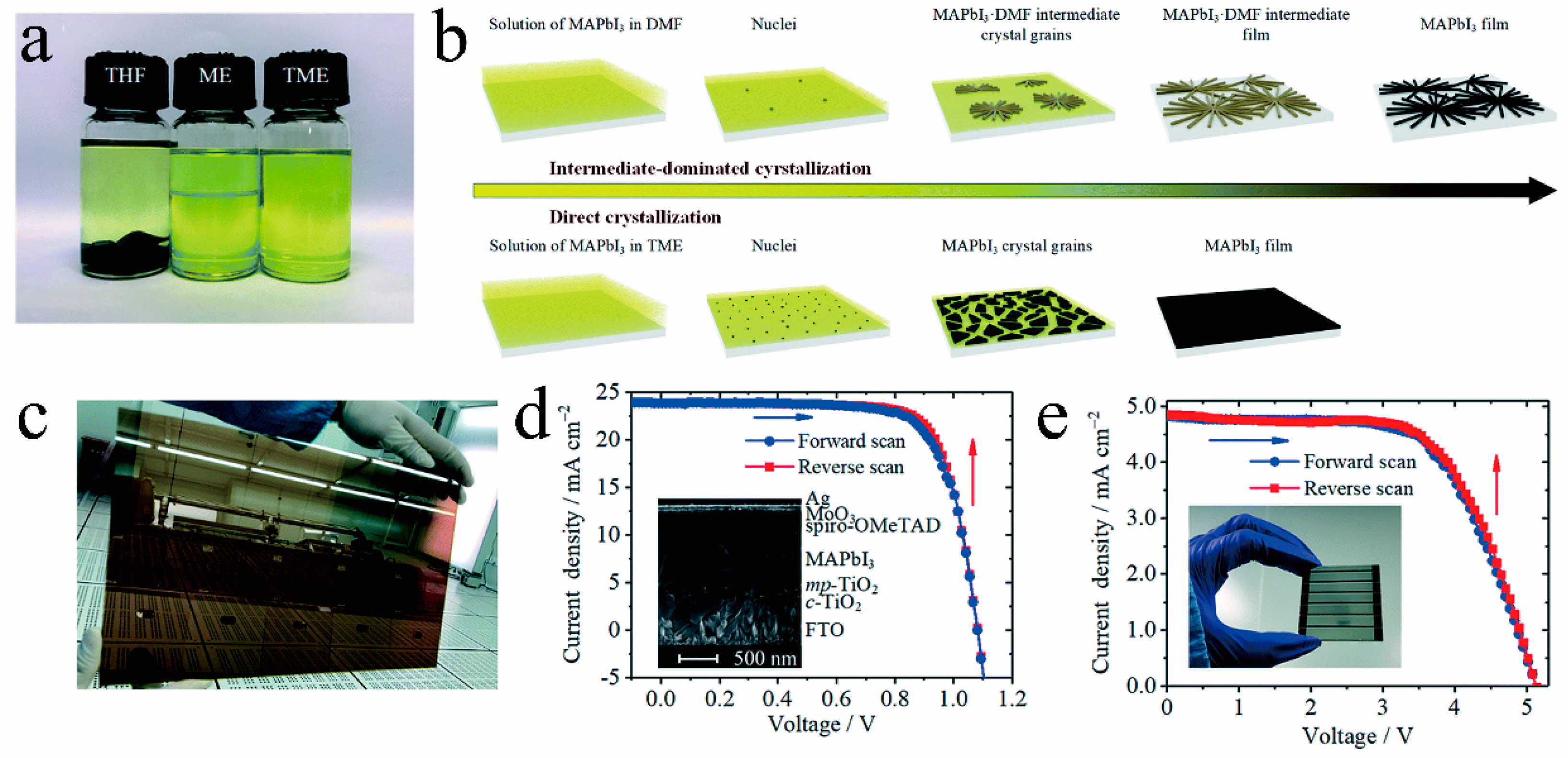
Figure 8. (a) Photos of MAPbI3 crystals dissolved in THF, ME, and TME (THF:ME = 0.9:1), with the expected concentration of 2.1 M. (b) Comparison of intermediate-dominated crystallization in DMF and direct crystallization in TME. (c) Large-area MAPbI3 film (190 mm × 320 mm) on FTO prepared by blade-coating a 0.6 M solution of MAPbI3 in TME at room temperature. J−V curves of the (d) 0.1 cm2 device and (e) 10 cm2 series module [42].
