It is likely that the ban will not be fully implemented for reasons of economic and social sustainability, but the current proposal, if not quickly withdrawn, will lead to a hasty search for substitutes that may perform worse than PFAS, be more expensive, and most likely be less characterized toxicologically.
It is difficult for material chemists to imagine possibly replacing all PFAS, within a few years, with alternative non-fluorinated compounds that have the same chemical and physical properties as the products they are intended to replace.
2. PFAS Are Not a Small Group of Chemicals with Similar Properties
A recent paper on PFAS terminology, developed within the Organization for Economic Cooperation and Development (OECD), highlights the tremendous heterogeneity in the chemical structures of different PFAS (
Figure 1) and the need to revise PFAS terminology
[9][17]. The term PFAS, which is commonly used to define the entire chemical class, is too general and likely to cause further confusion. The most studied PFAS that pose toxicological risks to humans and the environment are non-polymeric perfluoroalkyl carboxylic acids (PFCA), e.g., perfluorooctanoic acid, PFOA, and perfluorosulfonic acids (PFSA), e.g., perfluorooctane sulfonic acid and PFOS.
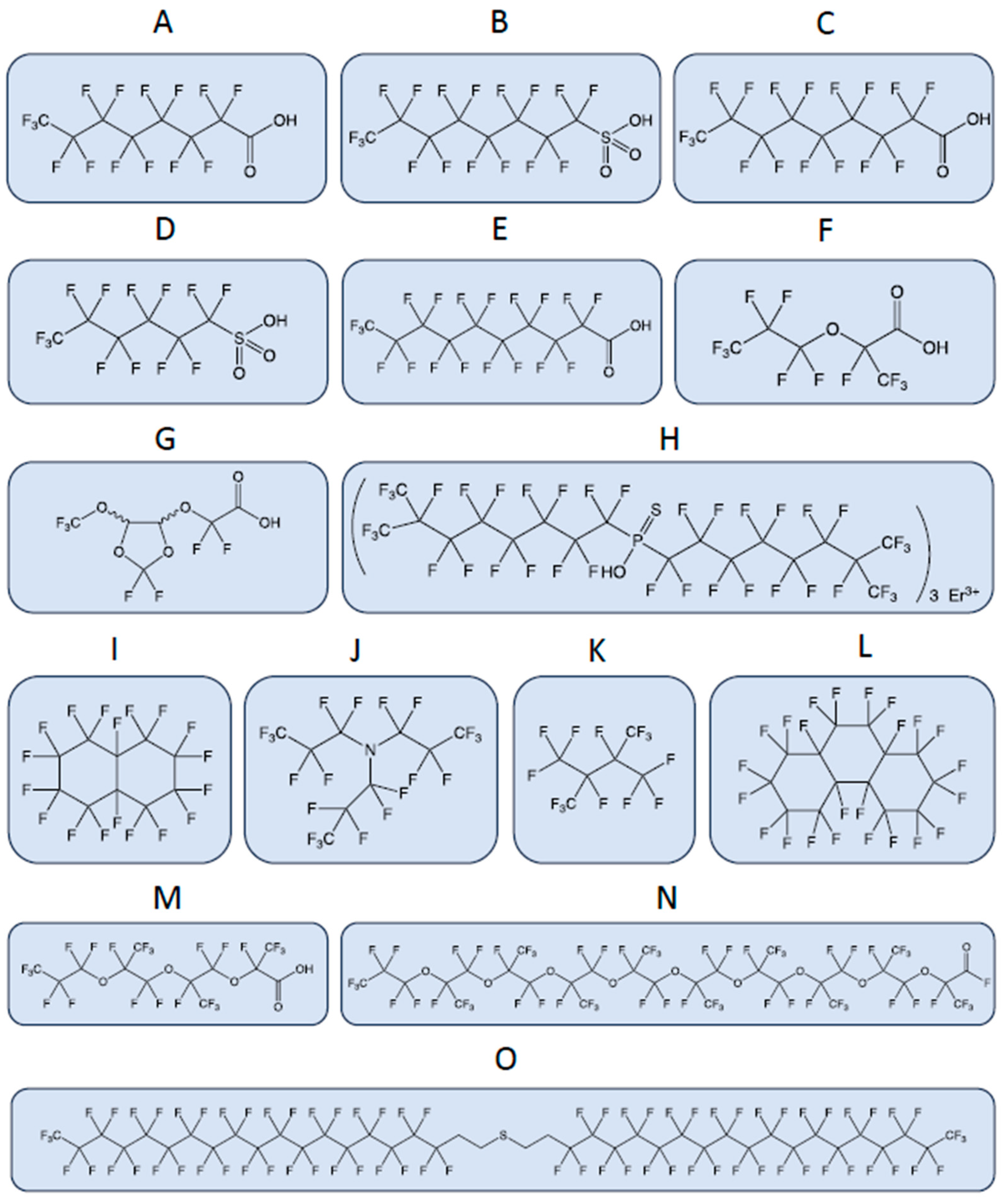
A few examples highlight the huge heterogeneity of chemical structures of PFAS, which poses challenges for their regulation. The physical and chemical properties of PFASs vary widely depending on their chain length, branching, and functional groups. These properties affect their environmental fate and transport, bioaccumulation potential, and toxicity. The bonds between the atoms are represented by lines. CAS, Chemical Abstracts Service Registry Number, is a numeric identifier assigned by the Chemical Abstracts Service (CAS) division of the American Chemical Society (ACS). (
) Perfluorooctanoic acid (PFOA), CAS: 335-67-1; (
) perfluorooctanesulfonic acid (PFOS), CAS: 1763-23-1; (
) perfluorononanoic acid (PFNA), CAS: 375-95-1; (
) perfluorohexanesulfonic acid (PFHxS), CAS: 355-46-4; (
) perfluorodecanoic acid (PFDA), CAS: 335-76-2; (
) 2,3,3,3-Tetrafluoro-2-(heptafluoropropoxy)propanoic acid (GenX or HFPO-DA), CAS: 13252-13-6; (
) 2,2-difluoro-2-{[2,2,4,5-tetrafluoro-5-(trifluoromethoxy)-1,3-dioxolan-4-yl]oxy}acetic acid (C6O4), CAS: 682-238-0; (
) P,P-Bis(perfluoro-7-methyloctyl) phosphinothioic acid erbium(3+) salt (3:1), CAS: 500776-89-6; (
) perfluorodecalin, CAS: 306-94-5; (
) perfluorotripropylamine, CAS: 338-83-0; (
) perfluoro-2,3-dimethylbutane, CAS: 354-96-1; (
) perfluoroperhydrophenanthrene, CAS: 306-91-2; (
) perfluoro-(2,5,8-trimethyl-3,6,9-trioxadodecanoic)acid, CAS: 65294-16-8; (
) dotriacontafluorononakis(trifluoromethyl)nonaoxatriacontanoyl fluoride, CAS: 65150-88-1; (
) bis 18:2 fluorotelomer thioether.
The chemical class of PFAS includes many substances that are uncharacterized to such an extent that their physical and chemical properties, including their solubility in water, are unknown. In fact, for some PFAS, the only available values for physical and chemical properties are estimates derived from mathematical models, such as quantitative structure–activity relationship (QSAR) models
