Breast cancer (BCa) is the most frequently diagnosed malignant tumor in women and is also one of the leading causes of cancer-related death. Most breast tumors are hormone-dependent and estrogen signaling plays a critical role in promoting the survival and malignant behaviors of these cells. Estrogen signaling involves ligand-activated cytoplasmic estrogen receptors that translocate to the nucleus with various co-regulators, such as steroid receptor co-activator (SRC) family members, and bind to the promoters of target genes and regulate their expression. SRC-3 is a member of this family that interacts with, and enhances, the transcriptional activity of the ligand activated estrogen receptor. Although SRC-3 has important roles in normal homeostasis and developmental processes, it has been shown to be amplified and overexpressed in breast cancer and to promote malignancy.
- breast cancer
- SRC-3
- estrogen signaling
- tumor microenvironment
- tumor cells
- malignant behavior
1. Introduction
2. SRC-3 Affects the Tumor Microenvironment
SRC-3 may affect the tumor microenvironment in multiple ways by affecting many immune system cells, as well as tumor cells. Although these effects generally result in an immunosuppressive phenotype, they may also contribute to the creation of an inflammatory environment. Tregs have a special place in the SRC-3-induced immunosuppressive tumor microenvironment. Indeed, Tregs have a strong SRC-3 expression and a high SRC-3 level is required for their immunosuppressive functions as described above [15]. In this context, it can be speculated that SRC-3 has a crucial role in establishing an immunosuppressive microenvironment in BCa. Although the factors that induce SRC-3 expression in Tregs are not yet understood, it is possible that this regulation may be dependent on some common mechanisms including the estrogen, retinoic acid (RA), and TGF-β signaling mechanisms. Silencing or inhibiting of SRC-3 in Tregs causes a decrease in the expression levels of both FoxP3 and PD-1 encoding genes [15]. FoxP3 and PD-1 levels are directly related to the immunosuppressive abilities of Tregs, and estrogen up-regulates the expressions of the genes that encode these proteins [81,82][32][33]. However, estrogen has the opposite effect on SRC-3 transcription and activity, and although estrogen inhibits SRC-3 transcription, it induces SRC-3 phosphortylations and consequently activates the SRC-3 protein [10,63][10][34]. Consequently, the phosphorylated SRC-3 binds to ERs to potentiate its genomic or non-genomic functions and thereby contributes to estrogen action. RA and TGF-β have important roles in the generation and differentiation of Tregs; RA induces FoxP3 expression in a TGF-β dependent manner and consequently promotes generation of Tregs [144,145][35][36]. Indeed, it has been shown that RA is a crucial factor in the TGF-β-mediated immune response that inhibits the IL-6-mediated induction of Th17 and promotes Treg differentiation [146][37]. Furthermore, it has been shown that RA and TGF-β increases SRC-3 transcription [10]. Therefore, it seems reasonable to hypothesize that SRC-3 may play a role in the RA- and TGF-β-mediated generation and/or differentiation of Tregs and Th17 cells. Indeed, SRC-3 has been shown to play an essential role in Th17 biology [147,148][38][39]. It has been demonstrated that SRC-3 interacts with RORα and RORγt and is involved in the activation of the expressions of RORγt-associated Th17 genes via IL-1/ILR1 signaling; thereby regulating pathogenic inflammation [149,150][40][41]. In concordance, Wang et al. recently showed that SRC-3 could shape the multiple myeloma microenvironment by inducing IL-17 expression in γδ T-cells. Mechanistically, they showed that the hypoxic microenvironment conditions in the multiple myeloma bone marrow niche stimulate SRC-3 expression in γδ T cells, and consequently SRC-3 interacts with RORγt and promotes IL-17 transcription. In concordance, they also demonstrated that inhibition of SRC-3 activity suppresses IL-17A expression in γδ T cells, reduces the multiple myeloma progression in mouse models and enhances the efficacy of bortezomib [151][42]. In further concordance, an association has been reported between high SRC-3 levels and poor outcomes in multiple myeloma patients treated with bortezomib, which suggests that targeting SRC-3 may be a promising approach to help overcome drug resistance [152][43]. Mechanistically, this effect was regulated mainly through NSD2 binding to SRC-3 to stabilize it [152][43]. NFκB has been shown to bind to the SRC-3 promoter and up-regulate its expression in response to TNF-α [153][44]. Although NFκB signaling is generally considered to be the mechanism that induces the differentiation of effector T-cells, it also promotes FoxP3 expression and has a role in the generation of Tregs [154,155,156][45][46][47]. SRC-3 expression has been shown to decrease in an AKT/mTOR-dependent manner in hypoxia conditions in preeclampsia, a complication of pregnancy [140][28]. Although it is unknown whether AKT/mTOR-dependent regulation of SRC-3 expression is a general mechanism in cells, including Tregs, it is possible that it is a general mechanism in the regulation of SRC-3 expression. Furthermore, the effects of SRC-3 in the induction of an anti-inflammatory environment have also been shown. Chen et al. have shown that SRC-3 inhibits the inflammation, and deletion of SRC-3 in mice which results in increased production of inflammatory cytokines such as TNF-α, IL-1β, and IL-6, and consequently, increased inflammation in the colon [157][48]. In concordance, induction of SRC-3 activity through the small molecule MCB-613 results in the enrichment of anti-inflammatory macrophages in mice [158][49]. The anti-inflammatory effect of SRC-3 has also been observed in vitro. In addition, stimulation of SRC-3 through MCB-613 in the RAW 264.7 macrophages results in decreasing expression of pro-inflammatory cytokine mRNAs including TNF-α, IL-1β, and IL-6 [158][49]. In concordance, it has been shown that LPS