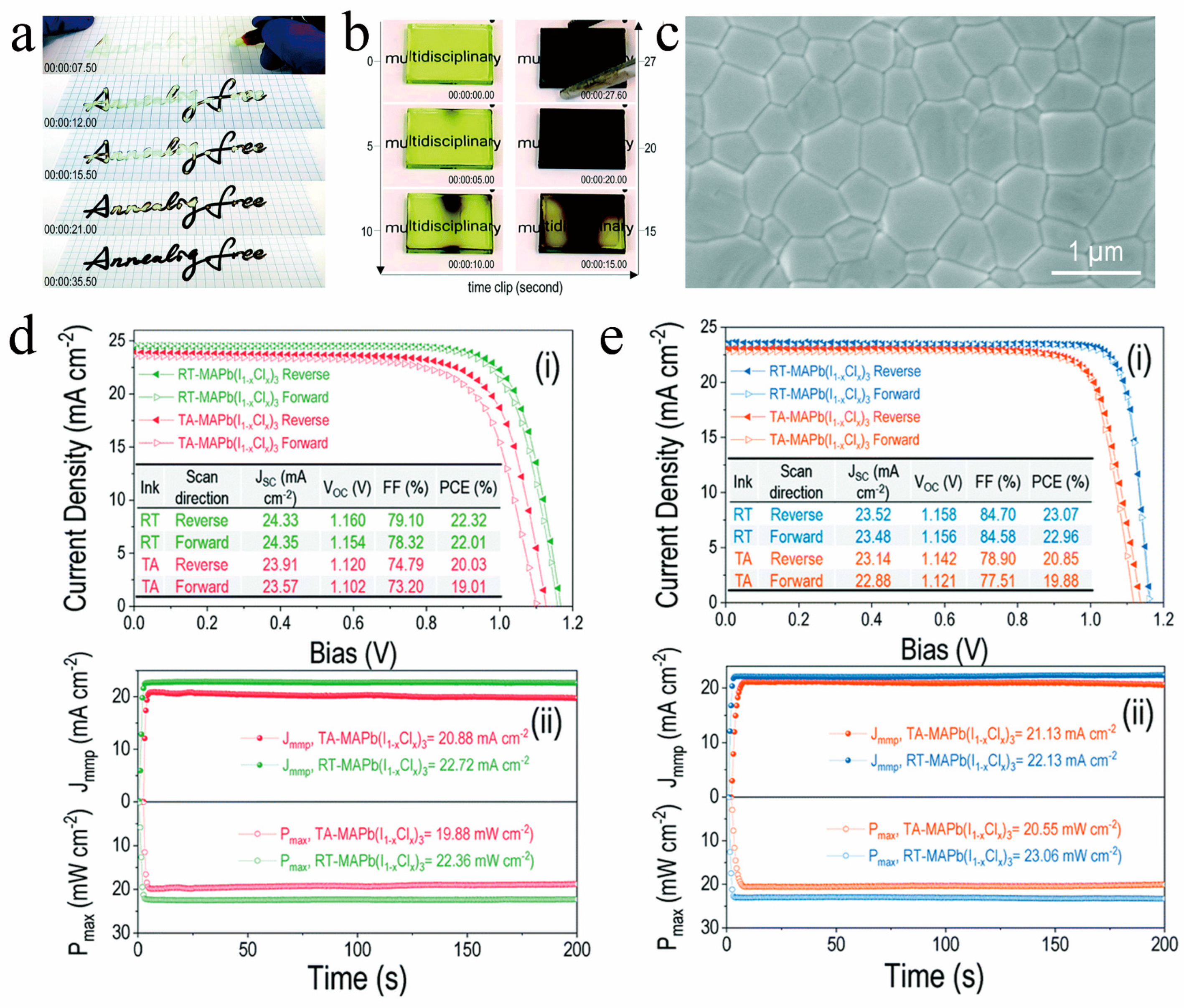
Figure 9. Snapshots showing the rapid RT isothermal crystallization (a) during handwriting on paper and (b) in a blade-coated film. (c) Microscopic features of the RT isothermally crystallized film in a top-view. (i) J−V characteristics and (ii) stabilized power output for RT perovskite- and TA perovskite-based (d) n-i-p and (e) p-i-n device. [44].
References
- McMeekin, D.P.; Sadoughi, G.; Rehman, W.; Eperon, G.E.; Saliba, M.; Hoerantner, M.T.; Haghighirad, A.; Sakai, N.; Korte, L.; Rech, B.; et al. A mixed-cation lead mixed-halide perovskite absorber for tandem solar cells. Science 2016, 351, 151–155.
