1. Introduction
Due to their low price, extreme durability, light weight, and good ductility, plastics are now widely used in the construction, healthcare, electronic components, automotive, agriculture, and food packaging industries
[1][2][3][4][1,2,3,4]. And because of the durability of plastics, they also pose a huge environmental hazard. Studies have shown that global plastic waste is expected to reach 270 million tons by 2060
[5]. Waste plastics undergo physical, chemical, biological, and other forms of wear, consumption, and decomposition, resulting in particles less than 5 mm in diameter being defined as microplastics (MPs) and particles less than 100 nm being defined as nanoparticles (NPs)
[6][7][8][9][6,7,8,9].
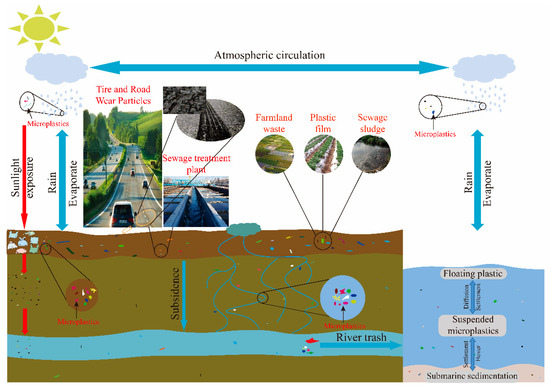
Figure 1 shows the source of microplastics. Plastic particles, cosmetics, laundry wastewater, sewage sludge, and atmospheric deposition generated during the friction between motor tires and road surfaces are also important sources of MPs
[10][11][12][10,11,12].
Figure 1. Source and migration characteristics of soil microplastics (MPs).
MPs can be absorbed by aquatic plants and animals and adversely affect their growth, development, and reproduction
[13][14][15][16][17][18][19][20][13,14,15,16,17,18,19,20]. On the other hand, MPs can absorb various pollutants in the ocean, such as antibiotics
[21][22][21,22], polycyclic aromatic hydrocarbons
[23][24][23,24], heavy metals
[25][26][27][25,26,27], organic compounds
[28][29][28,29], and pathogenic microorganisms
[30][31][32][30,31,32], and are capable of serving as carriers of pollutants that are fed on by aquatic organisms and thus entering the organisms. In addition, MPs can directly enter agricultural soils through sewage sludge, irrigation water, domestic water, and atmospheric deposition, or indirectly enter agricultural soils through the degradation of plastic residues (such as mulch films) in agricultural activities.
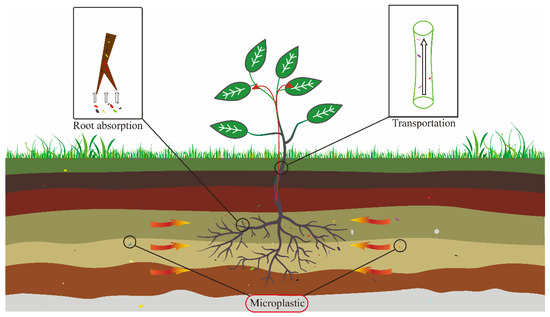
Figure 2 shows the pathway of MPs into plants. When absorbed by terrestrial plants, MPs will inhibit the growth and development of the plants and remain in the plant bodies. The accumulation of MPs in the plant body will eventually reach the human body along with the enrichment of the food chain, causing harm to the human body.
Figure 2. Absorption and transport of microplastics (MPs) in terrestrial plants. Arrows indicate microplastics absorbed by plants.