treatment leads to the increased secretion of pro-inflammatory cytokines, such as TNF-α, IL-6, and IL-1β, in SRC-3 deleted macrophages compared to wild-type macrophages [159][50]. Interestingly, the transcription of pro-inflammatory cytokines is nearly unchanged in SRC-3 deleted macrophages compared to wild type, but the translational efficiency of these cytokine mRNAs has been increased [159][50]. It has been shown that SRC-3-dependent regulation of this effect occurs at the post-transcriptional level, and SRC-3 exerts this effect by promoting the binding of some translational repressors to the 3’ UTR region of TNF-α mRNA to inhibit its translation. Furthermore, macrophages from SRC-3-deleted mice produce a high level of TNF-α protein in response to LPS stimulation without changing the TNF-α mRNA level [160][51]. SRC-3 may also affect the phagocytosis abilities of macrophages. It has been shown that the levels of the scavenger receptor A and catalase are lower in SRC-3 deficient macrophages, compared to wild-type macrophages [160][51]. In this context, SRC-3 directly contributes to the regulation of catalase transcription, and SRC-3 deficiency results in a decrease in catalase expression [160][51]. Catalase is an important enzyme in the regulation of reactive oxygen species and is responsible for the conversion of H2O2 to H2O [161][52]. It has been shown that both the ROS level and apoptotic index are higher in SRC-3 deleted macrophages compared to wild types [160][51]. Indeed, other studies also confirmed an inhibitory role of SRC-3 in both intrinsic and extrinsic apoptotic pathways [162,163][53][54]. On the other hand, it seems that SRC-3 is involved in both the activation and recruitment of neutrophils through the regulation of CXCL-2, in a NFκB dependent manner, and thereby SRC-3 may contribute to the creation and regulation of an inflammatory environment, at least in the neutrophil context [164][55]. Consistently, SRC-3 was shown to be an NFκB co-regulator that promotes NFκB-mediated transcriptional activity, and this activity is regulated by phosphorylation by IκB kinase [165,166][56][57]. The role of SRC-3 in regulation of NFκB was further supported by demonstration of a direct interaction between SRC-3 and Rel-A [166][57]. NFκB signaling is known to inhibit apoptosis, and therefore, SRC-3 dependent inhibition of apoptosis may be related to the activation of NFκB, at least partly. Moreover, SRC-3 is not only a co-activator for NFκB but is also a direct target, and inflammatory cytokines induce SRC-3 expression via direct binding of NFκB to the SRC-3 promoter [153][44]. It is probable that a feedback loop operates between SRC-3 and NFκB because SRC-3 also represses the translational efficiency of pro-inflammatory cytokines including TNF-α, IL-6, and IL-1β, and this effect is abolished in SRC-3 deficient mice, as described above [159][50].3. SRC-3 Promotes Stemness
SRC-3 was shown to drive the CSC phenotype, in concordance with its EMT promoting roles (Figure 1) [16]. In this context, cytoplasmic PELP1/SRC-3 complexes were shown to mediate the expansion of breast CSCs [167][58]. Indeed, PELP1/SRC-3 complexes regulate CSCs through modulating metabolic adaptation-associated gene expression programs [168][59]. Furthermore, SRC-3 is required for the maintenance and induction of the CSC phenotype; consequently, treatment of BCa cells with an SRC-3 inhibitor decreases SRC-3-induced CSCs in BCa [16,169][16][60]. The SRC-3 level is positively associated with ALDH+ CSCs in BCa [16]. ALDH1+ CSCs are especially important since they are associated with both tamoxifen resistance and early recurrence after anti-estrogen therapy in breast tumors [170,171][61][62]. Although use of disulfiram, an ALDH inhibitor, results in activating T cell immunity and consequently in the clearance of breast CSCs, it is not yet known whether this effect is associated with effects on SRC-3 [172,173][63][64]. Furthermore, SRC-3 interacts with SOX-2 and promotes its transcriptional activity [174][65]. SOX-2 expression increases during the development of tamoxifen resistance and a high SOX-2 level is important to maintain CSCs in BCa [175,176][66][67]. SRC-3 interacts with estrogen-related receptor β (ESRRB) and functions as a co-activator in inducting and sustaining embryonic stem cell (ESC) renewal and pluripotency [177,178,179][68][69][70]. Indeed, SRC-3 was shown to induce the expression of self-renewal and pluripotency related genes, including KLF-4, in ESCs [180][71]. Furthermore, SRC-3 is involved in the regulation of Hematopoietic stem cells (HSCs) by modulating their mitochondrial metabolism [181][72].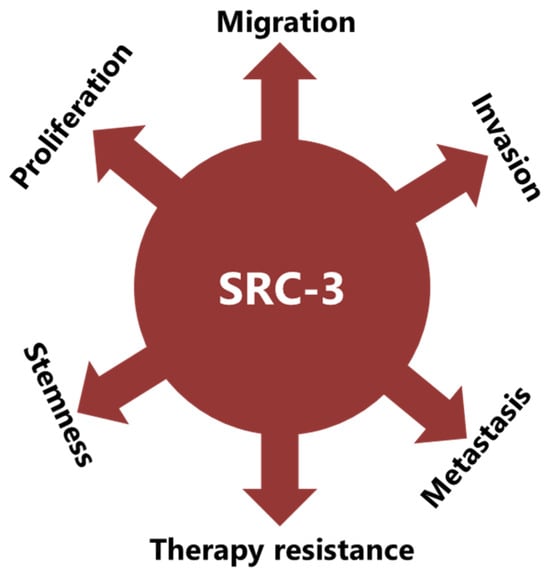
4. SRC-3 Promotes Malignant Behaviors of Tumor Cells
The implication of SRC-3 in the development and progression of many types of cancers has been reported [14,182][14][73]. Indeed, many reports have shown that SRC-3 is involved in carcinogenic processes via multiple pathways (Figure 1). However, SRC-3 has been most extensively studied in BCa. The story of a relationship between SRC-3 and BCa started about 20 years ago and SRC-3 is considered a proto-oncogene since its overexpression leads to BCa in mice [183][74]. Although both overexpression and amplification of SRC-3 are reported in BCa, its overexpression is much more common compared to gene amplification [11,184,185][11][75][76]. Furthermore, it has been shown that SRC-3 levels are higher in the advanced stages of the disease and that higher SRC-3 levels are associated with poor prognosis in ER(+) BCa [11,186,187,188,189,190][11][77][78][79][80][81]. The effects of SRC-3 in BCa pathogenesis were shown in mice in which SRC-3 was deleted or overexpressed. It was demonstrated that elevating SRC-3 abundance results in hyperplasia and consequently breast adenocarcinoma in mice [183,191,192][74][82][83]. Interestingly, even moderate overexpression of SRC-3 causes pre-malignant transformation in the mammary epithelium [193][84]. Conversely, SRC-3 deficiency inhibits both v-Ha-ras and chemical carcinogen-induced