[10][18], which can approximate the chemical and physical properties of compounds based on their chemical structure, rather values from direct measurements
[11][12][19,20].
At ambient temperature and pressure, long-chain PFAS typically exist in solid form as crystalline or amorphous powders. By contrast, short-chain PFAS, with 4–6 carbon atoms, are generally liquids at room temperature. Available data suggest that both the melting temperature and melting enthalpy of PFAS increase with the length of the fluorinated carbon chain
[12][13][20,21].
Vapor pressure, which is a measure of the volatility of a compound (the higher the vapor pressure, the more volatile the compound), is particularly relevant in evaluating the potential toxicity of PFAS. Highly volatile compounds have a greater potential for long-range transport because they are easily converted to the gas phase and can travel long distances in the atmosphere, whereas chemicals with low vapor pressures are more likely to remain in the solid and liquid forms and are generally transported through the soil, surface and groundwater, with reduced transport potential
[14][22]. The ambient vapor pressure of PFAS salts is significantly lower than that of the corresponding acidic forms. For example, the vapor pressure of the ammonium salt of PFOA is three orders of magnitude lower than that of its acid form. To accurately estimate the vapor pressure and environmental transport potential of PFAS, it is therefore necessary to determine their exact chemical nature in the environment, as different chemical forms can have very different vapor pressures
[15][23].
The chemical stability of a molecule greatly influences its persistence in the environment
[16][17][18][24,25,26]. The polar regions of PFAS, like the acid groups, can be susceptible to numerous chemical transformations. A recent study has shown that the carboxy terminal tail of PFCAs can facilitate a sodium hydroxide-mediated defluorination mechanism. This chemical degradation process occurs in the presence of the solvent dimethylsulfoxide and leads to highly reactive perfluoroalkyl intermediates that undergo further degradation, culminating in the final generation of fluoride ions
[19][27]. It is therefore scientifically inaccurate to consider the tens of thousands of PFAS as a single group of molecules with similar chemical and physical properties, biopersistence, bioaccumulation, and toxicity.
3. Bioaccumulation of PFAS: The Role of Chain Length
Bioaccumulation or biopersistence refers to the accumulation of a substance in an organism over time. Obviously, the bioavailability of a substance is a prerequisite for bioaccumulation. PFCAs and PFSAs, but not all PFAS, exhibit high stability and their lipophilicity depends on the length of the alkylic chain, resulting in their accumulation in several tissues
[20][56]. Moreover, they can bind to human serum albumin and other transporters in the blood
[21][57]. These properties make PFCA and PFSA potentially able to bioaccumulate in humans and animals.
