Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Sirius Huang and Version 1 by Agnieszka Wal.
Carnivorous plants attract animals, trap and kill them, and absorb nutrients from the digested bodies. This unusual (for autotrophs) type of nutrient acquisition evolved through the conversion of photosynthetically active leaves into specialised organs commonly called traps. The genus Nepenthes (pitcher plants) consists of approximately 169 species belonging to the group of carnivorous plants. Pitcher plants are characterised by specialised passive traps filled with a digestive fluid. The digestion that occurs inside the traps of carnivorous plants depends on the activities of many enzymes. Reactive oxygen species (ROS) and reactive nitrogen species (RNS) also participate in the digestive process.
- carnivorous plants
- Nepenthes
- reactive nitrogen species
- reactive oxygen species
1. Carnivorous Plants
Carnivorous plants (plantae carnivorae) are among the most intriguing autotrophic organisms which influence human imagination. The question arises as to how this life form evolved and resembles a kind of mythological “mermaid” that entices animals, kills them, and digests their bodies. The increased interest in these plants is related to the substances synthesised in the digestive process. Some of these compounds are flavonoids or naphthoquinones, which could have potent medical uses as inflammatory therapeutics [1,2][1][2]. Pitcher-shaped trap leaves create specific environments that are rich in microorganisms producing enzymes with potential industrial uses, such as lipases resistant to low pH conditions [3].
The phenomenon of animal-eating plants began to be investigated by biologists almost two centuries ago. Darwin described them as “the most wonderful plants in the world” [4,5][4][5]. Carnivorous plants do not have one ancestor and do not belong to one systematic group. Carnivory, as an adaptation, evolved independently in the plant kingdom at least six times in different geographical locations [5]. These plants primarily evolved in moist environments with low nutrient availability. Their typical habitats are swamps or peat bogs with waterlogged soil that is poorly aerated, which are unfavourable conditions for most higher plants [6]. Carnivory involved reprogramming standard leaf physiology from assimilates donor organs to traps. These modified leaves enabled nutrient absorption from organic matter released in the trap. The transformation of assimilatory organs into traps was accompanied by the development of specific features that attract animals to the trap, including altering the leaf colour, generating UV patterns, emitting volatile compounds, producing nectar, and generating specific shapes. These unique heterotrophs are primarily photosynthesising autotrophs that digest animals and absorb nutrients from their bodies [7,8,9][7][8][9]. The plant carnivory syndrome hypothesis considers the ability of plants to attract prey into a trap, keep it there, kill and digest the prey, and absorb the released nutrients [9,10][9][10]. Some plants that had been considered as carnivorous do not achieve all of the properties of the carnivory syndrome. Some do not attract prey, and others are unable to digest trapped organisms by themselves [10]. These plants are often called protocarnivorous or semicarnivorous.
The carnivorous plant group is estimated to contain approximately 860 species [11]. Carnivorous plants are found on almost every continent except for very cold regions [12]. The majority of carnivorous plants have developed different methods of obtaining essential mineral nutrients (e.g., N, P, or sulfur (S)) [13]. Nutrient uptake in carnivorous plants depends on specific glands that secrete digestive enzymes and (or the same) glands that enable nutrient absorption [9]. Traps are commonly grouped into five categories: sticky, adhesive traps (fly-traps), pitcher-shaped containers, moveable snap traps, suction bladders, and eel traps [14].
2. Carnivorous Pitcher Plants
Carnivorous plants that produce a pitfall-formed trap are called “pitcher plants”, including Sarraceniaceae, Cephalotaceae, and Nepenthaceae [15]. The Nepenthes (Nepenthaceae) create jug-shaped traps that acquire arthropods and other small animals [16]. Herein, the term “pitcher plants” is used exclusively for Nepenthes. Nepenthaceae are tropical plants that are primarily located in Indonesia, although some species occur in India (N. khasiana Hook. f.), Sri Lanka (N. distillatoria L.), Seychelles (N. pervillei Blume), and Madagascar (N. madagascarensis Poir. and N. masoalensis Schmid-Hollinger) [17,18][17][18]. Pitcher plants grow in habitats of very low nutrient availability, such as heath forests (kerangas), peat swamp forests, and montane forests [19].