- Green, M.A.; Ho-Baillie, A.; Snaith, H.J. The emergence of perovskite solar cells. Nat. Photonics 2014, 8, 506–514.
- Dong, Q.; Fang, Y.; Shao, Y.; Mulligan, P.; Qiu, J.; Cao, L.; Huang, J. Electron-hole diffusion lengths > 175 μm in solution-grown CH3NH3PbI3 single crystals. Science 2015, 347, 967–970.
- Stranks, S.D.; Eperon, G.E.; Grancini, G.; Menelaou, C.; Alcocer, M.J.P.; Leijtens, T.; Herz, L.M.; Petrozza, A.; Snaith, H.J. Electron-hole diffusion lengths exceeding 1 micrometer in an organometal trihalide perovskite absorber. Science 2013, 342, 341–344.
- Lin, Q.; Armin, A.; Nagiri, R.C.R.; Burn, P.L.; Meredith, P. Electro-optics of perovskite solar cells. Nat. Photonics 2014, 9, 106–112.
- D’Innocenzo, V.; Grancini, G.; Alcocer, M.J.; Kandada, A.R.; Stranks, S.D.; Lee, M.M.; Lanzani, G.; Snaith, H.J.; Petrozza, A. Excitons versus free charges in organo-lead tri-halide perovskites. Nat. Commun. 2014, 5, 3586.
- Yang, L.; Zhou, H.; Duan, Y.; Wu, M.; He, K.; Li, Y.; Xu, D.; Zou, H.; Yang, S.; Fang, Z.; et al. 25.24%-Efficiency FACsPbI3 perovskite solar cells enabled by intermolecular esterification reaction of DL-carnitine hydrochloride. Adv. Mater. 2023, 35, e2211545.
- Park, J.; Kim, J.; Yun, H.S.; Paik, M.J.; Noh, E.; Mun, H.J.; Kim, M.G.; Shin, T.J.; Seok, S.I. Controlled growth of perovskite layers with volatile alkylammonium chlorides. Nature 2023, 616, 724–730.
- Kim, H.S.; Lee, C.R.; Im, J.H.; Lee, K.B.; Moehl, T.; Marchioro, A.; Moon, S.J.; Humphry-Baker, R.; Yum, J.H.; Moser, J.E.; et al. Lead iodide perovskite sensitized all-solid-state submicron thin film mesoscopic solar cell with efficiency exceeding 9%. Sci. Rep. 2012, 2, 591.
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 2009, 131, 6050–6051.
- NREL Module Efficiency Chart. Available online: https://www.nrel.gov/pv/module-efficiency.html (accessed on 10 September 2023).
- Feng, S.-P.; Cheng, Y.; Yip, H.-L.; Zhong, Y.; Fong, P.W.K.; Li, G.; Ng, A.; Chen, C.; Castriotta, L.A.; Matteocci, F.; et al. Roadmap on commercialization of metal halide perovskite photovoltaics. J. Phys. Mater. 2023, 6, 032501.
- Rong, Y.; Hu, Y.; Mei, A.; Tan, H.; Saidaminov, M.I.; Seok, S.I.; McGehee, M.D.; Sargent, E.H.; Han, H. Challenges for commercializing perovskite solar cells. Science 2018, 361, eaat8235.
- Saki, Z.; Byranvand, M.M.; Taghavinia, N.; Kedia, M.; Saliba, M. Solution-processed perovskite thin-films: The journey from lab- to large-scale solar cells. Energy Environ. Sci. 2021, 14, 5690–5722.
- Li, Z.; Klein, T.R.; Kim, D.H.; Yang, M.; Berry, J.J.; van Hest, M.F.A.M.; Zhu, K. Scalable fabrication of perovskite solar cells. Nat. Rev. Mater. 2018, 3, 18017.
- Cui, J.; Yuan, H.; Li, J.; Xu, X.; Shen, Y.; Lin, H.; Wang, M. Recent progress in efficient hybrid lead halide perovskite solar cells. Sci. Technol. Adv. Mater. 2016, 16, 036004.
- Zhou, H.; Chen, Q.; Yang, Y. Vapor-assisted solution process for perovskite materials and solar cells. MRS Bull. 2015, 40, 667–673.
- Burschka, J.; Pellet, N.; Moon, S.J.; Humphry-Baker, R.; Gao, P.; Nazeeruddin, M.K.; Grätzel, M. Sequential deposition as a route to high-performance perovskite-sensitized solar cells. Nature 2013, 499, 316–319.