BCa [194,195][85][86]. Furthermore, SRC-3 directly interacts with ER-α in the presence of estrogen, recruits other co-regulators, and consequently increases the transcriptional activity of ER-α to promote cell proliferation [196,197,198][87][88][89]. Thereby, SRC-3 is involved in the pathogenesis of ER(+) BCa and promotes the malignant behavior of overexpressing cells. In this model, SRC-3 is the primary co-regulator for ER-α activity, and its binding allows the sequential binding of secondary co-regulators which are p300/CBP and CARM1 [199][90]. However, SRC-3 may interact with the mutant estrogen receptor, which is activated in a ligand-independent manner (in the absence of estrogen) [200,201][91][92]. In addition, if we discussing IMPC tumors in terms of SRC-3 activity and levels, although the direct effects of SRC-3 in IMPC pathogenesis are not yet known, it is highly likely that it is tumor-promoting, since it both controls the expression of HER2 and is a co-activator of the ER. HER2 status/level is a well-known prognostic biomarker for invasive BCa and its level was shown to be increased in SRC-3-overexpressing BCa cells [189,202,203][80][93][94]. Similarly, the SRC-3 level is higher in DCIS lesions compared to the corresponding normal breast tissue, and an elevated SRC-3 level in DCIS lesions causes an increase in the HER2 and HER3 levels and augments their corresponding signaling activities [204][95]. Furthermore, SRC-3 overexpression promotes ER(+) ADH lesions which have been considered as the earliest DCIS-related lesions in vivo [205][96]. In concordance, conditional knock out of SRC-3 results in a significant reduction in the populations of breast cancer initiating cells and myoepithelial progenitor cells, and consequently a decrease in the DCIS lesions [204][95]. It has been shown that SRC-3 is also involved in the production and secretion of growth factors, and thereby is involved in the regulation of growth factor signaling. For example, SRC-3 overexpression results in an increase in the IGF-I mRNA and protein levels, as well as the components of the IGF-I signaling mechanism, such as IGF-I receptor β (IGF-IRβ) [8,183,206][8][74][97]. In addition, the SRC-3 expression level is positively correlated with HER2, and this event is associated with tamoxifen resistance [189,203][80][94]. In concordance, breast tumorigenesis induced by HER2 was completely inhibited in SRC-3 deficient mice [202][93]. SRC-3 was implicated in the migration, invasion, and metastasis processes: it was namely shown that SRC-3 overexpression results in an increase in the MMP-7 and MMP-10 levels and thereby promotes metastasis [137][25]. SRC-3 promotes FAK activation, and also functions as an adapter molecule between EGFR and FAK and consequently promotes cell migration in BCa [207,208][98][99]. SRC-3 also promotes EMT in cancer cells through the classical cadherin switching mechanism, by which E-cadherin is replaced by N-cadherin [16]. This transition mechanism is crucial for tumor cells to gain migrative abilities and is considered as one of the initial steps in the invasion and metastasis processes of cancer cells [209][100]. Rohira et al. have shown that SRC-3 overexpression induces Snail 1 and Snail 2 expressions and thereby decreases E-cadherin level, and in concordance, Vimentin and N-cadherin levels increase in SRC-3 overexpressing cancer cells [16]. E-cadherin is a glycoprotein in epithelial cells and is crucial for the establishment of adherens junctions between neighboring cells [210][101]. Each E-cadherin molecule has a large extracellular region, a transmembrane region, and a short cytoplasmic domain [211][102]. The extracellular region consists of five extracellular cadherin domains and interacts with the extracellular region of cadherin in neighboring cells. The cytoplasmic domain of E-cadherin interacts with the cytoskeleton through catenin proteins. E-cadherin loss is observed in the advanced stages of many cancers, including BCa, and this event is a strong marker of EMT [212][103]. Cadherin switching in advanced stages of cancers is generally associated with an increase in the invasive and metastatic potential of cancer cells, and this event may be the result of various mechanisms that are triggered by genetic or epigenetic alterations [212,213][103][104]. Furthermore, it may also be a result of therapeutic approaches, such as androgen deprivation therapy in prostate cancer [214][105].References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249.
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48.
- Faltas, C.L.; LeBron, K.A.; Holz, M.K. Unconventional Estrogen Signaling in Health and Disease. Endocrinology 2020, 161, bqaa030.
- Clusan, L.; Ferriere, F.; Flouriot, G.; Pakdel, F. A Basic Review on Estrogen Receptor Signaling Pathways in Breast Cancer. Int. J. Mol. Sci. 2023, 24, 6834.
- Fuentes, N.; Silveyra, P. Estrogen receptor signaling mechanisms. Adv. Protein Chem. Struct. Biol. 2019, 116, 135–170.
- Xu, J.; Wu, R.C.; O’Malley, B.W. Normal and cancer-related functions of the p160 steroid receptor co-activator (SRC) family. Nat. Rev. Cancer 2009, 9, 615–630.
- Stashi, E.; York, B.; O’Malley, B.W. Steroid receptor coactivators: Servants and masters for control of systems metabolism. Trends Endocrinol. Metab. 2014, 25, 337–347.
- Xu, J.; Liao, L.; Ning, G.; Yoshida-Komiya, H.; Deng, C.; O’Malley, B.W. The steroid receptor coactivator SRC-3 (p/CIP/RAC3/AIB1/ACTR/TRAM-1) is required for normal growth, puberty, female reproductive function, and mammary gland development. Proc. Natl. Acad. Sci. USA 2000, 97, 6379–6384.
- Wu, R.C.; Qin, J.; Yi, P.; Wong, J.; Tsai, S.Y.; Tsai, M.J.; O’Malley, B.W. Selective phosphorylations of the SRC-3/AIB1 coactivator integrate genomic reponses to multiple cellular signaling pathways. Mol. Cell 2004, 15, 937–949.
- Zheng, F.F.; Wu, R.C.; Smith, C.L.; O’Malley, B.W. Rapid estrogen-induced phosphorylation of the SRC-3 coactivator occurs in an extranuclear complex containing estrogen receptor. Mol. Cell. Biol. 2005, 25, 8273–8284.