Nepenthes generate dimorphic traps on their leaf tips during ontogeny. The first trap type (terrestrial pitcher) rests on the ground and is commonly ovoid-shaped. The second trap type (funnel-shaped) is located above ground level and is known as an aerial pitcher [19,20][19][20]. The terrestrial pitcher trap is characteristic for young plants, which form compact rosettes and straight tendrils tipped with ovary pitchers. Mature plants are characterised by climbing stems with long internodes and curled tendrils, which enable attachment to surrounding plants [19]. Recently, a species of N. pudica [21] was also discovered that produces lower pitchers located only underground. Traps are present on wholly or partially achlorophyllous shoots. The pitchers resemble terrestrial pitchers in shape and structure [21].
Nepenthes leaves have similar morphologies, consisting of an extremely enlarged photosynthetically active leaf base called a lamina, and a tendril that carries the trap (Figure 1). Gravity is an important factor in the trapping process as an attracted prey falls into the hollow trap and has no way to climb out due to the trap structure [22]. The Nepenthes pitcher trap is divided into three functional parts (basic zones) [23]: a peristome (ribbed upper rim of the pitcher), a waxy zone [24], and a digestive zone [25] (Figure 1A). The trap lid is considered as a separate zone of the pitcher (the fourth functional zone), which primarily protects the trap against rain droplets [22,24][22][24] (Figure 1B). The top part of the trap is (usually) a hood-shaped appendix with glands that function to attract a potential prey. These glands are called extrafloral nectaries (EFNs) because they secrete sweet nectar and volatile compounds. EFNs also are located on the tendril [19]. The largest and most tightly packed glands may be present on the peristome [19]. The peristome surface is generally wet and slippery, with inward-pointing hairs that cause the attracted prey to fall into the trap. The outside part is usually rough and hairy and possesses longitudinal rims to facilitate the animal access to the attractive peristome of the trap [22].
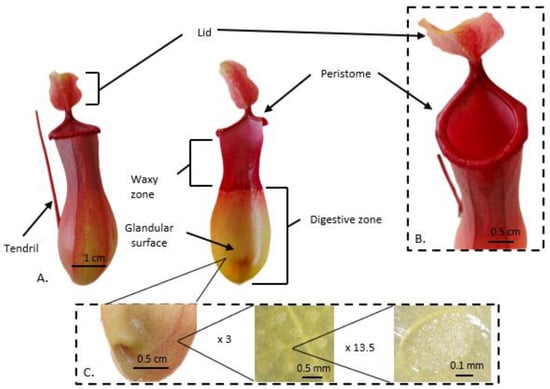
Figure 21. The aerial trap of the pitcher plant. (A) External and interior images show characteristics of the mature trap of Nepenthes ventrata Hort. ex Fleming (=N. ventricosa Blanco x N. alata Blanco). A lid is marked in the image of the whole (intact) trap tendril. (A) The peristome, glandular surface, and digestive zone are marked in the image of the trap cross-section. (B) Close-up image of the upper part of the trap. A peristome and a lid are marked. (C) shows images of the digestive glands at magnitudes of approximately 3× and 13.5×.
The upper part of the trap prevents the prey from climbing out because the inner surface is generally covered with downward-pointing hairs or loose wax crystals. The waxy zone contains modified stomata of hypertrophied guard cells (lunate cells) with curved surfaces that block climbing insects [24]. Insect escape is also made more complex by the anisotropic properties of the waxy zone of pitcher plants. The presence of moon cells causes insects to move at high speed and without innards towards the digestive zone, while their movement towards the peristome is difficult or even prevented [26]. The rough and non-cohesive surface of the wax crystals reduces insect adhesion to the waxy zone [25,27][25][27]. Even though the waxy zone plays such an essential role in reducing the adhesion of insects and preventing them from moving toward the trap opening, some pitcher plants, for example, N. ampullaria Jack and N. bicalcarata Hook.f. do not have this zone [28,29][28][29]. The released nutrients are adsorbed at the bottom of the trap, which is equipped with a permeable cuticle and glands (Figure 1C) that produce digestive enzymes. Transporters are located across the pitcher wall and actively transport liberated N into plant tissues [30]. The digestive fluid covers the lower part of this zone. The digestive fluid of pitcher plants, due to its unique physicochemical properties, plays not only an important role in digestion but also in the mechanical retention of the prey inside the trap [31]. In some species, e.g., N. rafflesiana Jack, the digestive fluid has viscoelastic properties that facilitate the prey drowning [19].