- Liu, M.; Johnston, M.B.; Snaith, H.J. Efficient planar heterojunction perovskite solar cells by vapour deposition. Nature 2013, 501, 395–398.
- Jeon, N.J.; Noh, J.H.; Kim, Y.C.; Yang, W.S.; Ryu, S.; Seok, S.I. Solvent engineering for high-performance inorganic-organic hybrid perovskite solar cells. Nat. Mater. 2014, 13, 897–903.
- Jiang, Q.; Zhao, Y.; Zhang, X.; Yang, X.; Chen, Y.; Chu, Z.; Ye, Q.; Li, X.; Yin, Z.; You, J. Surface passivation of perovskite film for efficient solar cells. Nat. Photonics 2019, 13, 460–466.
- Dunlap-Shohl, W.A.; Zhou, Y.; Padture, N.P.; Mitzi, D.B. Synthetic approaches for halide perovskite thin films. Chem. Rev. 2019, 119, 3193–3295.
- Kwon, N.; Lee, J.; Ko, M.J.; Kim, Y.Y.; Seo, J. Recent progress of eco-friendly manufacturing process of efficient perovskite solar cells. Nano Converg. 2023, 10, 28.
- Shi, P.; Ding, Y.; Ding, B.; Xing, Q.; Kodalle, T.; Sutter-Fella, C.M.; Yavuz, I.; Yao, C.; Fan, W.; Xu, J.; et al. Oriented nucleation in formamidinium perovskite for photovoltaics. Nature 2023, 620, 323–327.
- Jung, M.; Ji, S.G.; Kim, G.; Seok, S.I. Perovskite precursor solution chemistry: From fundamentals to photovoltaic applications. Chem. Soc. Rev. 2019, 48, 2011–2038.
- Abate, A.; Correa-Baena, J.-P.; Saliba, M.; Su’ait, M.S.; Bella, F. Perovskite solar cells: From the laboratory to the assembly line. Chem. Eur. J. 2018, 24, 3083–3100.
- Fang, Y.; Tian, T.; Yang, M.; Tan, Y.; Zhong, J.-X.; Huang, Y.; Wang, X.; Tao, J.; Yang, S.; Zou, C.; et al. Tailoring precursor chemistry enabled room temperature-processed perovskite films in ambient air for efficient and stable solar cells with improved reproducibility. Adv. Funct. Mater. 2023, 33, 2303674.
- Peng, B.; Kong, W.; Wang, T.; Qiao, L.; Zhang, L.; Yang, X. Room temperature crystallization and stability of halide perovskite thin films. Sci. Sin.-Phys. Mech. As. 2022, 52, 296811.
- Yang, Z.; Pan, J.; Liang, Y.; Li, Q.; Xu, D. Ambient air condition for room-temperature deposition of MAPbI3 films in highly efficient solar cells. Small 2018, 14, e1802240.
- Wei, X.; Zhang, M.; Liu, X.; Chen, F.; Lei, X.; Liu, H.; Meng, F.; Zeng, H.; Yang, S.; Liu, J. Semitransparent CH3NH3PbI3 films achieved by solvent engineering for annealing- and electron transport layer-free planar perovskite solar cells. Sol. RRL 2018, 2, 1700222.
- Liu, D.; Yang, C.; Bates, M.; Lunt, R.R. Room temperature processing of inorganic perovskite films to enable flexible solar cells. iScience 2018, 6, 272–279.
- Pan, J.; Mu, C.; Li, Q.; Li, W.; Ma, D.; Xu, D. Room-temperature, hydrochloride-assisted, one-step deposition for highly efficient and air-stable perovskite solar cells. Adv. Mater. 2016, 28, 8309–8314.
- Chen, Y.; Zhao, Y.; Liang, Z. Non-thermal annealing fabrication of efficient planar perovskite solar cells with inclusion of NH4Cl. Chem. Mater. 2015, 27, 1448–1451.
- Xiong, H.; Zabihi, F.; Wang, H.; Zhang, Q.; Eslamian, M. Grain engineering by ultrasonic substrate vibration post-treatment of wet perovskite films for annealing-free, high performance, and stable perovskite solar cells. Nanoscale 2018, 10, 8526–8535.
- Ahmadian-Yazdi, M.-R.; Eslamian, M. Toward scale-up of perovskite solar cells: Annealing-free perovskite layer by low-cost ultrasonic substrate vibration of wet films. Mater. Today Commun. 2018, 14, 151–159.