- Anzick, S.L.; Kononen, J.; Walker, R.L.; Azorsa, D.O.; Tanner, M.M.; Guan, X.Y.; Sauter, G.; Kallioniemi, O.P.; Trent, J.M.; Meltzer, P.S. AIB1, a steroid receptor coactivator amplified in breast and ovarian cancer. Science 1997, 277, 965–968.
- Guan, X.Y.; Xu, J.; Anzick, S.L.; Zhang, H.; Trent, J.M.; Meltzer, P.S. Hybrid selection of transcribed sequences from microdissected DNA: Isolation of genes within amplified region at 20q11-q13.2 in breast cancer. Cancer Res. 1996, 56, 3446–3450.
- Gojis, O.; Rudraraju, B.; Gudi, M.; Hogben, K.; Sousha, S.; Coombes, R.C.; Cleator, S.; Palmieri, C. The role of SRC-3 in human breast cancer. Nat. Rev. Clin. Oncol. 2010, 7, 83–89.
- Li, L.; Deng, C.X.; Chen, Q. SRC-3, a Steroid Receptor Coactivator: Implication in Cancer. Int. J. Mol. Sci. 2021, 22, 4760.
- Nikolai, B.C.; Jain, P.; Cardenas, D.L.; York, B.; Feng, Q.; McKenna, N.J.; Dasgupta, S.; Lonard, D.M.; O’Malley, B.W. Steroid receptor coactivator 3 (SRC-3/AIB1) is enriched and functional in mouse and human Tregs. Sci. Rep. 2021, 11, 3441.
- Rohira, A.D.; Yan, F.; Wang, L.; Wang, J.; Zhou, S.; Lu, A.; Yu, Y.; Xu, J.; Lonard, D.M.; O’Malley, B.W. Targeting SRC Coactivators Blocks the Tumor-Initiating Capacity of Cancer Stem-like Cells. Cancer Res. 2017, 77, 4293–4304.
- Kiliti, A.J.; Sharif, G.M.; Martin, M.B.; Wellstein, A.; Riegel, A.T. AIB1/SRC-3/NCOA3 function in estrogen receptor alpha positive breast cancer. Front. Endocrinol. 2023, 14, 1250218.
- Li, C.; Liang, Y.Y.; Feng, X.H.; Tsai, S.Y.; Tsai, M.J.; O’Malley, B.W. Essential phosphatases and a phospho-degron are critical for regulation of SRC-3/AIB1 coactivator function and turnover. Mol. Cell 2008, 31, 835–849.
- York, B.; Yu, C.; Sagen, J.V.; Liu, Z.; Nikolai, B.C.; Wu, R.C.; Finegold, M.; Xu, J.; O’Malley, B.W. Reprogramming the posttranslational code of SRC-3 confers a switch in mammalian systems biology. Proc. Natl. Acad. Sci. USA 2010, 107, 11122–11127.
- Liao, L.; Kuang, S.Q.; Yuan, Y.; Gonzalez, S.M.; O’Malley, B.W.; Xu, J. Molecular structure and biological function of the cancer-amplified nuclear receptor coactivator SRC-3/AIB1. J. Steroid Biochem. Mol. Biol. 2002, 83, 3–14.
- Wang, L.; Lonard, D.M.; O’Malley, B.W. The Role of Steroid Receptor Coactivators in Hormone Dependent Cancers and Their Potential as Therapeutic Targets. Horm. Cancer 2016, 7, 229–235.
- Yan, J.; Tsai, S.Y.; Tsai, M.J. SRC-3/AIB1: Transcriptional coactivator in oncogenesis. Acta Pharmacol. Sin. 2006, 27, 387–394.
- Wu, M.Y.; Fu, J.; Xu, J.; O’Malley, B.W.; Wu, R.C. Steroid receptor coactivator 3 regulates autophagy in breast cancer cells through macrophage migration inhibitory factor. Cell Res. 2012, 22, 1003–1021.
- Louie, M.C.; Zou, J.X.; Rabinovich, A.; Chen, H.W. ACTR/AIB1 functions as an E2F1 coactivator to promote breast cancer cell proliferation and antiestrogen resistance. Mol. Cell. Biol. 2004, 24, 5157–5171.
- Qin, L.; Liao, L.; Redmond, A.; Young, L.; Yuan, Y.; Chen, H.; O’Malley, B.W.; Xu, J. The AIB1 oncogene promotes breast cancer metastasis by activation of PEA3-mediated matrix metalloproteinase 2 (MMP2) and MMP9 expression. Mol. Cell. Biol. 2008, 28, 5937–5950.
- Goel, A.; Janknecht, R. Concerted activation of ETS protein ER81 by p160 coactivators, the acetyltransferase p300 and the receptor tyrosine kinase HER2/Neu. J. Biol. Chem. 2004, 279, 14909–14916.
- Myers, E.; Hill, A.D.; Kelly, G.; McDermott, E.W.; O’Higgins, N.J.; Buggy, Y.; Young, L.S. Associations and interactions between Ets-1 and Ets-2 and coregulatory proteins, SRC-1, AIB1, and NCoR in breast cancer. Clin. Cancer Res. 2005, 11, 2111–2122.
- He, C.; Shan, N.; Xu, P.; Ge, H.; Yuan, Y.; Liu, Y.; Zhang, P.; Wen, L.; Zhang, F.; Xiong, L.; et al. Hypoxia-induced Downregulation of SRC-3 Suppresses Trophoblastic Invasion and Migration Through Inhibition of the AKT/mTOR Pathway: Implications for the Pathogenesis of Preeclampsia. Sci. Rep. 2019, 9, 10349.
- Long, W.; Foulds, C.E.; Qin, J.; Liu, J.; Ding, C.; Lonard, D.M.; Solis, L.M.; Wistuba, I.I.; Qin, J.; Tsai, S.Y.; et al. ERK3 signals through SRC-3 coactivator to promote human lung cancer cell invasion. J. Clin. Invest. 2012, 122, 1869–1880.