Several digestive enzymes and peptides have been identified in the pitcher fluid of various Nepenthes species [2,10,30,32,33][2][10][30][32][33]. The presence of thaumatin-like proteins belonging to pathogenesis-related proteins (PRPs) has been reported. PRPs inhibit the growth of microbial competitors (also fungi) in the digestive fluid [2]. Other compounds identified in the digestive fluid are: naphthoquinones, among others plumbagin and droserone, [33,34][33][34] and flavonols such as quercetin and kaempferol [35].
Some Nepenthes species differ in the morphology and functionality of the pitcher zones and in trapping mechanisms, such as N. rafflesiana, which exists in diverse ecological and morphological forms [19]. These alterations enable the examination of the capture strategy of carnivorous plants, by the development of plant–animal cooperation [19] as described below. Some pitcher plants have abandoned the typical “predator” system of nutrient acquisition. Nepenthes absorb N from different sources of animal or plant origin [36]. For example, N. lowii Hook.f. and other Nepenthes produce dimorphic pitchers. Typical terrestrial trap structures that capture arthropods are formed only by immature carnivorous plants. When the mature plant gains the ability to develop aerial pitchers, it changes the method of N uptake. Aerial traps are highly lignified with a modified lid and secrete buttery white exudates that attract the mountain treeshrew (Tupaia montana). This small mammal consumes nectar from the pitcher lid and excretes faeces into the pitcher. Nitrogen delivered by treeshew faeces accounts for 57–100% of nutrients absorbed by the plant, and is the primary source of this one macronutrient for leaves [36]. The elongated traps of N. baramensis utilise N captured from the faeces of Hardwicke’s woolly bats (Kerivoula hardwickii) [16,37][16][37]. These bats provide approximately 34% of N absorbed by the plant leaves [16,37][16][37].
3. The Role of Reactive Oxygen Species in External Digestion by Carnivorous Plants
Reactive oxygen species (ROS) are products of the incomplete reduction or excitation of oxygen [58][38]. High ROS concentrations are primarily linked to inefficient antioxidant systems and lead to the induction of oxidative stress, which may result in cell death. ROS are necessary to perceive “normal” or “typical” functions of an organism because they act as crucial components of signalling transduction cascades [59][39]. At the physiological level, ROS participate in the removal of a toxic microbiome in animals and plants [60][40]. ROS include the superoxide anion (O2•−), hydroxyl radical (•OH), hydrogen peroxide (H2O2), and peroxyl, alkoxyl, and hydroperoxyl radicals [58][38]. In plant tissues, ROS are primarily produced in chloroplasts, mitochondria, peroxisomes, and the apoplastic space [61][41]. ROS are mainly formed in the Fenton and Haber-Weiss reactions [58,62][38][42]. The reactivity of •OH is limited to nearby molecules, whereas H2O2 has a longer half-life and can be translocated to another cellular compartment [63,64][43][44]. The increased activity of the plasma membrane respiratory burst oxidase homolog (Rboh), a nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, is linked to higher ROS generation [64][44]. ROS react with nucleic acids, proteins, lipids, and sugars [59][39], and are key factors of carcinogenesis in animals [65][45]. ROS attack on DNA structure results in single- or double-strand DNA breaks and DNA–protein cross-links [66][46]. Guanine oxidation at the C8 position leads to the formation of 8-hydroxy-2′-deoxyguanosine (8-OHdG) [67][47], which is mutagenic and related to mutations commonly observed in human cancers [65][45]. One of the most important modes of ROS action is their reactivity with amino acid residues in peptides/proteins, which leads to protein posttranslational modifications (PTMs). Carbonylation is an irreversible PTM occurring under physiological conditions [68][48]. Proteins with carbonyl groups formed on arginine, proline, threonine, or lysine residues usually lack normal function and are more prone to degradation [68][48]. The balance between ROS generation and decomposition is achieved by the antioxidant system, consisting of enzymatic and non-enzymatic components. The best