The bioaccumulation of PFCAs and PFSAs is, indeed, influenced by their chemical structure. Compounds with longer carbon chains, such as PFOA and PFOS, are the most persistent in the environment and can accumulate in living organisms
[18][22][23][26,58,59]. By contrast, short-chain PFAS are less likely to bioaccumulate
[23][24][25][26][59,60,61,62]. In fact, although short-chain PFAS have been detected in aquatic systems, their concentrations are generally lower than those of long-chain PFAS
[27][63]. In particular, long-chain PFAS are more likely to accumulate in the brain than short-chain PFAS due to their ability to cross brain barriers
[28][64].
A study analyzing PFAS profiles in drinking water and biological samples from airport workers exposed to contaminated groundwater found that ‘historical’ PFAS accounted for 50% of the total PFAS in drinking water and 90% in serum. Branched PFOS isomers had shorter half-lives than linear PFOS isomers, with half-lives generally decreasing with decreasing chain length
[29][65].
Fluoropolymers, on the other hand, do not pose a bioaccumulation risk because their high molecular weight prevents their absorption by the body, and thus their bioavailability
[30][66].
4. Fluoropolymers Are a Separate Class from Smaller PFAS Molecules
Although they fall into the PFAS category, fluoropolymers are a distinct class of chemical compounds characterized by much larger molecular sizes (typical molecular weights > 100,000 Da) and more complex structures than the smaller PFAS molecules. Fluoropolymers consist of long carbon chains with multiple repeating units and fluorine atoms, occasionally accompanied by branching or cross-linking between polymer chains. Compared to small PFAS molecules, the larger size and often complex structures of fluoropolymers likely limit their uptake by living organisms, thereby reducing their likelihood of bioaccumulation. In addition, the large size of fluoropolymers results in their lower solubility in water, further limiting their mobility and potential for dispersion in the environment
[30][31][66,67]. In fact, fluoropolymers can be classified as low-risk polymers (PLCs), as they meet all the requirements for this classification
[32][33][68,69].
Overall, size, structure, and water solubility play a key role in determining the biological fate and potential damage of fluorinated substances
[30][66].
Size Limits for Small-Molecule Biological Activity
Size plays, along with charge and structure, a critical role in determining the penetration of molecules across cell membranes. In the development of new drugs, 500 Da is often quoted as the maximum molecular weight parameter. However, it has been observed that molecules with higher molecular weights are also capable of being absorbed, and the limits of oral bioavailability appear to extend to about MW ≤ 1000 Da
[34][35][36][70,71,72].
Thus, data accumulated from extensive investigations of various pharmacological and non-pharmacological substances indicate that molecules with molecular weights above 1000 Da have very little, if any, ability to diffuse across cell membranes and, as a result, are not bioavailable when taken orally.
Therefore, substances with molecular weights greater than 1000 Da, such as fluoropolymers, which generally have molecular weights much greater than 1000 Da, have negligible bioavailability, resulting in limited potential toxicity and bioaccumulation.
5. Bioremediation of PFAS: Challenges and Opportunities
Recent advances in PFAS degradation via thermal and non-thermal methods have been recently reviewed. Along with physicochemical techniques
[19][27], bioremediation appears to be a successful solution for PFAS removal from the environment
[37][38][73,74].
Bioremediation is a process that utilizes the metabolic capabilities of microorganisms to degrade and detoxify contaminants. The microbial degradation of PFAS is emerging as a promising approach for the remediation of contaminated waters and sites. For example,
Acidimicrobium sp. strain A6 is capable of defluorinating PFOA and PFOS through a reaction in which iron is reduced and ammonium or hydrogen are used as electron donors; this reaction leads to the formation of shorter-chain perfluorinated products and acetate
[39][75]. Another study investigated the role of carbon–carbon double bonds in the biodegradation of unsaturated PFAS, showing that α,β unsaturation is critical for anaerobic reductive defluorination and highlighting the enhanced degradability of unsaturated fluorinated carboxylic acids with α/β-trifluoromethyl branches
[40][76]. Several microbial enzymes, including esterases, hydrolases, oxidases, reductases, and dehalogenases, play key roles in PFAS biodegradation, and advances in enzyme engineering and biocatalysis offer the potential for the development of efficient and sustainable PFAS bioremediation strategies
[41][42][77,78].
However, the diversity of PFAS structures poses a challenge for bioremediation. Long-chain PFCAs and PFSAs may be more resistant to biodegradation than their short-chain counterparts. Despite these challenges, bioremediation offers several advantages over other remediation methods, such as chemical treatment and incineration. Bioremediation has very low costs and is environmentally friendly because it does not require expensive equipment and does not produce harmful byproducts
[43][79].
More research is needed to determine the feasibility of bioremediation as an effective strategy for PFAS remediation and to optimize the degradation of PFAS with different chemical structures. Should PFAS bioremediation techniques demonstrate their effectiveness, the depiction in media and the emphasis in regulatory proposals that currently categorize PFAS contaminants as ‘forever chemicals’ to underscore their environmental risk may need to be reevaluated.