- Louet, J.F.; Coste, A.; Amazit, L.; Tannour-Louet, M.; Wu, R.C.; Tsai, S.Y.; Tsai, M.J.; Auwerx, J.; O’Malley, B.W. Oncogenic steroid receptor coactivator-3 is a key regulator of the white adipogenic program. Proc. Natl. Acad. Sci. USA 2006, 103, 17868–17873.
- Coste, A.; Louet, J.F.; Lagouge, M.; Lerin, C.; Antal, M.C.; Meziane, H.; Schoonjans, K.; Puigserver, P.; O’Malley, B.W.; Auwerx, J. The genetic ablation of SRC-3 protects against obesity and improves insulin sensitivity by reducing the acetylation of PGC-1alpha. Proc. Natl. Acad. Sci. USA 2008, 105, 17187–17192.
- Polanczyk, M.J.; Hopke, C.; Vandenbark, A.A.; Offner, H. Treg suppressive activity involves estrogen-dependent expression of programmed death-1 (PD-1). Int. Immunol. 2007, 19, 337–343.
- Prieto, G.A.; Rosenstein, Y. Oestradiol potentiates the suppressive function of human CD4 CD25 regulatory T cells by promoting their proliferation. Immunology 2006, 118, 58–65.
- Lauritsen, K.J.; List, H.J.; Reiter, R.; Wellstein, A.; Riegel, A.T. A role for TGF-beta in estrogen and retinoid mediated regulation of the nuclear receptor coactivator AIB1 in MCF-7 breast cancer cells. Oncogene 2002, 21, 7147–7155.
- Hill, J.A.; Hall, J.A.; Sun, C.M.; Cai, Q.; Ghyselinck, N.; Chambon, P.; Belkaid, Y.; Mathis, D.; Benoist, C. Retinoic acid enhances Foxp3 induction indirectly by relieving inhibition from CD4+CD44hi Cells. Immunity 2008, 29, 758–770.
- Xiao, S.; Jin, H.; Korn, T.; Liu, S.M.; Oukka, M.; Lim, B.; Kuchroo, V.K. Retinoic acid increases Foxp3+ regulatory T cells and inhibits development of Th17 cells by enhancing TGF-beta-driven Smad3 signaling and inhibiting IL-6 and IL-23 receptor expression. J. Immunol. 2008, 181, 2277–2284.
- Mucida, D.; Park, Y.; Kim, G.; Turovskaya, O.; Scott, I.; Kronenberg, M.; Cheroutre, H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science 2007, 317, 256–260.
- He, Z.; Zhang, J.; Du, Q.; Xu, J.; Gwack, Y.; Sun, Z. SRC3 Is a Cofactor for RORgammat in Th17 Differentiation but Not Thymocyte Development. J. Immunol. 2019, 202, 760–769.
- Gilad, Y.; Lonard, D.M.; O’Malley, B.W. Steroid receptor coactivators—Their role in immunity. Front. Immunol. 2022, 13, 1079011.
- Tanaka, K.; Martinez, G.J.; Yan, X.; Long, W.; Ichiyama, K.; Chi, X.; Kim, B.S.; Reynolds, J.M.; Chung, Y.; Tanaka, S.; et al. Regulation of Pathogenic T Helper 17 Cell Differentiation by Steroid Receptor Coactivator-3. Cell Rep. 2018, 23, 2318–2329.
- Moraitis, A.N.; Giguere, V.; Thompson, C.C. Novel mechanism of nuclear receptor corepressor interaction dictated by activation function 2 helix determinants. Mol. Cell. Biol. 2002, 22, 6831–6841.
- Wang, J.; Peng, Z.; Guo, J.; Wang, Y.; Wang, S.; Jiang, H.; Wang, M.; Xie, Y.; Li, X.; Hu, M.; et al. CXCL10 Recruitment of gammadelta T Cells into the Hypoxic Bone Marrow Environment Leads to IL17 Expression and Multiple Myeloma Progression. Cancer Immunol. Res. 2023, 11, 1384–1399.
- Liu, J.; Xie, Y.; Guo, J.; Li, X.; Wang, J.; Jiang, H.; Peng, Z.; Wang, J.; Wang, S.; Li, Q.; et al. Targeting NSD2-mediated SRC-3 liquid-liquid phase separation sensitizes bortezomib treatment in multiple myeloma. Nat. Commun. 2021, 12, 1022.
- Alvarado, C.V.; Rubio, M.F.; Fernandez Larrosa, P.N.; Panelo, L.C.; Azurmendi, P.J.; Ruiz Grecco, M.; Martinez-Noel, G.A.; Costas, M.A. The levels of RAC3 expression are up regulated by TNF in the inflammatory response. FEBS Open Bio 2014, 4, 450–457.
- Grinberg-Bleyer, Y.; Caron, R.; Seeley, J.J.; De Silva, N.S.; Schindler, C.W.; Hayden, M.S.; Klein, U.; Ghosh, S. The Alternative NF-kappaB Pathway in Regulatory T Cell Homeostasis and Suppressive Function. J. Immunol. 2018, 200, 2362–2371.
- Long, M.; Park, S.G.; Strickland, I.; Hayden, M.S.; Ghosh, S. Nuclear factor-kappaB modulates regulatory T cell development by directly regulating expression of Foxp3 transcription factor. Immunity 2009, 31, 921–931.
- Ruan, Q.; Kameswaran, V.; Tone, Y.; Li, L.; Liou, H.C.; Greene, M.I.; Tone, M.; Chen, Y.H. Development of Foxp3+ regulatory t cells is driven by the c-Rel enhanceosome. Immunity 2009, 31, 932–940.
- Chen, W.; Zhuo, M.; Lu, X.; Xia, X.; Zhao, Y.; Huang, Z.; Xu, J.; Li, W.; Yu, C. SRC-3 protects intestine from DSS-induced colitis by inhibiting inflammation and promoting goblet cell differentiation through enhancement of KLF4 expression. Int. J. Biol. Sci. 2018, 14, 2051–2064.
- Mullany, L.K.; Rohira, A.D.; Leach, J.P.; Kim, J.H.; Monroe, T.O.; Ortiz, A.R.; Stork, B.; Gaber, M.W.; Sarkar, P.; Sikora, A.G.; et al. A steroid receptor coactivator stimulator (MCB-613) attenuates adverse remodeling after myocardial infarction. Proc. Natl. Acad. Sci. USA 2020, 117, 31353–31364.