known enzymatic ROS modulators are isoforms of superoxide dismutases, catalases, ascorbate peroxidases, glutathione peroxidases, and glutathione reductases. Thioredoxins, glutaredoxins, and peroxiredoxins are other proteins acting as ROS modulators [69][49]. Among non-enzymatic antioxidants are reduced ascorbic acid (ASA), reduced glutathione, carotenoids, and α-tocopherols [58,69,70][38][49][50]. The adverse effects of ROS in human digestion have been examined. ROS participate in tissue lesions during inflammatory processes [71][51]. Important sources of ROS include gastric epithelial cells and activated inflammatory cells such as neutrophils in infected tissues [72][52]. Carcinogenesis in the digestive system is strongly affected by ROS. Oxidative stress and ROS overproduction is coupled to Helicobacter pylori infection, one of the factors in cancer development [65][45]. The link between ROS generation in mitochondria and several environmental risks for the incidence of development gastric cancer has been proposed. ROS are implicated in gastric cancer invasion and metastasis [65][45]; their levels increase in chronic gastritis, inflammatory bowel diseases, and chronic liver diseases [73][53]. The results of animal studies indicate that gastroesophageal reflux stimulates ROS formation and leads to lesions of the oesophageal mucosa [74,75][54][55]. The consumption/digestion of meat (consisting of fat, proteins, and free and bound iron) by a human is strongly coupled to oxidative processes. Unstable ROS products of the Fenton reaction generate cytotoxic and genotoxic lipid oxidation products like malondialdehyde or 4-hydroxynonenal [76][56]. These compounds participate in protein carbonylation. Ageing tissues accumulate carbonylated protein aggregates [68][48]. ROS also contribute directly to protein degradation, especially of oxidatively modified proteins [77][57]. Protease activity in vitro is higher when the protein reacts with free radicals [78][58]. In N. alata Blanco pitcher fluid, the enzymatic digestion of products of the exogenous oxidised B chain of bovine insulin was observed [52][59]. Free radicals are assumed to accelerate protein breakdown, probably by their special modification (e.g., carbonylation) followed by degradation [68,77][48][57]. In plants, ROS-dependent changes in the activities of acidic proteases, chymotrypsin-like, peptidyl-glutamyl-peptidehydrolase, caseinolytic-specific, and trypsin-like proteases were reported in sugar-deprived maize (Zea mays L.) root tips [79][60], and germinating apple (Malus domestica Borkh.) embryos [80][61]. ROS are involved in the prey trapping and digestion in the carnivorous plant N. gracilis Korth. [81][62] (Figure 2). Electron paramagnetic resonance (EPR) spin trapping analysis demonstrated that the first step of the prey digestion is accompanied by the generation of free radicals (primarily •OH) in the pitcher fluid. To prove that ROS induce protein degradation in the trap digestive fluid, myosin (an abundant protein in insect bodies) was added to the fluid of a young, closed trap along with a cocktail of protease inhibitors, and gel electrophoresis of the fluid protein fraction was performed. Myosin light chains were degraded, indicating that free radicals are beneficial for prey digestion [81][62]. In the N. ventrata Hort. ex Fleming digestive fluid, the presence of O2•− was confirmed during the whole ontogeny of the trap, starting from the closed organs [44][63]. The researchers proposed that radical forms of ROS are involved not only in the digestion per se but also play a role as compounds regulating the trap microbiome, although this needs further investigation.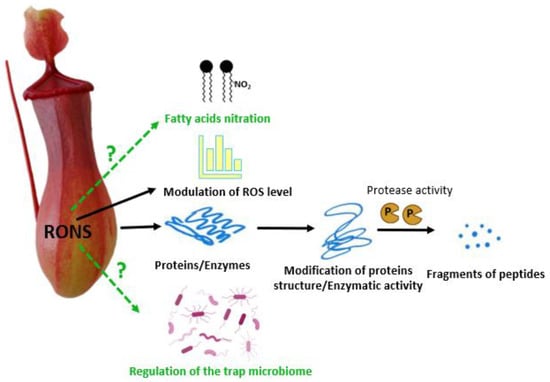
Figure 2. A scheme shows the role that RONS presumably perform (solid black lines) or may perform (dashed green lines with a question mark) in the digestion of prey by pitcher plants. RONS influence the activity of enzymes responsible for the prey decomposition, modify proteins, and facilitate their proteolysis. RNS modify the concentration of ROS in the digestive fluid. RONS alter cellular compounds, which may participate in fatty acid nitration. RNS may also have antimicrobial effects and control the growth of the microbiome in the digestive fluid in the pitcher plant’s trap.