- Yang, B.; Dyck, O.; Poplawsky, J.; Keum, J.; Das, S.; Puretzky, A.; Aytug, T.; Joshi, P.C.; Rouleau, C.M.; Duscher, G.; et al. Controllable growth of perovskite films by room-temperature air exposure for efficient planar heterojunction photovoltaic cells. Angew. Chem. Int. Ed. 2015, 54, 14862–14865.
- Zhou, Y.; Yang, M.; Wu, W.; Vasiliev, A.L.; Zhu, K.; Padture, N.P. Room-temperature crystallization of hybrid-perovskite thin films via solvent–solvent extraction for high-performance solar cells. J. Mater. Chem. A 2015, 3, 8178–8184.
- Wang, B.B.; Zhang, Z.G.; Ye, S.Y.; Rao, H.X.; Bian, Z.Q.; Huang, C.H.; Li, Y.F. Room-temperature water-vapor annealing for high-performance planar perovskite solar cells. J. Mater. Chem. A 2016, 4, 17267–17273.
- Fang, X.; Wu, Y.; Lu, Y.; Sun, Y.; Zhang, S.; Zhang, J.; Zhang, W.; Yuan, N.; Ding, J. Annealing-free perovskite films based on solvent engineering for efficient solar cells. J. Mater. Chem. C 2017, 5, 842–847.
- Matsui, T.; Seo, J.-Y.; Saliba, M.; Zakeeruddin, S.M.; Grätzel, M. Room-temperature formation of highly crystalline multication perovskites for efficient, low-cost solar cells. Adv. Mater. 2017, 29, 1606258.
- Zhang, H.; Cheng, J.; Li, D.; Lin, F.; Mao, J.; Liang, C.; Jen, A.K.; Grätzel, M.; Choy, W.C. Toward all room-temperature, solution-processed, high-performance planar perovskite solar cells: A new scheme of pyridine-promoted perovskite formation. Adv. Mater. 2017, 29, 1604695.
- Liu, Q.; Zhao, Y.; Ma, Y.; Sun, X.; Ge, W.; Fang, Z.; Bai, H.; Tian, Q.; Fan, B.; Zhang, T. A mixed solvent for rapid fabrication of large-area methylammonium lead iodide layers by one-step coating at room temperature. J. Mater. Chem. A 2019, 7, 18275–18284.
- Chen, Z.; Zhang, H.; Yao, F.; Tao, C.; Fang, G.; Li, G. Room temperature formation of semiconductor grade alpha-FAPbI3 films for efficient perovskite solar cells. Cell Rep. Phys. Sci. 2020, 1, 100205.
- Wang, K.; Wu, C.; Hou, Y.; Yang, D.; Ye, T.; Yoon, J.; Sanghadasa, M.; Priya, S. Isothermally crystallized perovskites at room-temperature. Energy Environ. Sci. 2020, 13, 3412–3422.
- Ahn, N.; Son, D.-Y.; Jang, I.-H.; Kang, S.M.; Choi, M.; Park, N.-G. Highly Reproducible Perovskite Solar Cells with Average Efficiency of 18.3% and Best Efficiency of 19.7% Fabricated via Lewis Base Adduct of Lead(II) Iodide. J. Am. Chem. Soc. 2015, 137, 8696–8699.
- Mahon, N.S.; Korolik, O.V.; Khenkin, M.V.; Arnaoutakis, G.E.; Galagan, Y.; Soriūtė, V.; Litvinas, D.; Ščajev, P.; Katz, E.A.; Mazanik, A.V. Photoluminescence kinetics for monitoring photoinduced processes in perovskite solar cells. Sol. Energy 2020, 195, 114–120.
- Zhou, Y.; Yang, M.; Game, O.S.; Wu, W.; Kwun, J.; Strauss, M.A.; Yan, Y.; Huang, J.; Zhu, K.; Padture, N.P. Manipulating crystallization of organolead mixed-halide thin films in antisolvent baths for wide-bandgap perovskite solar cells. ACS Appl. Mater. Interfaces 2016, 8, 2232–2237.
- Zheng, X.J.; Chen, B.; Wu, C.C.; Priya, S. Room temperature fabrication of CH3NH3PbBr3 by anti-solvent assisted crystallization approach for perovskite solar cells with fast response and small J-V hysteresis. Nano Energy 2015, 17, 269–278.