- Yu, C.; York, B.; Wang, S.; Feng, Q.; Xu, J.; O’Malley, B.W. An essential function of the SRC-3 coactivator in suppression of cytokine mRNA translation and inflammatory response. Mol. Cell 2007, 25, 765–778.
- Chen, Q.; Chen, T.; Xu, Y.; Zhu, J.; Jiang, Y.; Zhao, Y.; Xu, J.; Yu, C. Steroid receptor coactivator 3 is required for clearing bacteria and repressing inflammatory response in Escherichia coli-induced septic peritonitis. J. Immunol. 2010, 185, 5444–5452.
- Alfonso-Prieto, M.; Biarnes, X.; Vidossich, P.; Rovira, C. The molecular mechanism of the catalase reaction. J. Am. Chem. Soc. 2009, 131, 11751–11761.
- Colo, G.P.; Rosato, R.R.; Grant, S.; Costas, M.A. RAC3 down-regulation sensitizes human chronic myeloid leukemia cells to TRAIL-induced apoptosis. FEBS Lett. 2007, 581, 5075–5081.
- Colo, G.P.; Rubio, M.F.; Nojek, I.M.; Werbajh, S.E.; Echeverria, P.C.; Alvarado, C.V.; Nahmod, V.E.; Galigniana, M.D.; Costas, M.A. The p160 nuclear receptor co-activator RAC3 exerts an anti-apoptotic role through a cytoplasmatic action. Oncogene 2008, 27, 2430–2444.
- Chen, W.; Lu, X.; Chen, Y.; Li, M.; Mo, P.; Tong, Z.; Wang, W.; Wan, W.; Su, G.; Xu, J.; et al. Steroid Receptor Coactivator 3 Contributes to Host Defense against Enteric Bacteria by Recruiting Neutrophils via Upregulation of CXCL2 Expression. J. Immunol. 2017, 198, 1606–1615.
- Werbajh, S.; Nojek, I.; Lanz, R.; Costas, M.A. RAC-3 is a NF-kappa B coactivator. FEBS Lett. 2000, 485, 195–199.
- Wu, R.C.; Qin, J.; Hashimoto, Y.; Wong, J.; Xu, J.; Tsai, S.Y.; Tsai, M.J.; O’Malley, B.W. Regulation of SRC-3 (pCIP/ACTR/AIB-1/RAC-3/TRAM-1) Coactivator activity by I kappa B kinase. Mol. Cell. Biol. 2002, 22, 3549–3561.
- Truong, T.H.; Hu, H.; Temiz, N.A.; Hagen, K.M.; Girard, B.J.; Brady, N.J.; Schwertfeger, K.L.; Lange, C.A.; Ostrander, J.H. Cancer Stem Cell Phenotypes in ER+ Breast Cancer Models Are Promoted by PELP1/AIB1 Complexes. Mol. Cancer Res. 2018, 16, 707–719.
- Truong, T.H.; Benner, E.A.; Hagen, K.M.; Temiz, N.A.; Kerkvliet, C.P.; Wang, Y.; Cortes-Sanchez, E.; Yang, C.H.; Trousdell, M.C.; Pengo, T.; et al. PELP1/SRC-3-dependent regulation of metabolic PFKFB kinases drives therapy resistant ER+ breast cancer. Oncogene 2021, 40, 4384–4397.
- Panelo, L.C.; Machado, M.S.; Rubio, M.F.; Jaworski, F.; Alvarado, C.V.; Paz, L.A.; Urtreger, A.J.; Vazquez, E.; Costas, M.A. High RAC3 expression levels are required for induction and maintaining of cancer cell stemness. Oncotarget 2018, 9, 5848–5860.
- Dubrovska, A.; Hartung, A.; Bouchez, L.C.; Walker, J.R.; Reddy, V.A.; Cho, C.Y.; Schultz, P.G. CXCR4 activation maintains a stem cell population in tamoxifen-resistant breast cancer cells through AhR signalling. Br. J. Cancer 2012, 107, 43–52.
- Miyoshi, Y.; Shien, T.; Ogiya, A.; Ishida, N.; Yamazaki, K.; Horii, R.; Horimoto, Y.; Masuda, N.; Yasojima, H.; Inao, T.; et al. Differences in expression of the cancer stem cell marker aldehyde dehydrogenase 1 among estrogen receptor-positive/human epidermal growth factor receptor type 2-negative breast cancer cases with early, late, and no recurrence. Breast Cancer Res. 2016, 18, 73.
- Dancik, G.M.; Voutsas, I.F.; Vlahopoulos, S. Aldehyde Dehydrogenase Enzyme Functions in Acute Leukemia Stem Cells. Front. Biosci. (Schol. Ed.) 2022, 14, 8.
- Liu, C.; Qiang, J.; Deng, Q.; Xia, J.; Deng, L.; Zhou, L.; Wang, D.; He, X.; Liu, Y.; Zhao, B.; et al. ALDH1A1 Activity in Tumor-Initiating Cells Remodels Myeloid-Derived Suppressor Cells to Promote Breast Cancer Progression. Cancer Res. 2021, 81, 5919–5934.
- Huang, Y.; Duan, X.; Wang, Z.; Sun, Y.; Guan, Q.; Kang, L.; Zhang, Q.; Fang, L.; Li, J.; Wong, J. An acetylation-enhanced interaction between transcription factor Sox2 and the steroid receptor coactivators facilitates Sox2 transcriptional activity and function. J. Biol. Chem. 2021, 297, 101389.
- Domenici, G.; Aurrekoetxea-Rodriguez, I.; Simoes, B.M.; Rabano, M.; Lee, S.Y.; Millan, J.S.; Comaills, V.; Oliemuller, E.; Lopez-Ruiz, J.A.; Zabalza, I.; et al. A Sox2-Sox9 signalling axis maintains human breast luminal progenitor and breast cancer stem cells. Oncogene 2019, 38, 3151–3169.
- Leung, E.Y.; Askarian-Amiri, M.E.; Sarkar, D.; Ferraro-Peyret, C.; Joseph, W.R.; Finlay, G.J.; Baguley, B.C. Endocrine Therapy of Estrogen Receptor-Positive Breast Cancer Cells: Early Differential Effects on Stem Cell Markers. Front. Oncol. 2017, 7, 184.