References
- Melzig, M.F.; Pertz, H.H.; Krenn, L. Anti-Inflammatory and Spasmolytic Activity of Extracts from Droserae herba. Phytomedicine 2001, 8, 225–229.
- Mithöfer, A. Carnivorous Pitcher Plants: Insights in an Old Topic. Phytochemistry 2011, 72, 1678–1682.
- Morohoshi, T.; Oikawa, M.; Sato, S.; Kikuchi, N.; Kato, N.; Ikeda, T. Isolation and Characterization of Novel Lipases from a Metagenomic Library of the Microbial Community in the Pitcher Fluid of the Carnivorous Plant Nepenthes hybrida. J. Biosci. Bioeng. 2011, 112, 315–320.
- Darwin, C. Insectivorous Plants; D. Appleton and Company: New York, NY, USA, 1875.
- Ellison, A.M.; Gotelli, N.J. Energetics and the Evolution of Carnivorous Plants—Darwin’s ‘Most Wonderful Plants in the World’. J. Exp. Bot. 2009, 60, 19–42.
- Armstrong, W. Aeration in Higher Plants. Adv. Bot. Res. 1979, 7, 225–332.
- Jürgens, A.; El-Sayed, A.M.; Suckling, D.M. Do Carnivorous Plants Use Volatiles for Attracting Prey Insects? Funct. Ecol. 2009, 23, 875–887.
- Adlassnig, W.; Steinhauser, G.; Peroutka, M.; Musilek, A.; Sterba, J.H.; Lichtscheidl, I.K.; Bichler, M. Expanding the Menu for Carnivorous Plants: Uptake of Potassium, Iron and Manganese by Carnivorous Pitcher Plants. Appl. Radiat. Isot. 2009, 67, 2117–2122.
- Król, E.; Płachno, B.J.; Adamec, L.; Stolarz, M.; Dziubińska, H.; Trębacz, K. Quite a Few Reasons for Calling Carnivores ‘the Most Wonderful Plants in the World’. Ann. Bot. 2012, 109, 47–64.
- Płachno, B.J.; Adamec, L.; Huet, H. Mineral Nutrient Uptake from Prey and Glandular Phosphatase Activity as a Dual Test of Carnivory in Semi-Desert Plants with Glandular Leaves Suspected of Carnivory. Ann. Bot. 2009, 104, 649–654.
- Cross, A.T.; Krueger, T.A.; Gonella, P.M.; Robinson, A.S.; Fleischmann, A.S. Conservation of Carnivorous Plants in the Age of Extinction. Glob. Ecol. Conserv. 2020, 24, e01272.
- Millett, J.; Svensson, B.M.; Newton, J.; Rydin, H. Reliance on Prey-Derived Nitrogen by the Carnivorous Plant Drosera rotundifolia Decreases with Increasing Nitrogen Deposition. New Phytol. 2012, 195, 182–188.
- Adlassnig, W.; Peroutka, M.; Lambers, H.; Lichtscheidl, I.K. The Roots of Carnivorous Plants. Plant Soil. 2005, 274, 127–140.
- Lloyd, F.E. The Carnivorous Plants; Chronica Botanica Company: Waltham, MA, USA, 1942.
- Albert, V.A.; Williams, S.E.; Chase, M.W. Carnivorous Plants: Phylogeny and Structural Evolution. Science (1979) 1992, 257, 1491–1495.
- Clarke, C.; Moran, J.A.; Lee, C.C. Nepenthes baramensis (Nepenthaceae)—A New Species from North-Western Borneo. Blumea Biodivers. Evol. Biogeogr. Plants 2011, 56, 229–233.