- Yin, M.S.; Xie, F.X.; Chen, H.; Yang, X.D.; Ye, F.; Bi, E.B.; Wu, Y.Z.; Cai, M.T.; Han, L.Y. Annealing-free perovskite films by instant crystallization for efficient solar cells. J. Mater. Chem. A 2016, 4, 8548–8553.
- Zhu, W.; Chen, D.; Zhou, L.; Zhang, C.; Chang, J.; Lin, Z.; Zhang, J.; Hao, Y. Intermediate phase intermolecular exchange triggered defect elimination in CH3NH3PbI3 toward room-temperature fabrication of efficient perovskite solar cells. ACS Appl. Mater. Interfaces 2017, 9, 40378–40385.
- Lv, X.; Dong, X.; Ye, Z.; Zhou, J.; Deng, F.; Zheng, Y.-Z.; Tao, X. Bromide induced room-temperature formation of photoactive formamidinium-based perovskite for high-efficiency, low-cost solar cells. Sol. RRL 2019, 3, 1800313.
- Dubey, A.; Kantack, N.; Adhikari, N.; Reza, K.M.; Venkatesan, S.; Kumar, M.; Khatiwada, D.; Darling, S.; Qiao, Q.Q. Room temperature, air crystallized perovskite film for high performance solar cells. J. Mater. Chem. A 2016, 4, 10231–10240.
- Yu, H.; Liu, X.; Xia, Y.; Dong, Q.; Zhang, K.; Wang, Z.; Zhou, Y.; Song, B.; Li, Y. Room-temperature mixed-solvent-vapor annealing for high performance perovskite solar cells. J. Mater. Chem. A 2016, 4, 321–326.
- Peled, S.S.; Perez, M.; Meron, D.; Osherov, A.; Bulovic, V.; Katz, E.A.; Golan, Y. Morphology control of perovskite films: A two-step, all solution process for conversion of lead selenide into methylammonium lead iodide. Mater. Chem. Front. 2021, 5, 1410–1417.
- Zhang, H.; Li, D.; Cheng, J.; Lin, F.; Mao, J.; Jen, A.K.Y.; Grätzel, M.; Choy, W.C.H. Room temperature formation of organic-inorganic lead halide perovskites: Design of nanostructured and highly reactive intermediates. J. Mater. Chem. A 2017, 5, 3599–3608.
- Zhang, L.; Zuo, C.; Ding, L. Efficient MAPbI3 solar cells made via drop-coating at room temperature. J. Semicond. 2021, 42, 072201.
- Wang, Z.; Zhu, X.; Feng, J.; Wang, C.; Zhang, C.; Ren, X.; Priya, S.; Liu, S.; Yang, D. Antisolvent- and annealing-free deposition for highly stable efficient perovskite solar cells via modified ZnO. Adv. Sci. 2021, 8, 2002860.
- Wang, G.; Liao, L.; Chen, L.; Xu, C.; Yao, Y.; Liu, D.; Li, P.; Deng, J.; Song, Q. Perovskite solar cells fabricated under ambient air at room temperature without any post-treatment. Org. Electron. 2020, 86, 105918.
- Dou, B.; Whitaker, J.B.; Bruening, K.; Moore, D.T.; Wheeler, L.M.; Ryter, J.; Breslin, N.J.; Berry, J.J.; Garner, S.M.; Barnes, F.S.; et al. Roll-to-Roll printing of perovskite solar cells. ACS Energy Lett. 2018, 3, 2558–2565.
- Noel, N.K.; Habisreutinger, S.N.; Wenger, B.; Klug, M.T.; Hörantner, M.T.; Johnston, M.B.; Nicholas, R.J.; Moore, D.T.; Snaith, H.J. A low viscosity, low boiling point, clean solvent system for the rapid crystallisation of highly specular perovskite films. Energy Environ. Sci. 2017, 10, 145–152.
- Chalkias, D.A.; Mourtzikou, A.; Katsagounos, G.; Kalarakis, A.N.; Stathatos, E. Development of greener and stable inkjet-printable perovskite precursor inks for all-printed annealing-free perovskite solar mini-modules manufacturing. Small Methods 2023, 7, e2300664.
- Wang, J.; Yao, X.; Xiao, W.J.; Wang, S.; Xu, G.; Chen, X.Q.; Wu, S.C.; Visoly-Fisher, I.; Katz, E.A.; Li, Y.; et al. Mutual composition transformations among 2D/3D organolead halide perovskites and mechanisms behind. Sol. RRL 2018, 2, 1800125.
More