- Percharde, M.; Azuara, V. Essential roles for the nuclear receptor coactivator Ncoa3 in pluripotency. Cell Cycle 2013, 12, 195–196.
- Percharde, M.; Lavial, F.; Ng, J.H.; Kumar, V.; Tomaz, R.A.; Martin, N.; Yeo, J.C.; Gil, J.; Prabhakar, S.; Ng, H.H.; et al. Ncoa3 functions as an essential Esrrb coactivator to sustain embryonic stem cell self-renewal and reprogramming. Genes Dev. 2012, 26, 2286–2298.
- Wu, Z.; Yang, M.; Liu, H.; Guo, H.; Wang, Y.; Cheng, H.; Chen, L. Role of nuclear receptor coactivator 3 (Ncoa3) in pluripotency maintenance. J. Biol. Chem. 2012, 287, 38295–38304.
- Chitilian, J.M.; Thillainadesan, G.; Manias, J.L.; Chang, W.Y.; Walker, E.; Isovic, M.; Stanford, W.L.; Torchia, J. Critical components of the pluripotency network are targets for the p300/CBP interacting protein (p/CIP) in embryonic stem cells. Stem Cells 2014, 32, 204–215.
- Hu, M.; Zeng, H.; Chen, S.; Xu, Y.; Wang, S.; Tang, Y.; Wang, X.; Du, C.; Shen, M.; Chen, F.; et al. SRC-3 is involved in maintaining hematopoietic stem cell quiescence by regulation of mitochondrial metabolism in mice. Blood 2018, 132, 911–923.
- Ma, G.; Ren, Y.; Wang, K.; He, J. SRC-3 has a role in cancer other than as a nuclear receptor coactivator. Int. J. Biol. Sci. 2011, 7, 664–672.
- Torres-Arzayus, M.I.; Font de Mora, J.; Yuan, J.; Vazquez, F.; Bronson, R.; Rue, M.; Sellers, W.R.; Brown, M. High tumor incidence and activation of the PI3K/AKT pathway in transgenic mice define AIB1 as an oncogene. Cancer Cell 2004, 6, 263–274.
- Reiter, R.; Oh, A.S.; Wellstein, A.; Riegel, A.T. Impact of the nuclear receptor coactivator AIB1 isoform AIB1-Delta3 on estrogenic ligands with different intrinsic activity. Oncogene 2004, 23, 403–409.
- Reiter, R.; Wellstein, A.; Riegel, A.T. An isoform of the coactivator AIB1 that increases hormone and growth factor sensitivity is overexpressed in breast cancer. J. Biol. Chem. 2001, 276, 39736–39741.
- Bautista, S.; Valles, H.; Walker, R.L.; Anzick, S.; Zeillinger, R.; Meltzer, P.; Theillet, C. In breast cancer, amplification of the steroid receptor coactivator gene AIB1 is correlated with estrogen and progesterone receptor positivity. Clin. Cancer Res. 1998, 4, 2925–2929.
- Hudelist, G.; Czerwenka, K.; Kubista, E.; Marton, E.; Pischinger, K.; Singer, C.F. Expression of sex steroid receptors and their co-factors in normal and malignant breast tissue: AIB1 is a carcinoma-specific co-activator. Breast Cancer Res. Treat. 2003, 78, 193–204.
- Lee, K.; Lee, A.; Song, B.J.; Kang, C.S. Expression of AIB1 protein as a prognostic factor in breast cancer. World J. Surg. Oncol. 2011, 9, 139.
- Osborne, C.K.; Bardou, V.; Hopp, T.A.; Chamness, G.C.; Hilsenbeck, S.G.; Fuqua, S.A.; Wong, J.; Allred, D.C.; Clark, G.M.; Schiff, R. Role of the estrogen receptor coactivator AIB1 (SRC-3) and HER-2/neu in tamoxifen resistance in breast cancer. J. Natl. Cancer Inst. 2003, 95, 353–361.
- Zhao, C.; Yasui, K.; Lee, C.J.; Kurioka, H.; Hosokawa, Y.; Oka, T.; Inazawa, J. Elevated expression levels of NCOA3, TOP1, and TFAP2C in breast tumors as predictors of poor prognosis. Cancer 2003, 98, 18–23.
- Nakles, R.E.; Shiffert, M.T.; Diaz-Cruz, E.S.; Cabrera, M.C.; Alotaiby, M.; Miermont, A.M.; Riegel, A.T.; Furth, P.A. Altered AIB1 or AIB1Delta3 expression impacts ERalpha effects on mammary gland stromal and epithelial content. Mol. Endocrinol. 2011, 25, 549–563.
- Tilli, M.T.; Reiter, R.; Oh, A.S.; Henke, R.T.; McDonnell, K.; Gallicano, G.I.; Furth, P.A.; Riegel, A.T. Overexpression of an N-terminally truncated isoform of the nuclear receptor coactivator amplified in breast cancer 1 leads to altered proliferation of mammary epithelial cells in transgenic mice. Mol. Endocrinol. 2005, 19, 644–656.
- Avivar, A.; Garcia-Macias, M.C.; Ascaso, E.; Herrera, G.; O’Connor, J.E.; Font de Mora, J. Moderate overexpression of AIB1 triggers pre-neoplastic changes in mammary epithelium. FEBS Lett. 2006, 580, 5222–5226.
- Kuang, S.Q.; Liao, L.; Wang, S.; Medina, D.; O’Malley, B.W.; Xu, J. Mice lacking the amplified in breast cancer 1/steroid receptor coactivator-3 are resistant to chemical carcinogen-induced mammary tumorigenesis. Cancer Res. 2005, 65, 7993–8002.
- Kuang, S.Q.; Liao, L.; Zhang, H.; Lee, A.V.; O’Malley, B.W.; Xu, J. AIB1/SRC-3 deficiency affects insulin-like growth factor I signaling pathway and suppresses v-Ha-ras-induced breast cancer initiation and progression in mice. Cancer Res. 2004, 64, 1875–1885.