- Meimberg, H.; Wistuba, A.; Dittrich, P.; Heubl, G. Molecular Phylogeny of Nepenthaceae Based on Cladistic Analysis of Plastid TrnK Intron Sequence Data. Plant Biol. 2001, 3, 164–175.
- Meimberg, H.; Heubl, G. Introduction of a Nuclear Marker for Phylogenetic Analysis of Nepenthaceae. Plant Biol. 2006, 8, 831–840.
- Bauer, U.; Grafe, T.U.; Federle, W. Evidence for Alternative Trapping Strategies in Two Forms of the Pitcher Plant. Nepenthes rafflesiana. J. Exp. Bot. 2011, 62, 3683–3692.
- Clarke, C. Nepenthes of Borneo; Natural History Publications (Borneo): Kota Kinabalu, Malaysia, 1997; ISBN 9789838120159.
- Dančák, M.; Majeský, Ľ.; Čermák, V.; Golos, M.R.; Płachno, B.J.; Tjiasmanto, W. First Record of Functional Underground Traps in a Pitcher Plant: Nepenthes pudica (Nepenthaceae), a New Species from North Kalimantan, Borneo. PhytoKeys 2022, 201, 77–97.
- Adlassnig, W.; Peroutka, M.; Lendl, T. Traps of Carnivorous Pitcher Plants as a Habitat: Composition of the Fluid, Biodiversity and Mutualistic Activities. Ann. Bot. 2011, 107, 181–194.
- Gaume, L.; Di Giusto, B. Adaptive Significance and Ontogenetic Variability of the Waxy Zone in Nepenthes rafflesiana. Ann. Bot. 2009, 104, 1281–1291.
- Gaume, L.; Gorb, S.; Rowe, N. Function of Epidermal Surfaces in the Trapping Efficiency of Nepenthes alata Pitchers. New Phytol. 2002, 156, 479–489.
- Gaume, L.; Perret, P.; Gorb, E.; Gorb, S.; Labat, J.J.; Rowe, N. How Do Plant Waxes Cause Flies to Slide? Experimental Tests of Wax-Based Trapping Mechanisms in Three Pitfall Carnivorous Plants. Arthropod Struct. Dev. 2004, 33, 103–111.
- Wang, L.; Tao, D.; Dong, S.; Li, S.; Tian, Y. Contributions of Lunate Cells and Wax Crystals to the Surface Anisotropy of Nepenthes Slippery Zone. R. Soc. Open Sci. 2018, 5, 180766.
- Gorb, E.; Haas, K.; Henrich, A.; Enders, S.; Barbakadze, N.; Gorb, S. Composite Structure of the Crystalline Epicuticular Wax Layer of the Slippery Zone in the Pitchers of the Carnivorous Plant Nepenthes alata and Its Effect on Insect Attachment. J. Exp. Biol. 2005, 208, 4651–4662.
- Moran, J.A.; Clarke, C.M.; Hawkins, B.J. From Carnivore to Detritivore? Isotopic Evidence for Leaf Litter Utilization by the Tropical Pitcher Plant Nepenthes ampullaria. Int. J. Plant Sci. 2003, 164, 635–639.
- Bohn, H.F.; Federle, W. Insect Aquaplaning: Nepenthes Pitcher Plants Capture Prey with the Peristome, a Fully Wettable Water-Lubricated Anisotropic Surface. Proc. Natl. Acad. Sci. USA 2004, 101, 14138–14143.
- Moran, J.A.; Clarke, C.M. The Carnivorous Syndrome in Nepenthes Pitcher Plants. Plant Signal Behav. 2010, 5, 644–648.
- Kang, V.; Isermann, H.; Sharma, S.; Wilson, D.I.; Federle, W. How a Sticky Fluid Facilitates Prey Retention in a Carnivorous Pitcher Plant (Nepenthes rafflesiana). Acta Biomater. 2021, 128, 357–369.
- Hatano, N.; Hamada, T. Proteomic Analysis of Secreted Protein Induced by a Component of Prey in Pitcher Fluid of the Carnivorous Plant Nepenthes alata. J. Proteom. 2012, 75, 4844–4852.
- Eilenberg, H.; Pnini-Cohen, S.; Rahamim, Y.; Sionov, E.; Segal, E.; Carmeli, S.; Zilberstein, A. Induced Production of Antifungal Naphthoquinones in the Pitchers of the Carnivorous Plant Nepenthes khasiana. J. Exp. Bot. 2010, 61, 911–922.
- Raj, G.; Kurup, R.; Hussain, A.A.; Baby, S. Distribution of Naphthoquinones, Plumbagin, Droserone, and 5-O-Methyl Droserone in Chitin-Induced and Uninduced Nepenthes khasiana: Molecular Events in Prey Capture. J. Exp. Bot. 2011, 62, 5429–5436.
- Aung, H.H.; Chia, L.S.; Goh, N.K.; Chia, T.F.; Ahmed, A.A.; Pare, P.W.; Mabry, T.J. Phenolic Constituents from the Leaves of the Carnivorous Plant Nepenthes gracilis. Fitoterapia 2002, 73, 445–447.
- Clarke, C.M.; Bauer, U.; Lee, C.C.; Tuen, A.A.; Rembold, K.; Moran, J.A. Tree Shrew Lavatories: A Novel Nitrogen Sequestration Strategy in a Tropical Pitcher Plant. Biol. Lett. 2009, 5, 632–635.
- Grafe, T.U.; Schöner, C.R.; Kerth, G.; Junaidi, A.; Schöner, M.G. A Novel Resource-Service Mutualism between Bats and Pitcher Plants. Biol. Lett. 2011, 7, 436–439.
- Mittler, R. ROS Are Good. Trends Plant Sci. 2017, 22, 11–19.
- Ciacka, K.; Krasuska, U.; Staszek, P.; Wal, A.; Zak, J.; Gniazdowska, A. Effect of Nitrogen Reactive Compounds on Aging in Seed. Front. Plant Sci. 2020, 11, 1011.
- Horn, A.; Jaiswal, J.K. Cellular Mechanisms and Signals That Coordinate Plasma Membrane Repair. Cell. Mol. Life Sci. 2018, 75, 3751–3770.
- Corpas, F.J.; Gupta, D.K.; Palma, J.M. Production Sites of Reactive Oxygen Species (ROS) in Organelles from Plant Cells. In Reactive Oxygen Species and Oxidative Damage in Plants under Stress; Corpas, F.J., Gupta, D.K., Palma, J.M., Eds.; Springer: Cham, Switzerland, 2015; pp. 1–22.
- Thiel, J.; Rolletschek, H.; Friedel, S.; Lunn, J.E.; Nguyen, T.H.; Feil, R.; Tschiersch, H.; Müller, M.; Borisjuk, L. Seed-Specific Elevation of Non-Symbiotic Hemoglobin AtHb1: Beneficial Effects and Underlying Molecular Networks in Arabidopsis thaliana. BMC Plant Biol. 2011, 11, 48.
- Møller, I.M.; Rogowska-Wrzesinska, A.; Rao, R.S.P. Protein Carbonylation and Metal-Catalyzed Protein Oxidation in a Cellular Perspective. J. Proteomics 2011, 74, 2228–2242.
- Kumar, J.S.P.; Rajendra Prasad, S.; Banerjee, R.; Thammineni, C. Seed Birth to Death: Dual Functions of Reactive Oxygen Species in Seed Physiology. Ann. Bot. 2015, 116, 663–668.
- Tamura, M.; Mutoh, M.; Fujii, G.; Matsui, H. Involvement of Mitochondrial Reactive Oxygen Species in Gastric Carcinogenesis. J. Gastrointest. Dig. Syst. 2013, 3, 150.
- Toyokuni, S.; Mori, T.; Hiai, H.; Dizdaroglu, M. Treatment of Wistar Rats with a Renal Carcinogen, Ferric Nitrilotriacetate, Causes DNA-protein Cross-linking between Thymine and Tyrosine in Their Renal Chromatin. Int. J. Cancer 1995, 62, 309–313.
- Kasai, H.; Nishimura, S. Hydroxylation of Deoxyguanosine at the C-8 Position by Ascorbic Acid and Other Reducing Agents. Nucleic Acids Res. 1984, 12, 2137–2145.