- Planas-Silva, M.D.; Shang, Y.; Donaher, J.L.; Brown, M.; Weinberg, R.A. AIB1 enhances estrogen-dependent induction of cyclin D1 expression. Cancer Res. 2001, 61, 3858–3862.
- Suen, C.S.; Berrodin, T.J.; Mastroeni, R.; Cheskis, B.J.; Lyttle, C.R.; Frail, D.E. A transcriptional coactivator, steroid receptor coactivator-3, selectively augments steroid receptor transcriptional activity. J. Biol. Chem. 1998, 273, 27645–27653.
- Tikkanen, M.K.; Carter, D.J.; Harris, A.M.; Le, H.M.; Azorsa, D.O.; Meltzer, P.S.; Murdoch, F.E. Endogenously expressed estrogen receptor and coactivator AIB1 interact in MCF-7 human breast cancer cells. Proc. Natl. Acad. Sci. USA 2000, 97, 12536–12540.
- Yi, P.; Wang, Z.; Feng, Q.; Chou, C.K.; Pintilie, G.D.; Shen, H.; Foulds, C.E.; Fan, G.; Serysheva, I.; Ludtke, S.J.; et al. Structural and Functional Impacts of ER Coactivator Sequential Recruitment. Mol. Cell 2017, 67, 733–743.e4.
- Fanning, S.W.; Mayne, C.G.; Dharmarajan, V.; Carlson, K.E.; Martin, T.A.; Novick, S.J.; Toy, W.; Green, B.; Panchamukhi, S.; Katzenellenbogen, B.S.; et al. Estrogen receptor alpha somatic mutations Y537S and D538G confer breast cancer endocrine resistance by stabilizing the activating function-2 binding conformation. eLife 2016, 5, e12792.
- Zhao, Y.; Laws, M.J.; Guillen, V.S.; Ziegler, Y.; Min, J.; Sharma, A.; Kim, S.H.; Chu, D.; Park, B.H.; Oesterreich, S.; et al. Structurally Novel Antiestrogens Elicit Differential Responses from Constitutively Active Mutant Estrogen Receptors in Breast Cancer Cells and Tumors. Cancer Res. 2017, 77, 5602–5613.
- Fereshteh, M.P.; Tilli, M.T.; Kim, S.E.; Xu, J.; O’Malley, B.W.; Wellstein, A.; Furth, P.A.; Riegel, A.T. The nuclear receptor coactivator amplified in breast cancer-1 is required for Neu (ErbB2/HER2) activation, signaling, and mammary tumorigenesis in mice. Cancer Res. 2008, 68, 3697–3706.
- Kirkegaard, T.; McGlynn, L.M.; Campbell, F.M.; Muller, S.; Tovey, S.M.; Dunne, B.; Nielsen, K.V.; Cooke, T.G.; Bartlett, J.M. Amplified in breast cancer 1 in human epidermal growth factor receptor—Positive tumors of tamoxifen-treated breast cancer patients. Clin. Cancer Res. 2007, 13, 1405–1411.
- Ory, V.; Tassi, E.; Cavalli, L.R.; Sharif, G.M.; Saenz, F.; Baker, T.; Schmidt, M.O.; Mueller, S.C.; Furth, P.A.; Wellstein, A.; et al. The nuclear coactivator amplified in breast cancer 1 maintains tumor-initiating cells during development of ductal carcinoma in situ. Oncogene 2014, 33, 3033–3042.
- Torres-Arzayus, M.I.; Zhao, J.; Bronson, R.; Brown, M. Estrogen-dependent and estrogen-independent mechanisms contribute to AIB1-mediated tumor formation. Cancer Res. 2010, 70, 4102–4111.
- Wang, Z.; Rose, D.W.; Hermanson, O.; Liu, F.; Herman, T.; Wu, W.; Szeto, D.; Gleiberman, A.; Krones, A.; Pratt, K.; et al. Regulation of somatic growth by the p160 coactivator p/CIP. Proc. Natl. Acad. Sci. USA 2000, 97, 13549–13554.
- Long, W.; Yi, P.; Amazit, L.; LaMarca, H.L.; Ashcroft, F.; Kumar, R.; Mancini, M.A.; Tsai, S.Y.; Tsai, M.J.; O’Malley, B.W. SRC-3Delta4 mediates the interaction of EGFR with FAK to promote cell migration. Mol. Cell 2010, 37, 321–332.
- Yan, J.; Erdem, H.; Li, R.; Cai, Y.; Ayala, G.; Ittmann, M.; Yu-Lee, L.Y.; Tsai, S.Y.; Tsai, M.J. Steroid receptor coactivator-3/AIB1 promotes cell migration and invasiveness through focal adhesion turnover and matrix metalloproteinase expression. Cancer Res. 2008, 68, 5460–5468.
- Loh, C.Y.; Chai, J.Y.; Tang, T.F.; Wong, W.F.; Sethi, G.; Shanmugam, M.K.; Chong, P.P.; Looi, C.Y. The E-Cadherin and N-Cadherin Switch in Epithelial-to-Mesenchymal Transition: Signaling, Therapeutic Implications, and Challenges. Cells 2019, 8, 1118.
- Pecina-Slaus, N. Tumor suppressor gene E-cadherin and its role in normal and malignant cells. Cancer Cell Int. 2003, 3, 17.
- Oda, H.; Takeichi, M. Evolution: Structural and functional diversity of cadherin at the adherens junction. J. Cell. Biol. 2011, 193, 1137–1146.
- Kaszak, I.; Witkowska-Pilaszewicz, O.; Niewiadomska, Z.; Dworecka-Kaszak, B.; Ngosa Toka, F.; Jurka, P. Role of Cadherins in Cancer-A Review. Int. J. Mol. Sci. 2020, 21, 7624.
- Varisli, L.; Tolan, V. Increased ROS alters E-/N-cadherin levels and promotes migration in prostate cancer cells. Bratisl. Lek. Listy 2022, 123, 752–757.
- Varisli, L.; Tolan, V.; Cen, J.H.; Vlahopoulos, S.; Cen, O. Dissecting the effects of androgen deprivation therapy on cadherin switching in advanced prostate cancer: A molecular perspective. Oncol. Res. 2022, 30, 137–155.
 Encyclopedia
Encyclopedia