- Ciacka, K.; Tymiński, M.; Gniazdowska, A.; Krasuska, U. Carbonylation of Proteins—An Element of Plant Ageing. Planta 2020, 252, 12.
- Morscher, R.J.; Aminzadeh-Gohari, S.; Feichtinger, R.G.; Mayr, J.A.; Lang, R.; Neureiter, D.; Sperl, W.; Kofler, B. Inhibition of Neuroblastoma Tumor Growth by Ketogenic Diet and/or Calorie Restriction in a CD1-Nu Mouse Model. PLoS ONE 2015, 10, e0129802.
- Kranner, I.; Birtić, S.; Anderson, K.M.; Pritchard, H.W. Glutathione Half-Cell Reduction Potential: A Universal Stress Marker and Modulator of Programmed Cell Death? Free Radic. Biol. Med. 2006, 40, 2155–2165.
- Landriscina, M.; Maddalena, F.; Laudiero, G.; Esposito, F. Adaptation to Oxidative Stress, Chemoresistance, and Cell Survival. Antioxid. Redox Signal. 2009, 11, 2701–2716.
- Babior, B.M. Oxidants from Phagocytes: Agents of Defense and Destruction. Blood 1984, 64, 959–966.
- Takaki, A.; Kawano, S.; Uchida, D.; Takahara, M.; Hiraoka, S.; Okada, H. Paradoxical Roles of Oxidative Stress Response in the Digestive System before and after Carcinogenesis. Cancers 2019, 11, 213.
- Wetscher, G.J.; Hinder, P.R.; Bagchi, D.; Perdikis, G.; Redmond, E.J.; Glaser, K.; Adrian, T.E.; Hinder, R.A. Free Radical Scavengers Prevent Reflux Esophagitis in Rats. Dig. Dis. Sci. 1995, 40, 1292–1296.
- Meining, A.; Classen, M. The Role of Diet and Lifestyle Measures in the Pathogenesis and Treatment of Gastroesophageal Reflux Disease. Am. J. Gastroenterol. 2000, 95, 2692–2697.
- Van Hecke, T.; Van Camp, J.; De Smet, S. Oxidation during Digestion of Meat: Interactions with the Diet and Helicobacter Pylori Gastritis, and Implications on Human Health. Compr. Rev. Food Sci. Food Saf. 2017, 16, 214–233.
- Davies, K.J. Protein Damage and Degradation by Oxygen Radicals. I. General. Aspects. J. Biol. Chem. 1987, 262, 9895–9901.
- Davies, K.J.A.; Lin, S.W. Degradation of Oxidatively Denatured Proteins in Escherichia Coli. Free Radic. Biol. Med. 1988, 5, 215–223.
- An, C.I.; Fukusaki, E.I.; Kobayashi, A. Aspartic Proteinases Are Expressed in Pitchers of the Carnivorous Plant Nepenthes alata Blanco. Planta 2002, 214, 661–667.
- Basset, G.; Raymond, P.; Malek, L.; Brouquisse, R. Changes in the Expression and the Enzymic Properties of the 20S Proteasome in Sugar-Starved Maize Roots. Evidence for an in Vivo Oxidation of the Proteasome. Plant Physiol. 2002, 128, 1149–1162.
- Krasuska, U.; Ciacka, K.; Dębska, K.; Bogatek, R.; Gniazdowska, A. Dormancy Alleviation by NO or HCN Leading to Decline of Protein Carbonylation Levels in Apple (Malus domestica Borkh.). Embryos. J. Plant Physiol. 2014, 171, 1132–1141.
- Chia, T.F.; Aung, H.H.; Osipov, A.N.; Goh, N.K.; Chia, L.S. Carnivorous Pitcher Plant Uses Free Radicals in the Digestion of Prey. Redox Report 2004, 9, 255–261.
- Wal, A.; Staszek, P.; Pakula, B.; Paradowska, M.; Krasuska, U. ROS and RNS Alterations in the Digestive Fluid of Nepenthes × Ventrata Trap at Different Developmental Stages. Plants 2022, 11, 3304.
More