At present, most of the waste plastics in the world are disposed of in landfills
[33], which require a large amount of land. Due to the strong stability of plastic, it is not easy to decompose in the soil, which seriously affects the sustainable use of the soil. Moreover, a large number of microorganisms breed in the soil, producing harmful gases that adversely affect the surrounding air and environment
[34]. Not only that, but the leachate from plastic waste will also enter the river through groundwater, causing harm to the environment and ecology. With the deepening of research, there are an increasing number of treatment methods for waste plastics. The MPs contained in the sewage treated by the sewage treatment plant are reduced, but there are still many small particles that are difficult to remove. Adsorption, advanced oxidation processes (AOPs), and biodegradation can accelerate the degradation of MPs through a series of physical and chemical reactions, thus increasing their degradation rate
[35][36][35,36]. Among them, AOPs include direct photodegradation, photocatalytic oxidation, and electrochemical oxidation. However, there are shortcomings to these methods. The process of photodegradation is uncontrollable. Even under laboratory conditions, the degree of photoaging and the types of intermediates in the photochemical system cannot be completely determined. In addition, photodegradation consumes more energy. Prolonged exposure to sunlight may also cause light pollution
[37]. For photocatalytic oxidation, although it uses free solar energy, most plastics can only be partially degraded under ultraviolet radiation, and the degree of degradation is not up to the requirements. The catalyst added in the reaction is also difficult to recover and can easily result in secondary pollution
[35]. Electrochemical oxidation has broad application prospects in the treatment of degradable plastics due to its strong controllability, simple operation, and low secondary pollution. However, for these processes, the intermediate products obtained by their degradation are uncertain as to whether they are harmful or not, and it is difficult to control the reaction process. Therefore, much research is being conducted on the biodegradation process. MPs can serve as substrates for microbial biofilm growth and provide energy for microbial growth and reproduction. And the selection of biodegradation conditions is a key factor in improving the efficiency of degradation. pH is a critical factor for the survival of microorganisms, as it has a key influence on their life activities and substance metabolism
[38]. An increase or decrease in pH during biodegradation may be due to the production and accumulation of alkaline aromatic compounds or other metabolites during degradation. As the biofilm grows, the plastic structure breaks, and during the assimilation process, it is taken up by the microorganisms (bacteria, fungi) in the biofilm and finally decomposed into smaller molecules (CO, N
2, H
2, H
2O, H
2S)
[39]. These molecules are further used by microorganisms as a usable energy source and eventually returned to the atmosphere, completing the conversion from small molecules to usable products
[35]. Not only is it better than other processes in terms of energy savings, environmental pollution, and degradation efficiency, but the biodegradation of its intermediate products and final products will not cause secondary pollution, which is a more efficient and ideal degradation process. Therefore, bioremediation is also considered to be the most ideal method for removing MP contamination.
2. Physical and Chemical Processes
MPs are considered more serious persistent pollutants than plastics
[40]. In the past ten years, China and European countries have taken corresponding measures to limit the use of plastics by issuing laws and regulations to reduce the pollution of MPs from the source
[35]. However, the situation of plastic pollution around the world is still serious. In recent years, many studies have reported the degradation processes of MPs. These include physical and chemical methods.
2.1. Physical Law
Physical methods mainly include sol–gel, coagulation filtration, and adsorption. Structural composite silica gel is obtained by the sol–gel process to polymerize and interact with MPs
[41][42][41,42], and then separation technology is used to eliminate these agglomerates to eliminate the MPs. Coagulation filtration causes MPs to form larger agglomerates through coagulation to achieve the separation effect. The sol–gel process exhibits a pH-induced reaction and is more suitable for application in liquids
[43]. Excessive use of coagulants will cause secondary pollution and harm organisms. Leppänen et al.
[44] captured microplastics in the water column using a hygroscopic nanocellulose network. In addition, for a long time, biochar has been regarded as the most promising adsorbent due to its porous structure and easy fabrication, and the adsorption of pollutants has been widely studied
[45][46][45,46]. MPs can be adsorbed by different adsorbent materials through mechanisms including electrostatic interactions, hydrogen bonding interactions, and π-π interactions. Wang et al.
[47] studied a highly efficient Mg/Zn-modified magnetic biochar adsorbent for the removal of MPs from aqueous solutions with a maximum efficiency of 99.46%. Tiwari et al.
[48] studied the interaction between a Zn–Al layered double hydroxide (LDH) and MPs, indicating that the Zn–Al–LDH can adsorb MPs in water and that its efficiency can reach 164.49 mg/g. Sun et al.
[49] found that a sponge made of chitin and graphene oxide (ChGO) as raw materials can effectively adsorb different types of MPs. Even after multiple adsorption cycles, its efficiency can still reach 89.8%. Both magnetic and composite adsorbents have ideal removal efficiencies, but their material synthesis is complex and the cost is high; additionally, more research is needed to explore the adsorption mechanism of MPs; therefore, the development of MPs adsorbents will still be the focus of attention
[50].
2.2. Advanced Oxidation Processes (AOPs) Degradation
Some recent studies have shown that AOPs are an efficient chemical elimination technology that can lead to the formation of various reactive oxygen species (ROS), chemical chain scission, or cross-linking and exhibit excellent performance in degrading MPs
[51][52][51,52]. AOPs include three main methods: Direct photodegradation, photocatalytic oxidation, and electrochemical oxidation. The oxidation process, degradation mechanism, advantages and disadvantages, and future application prospects of the three processes will be discussed in detail below.
2.2.1. Direct Photodegradation
Direct photodegradation is the main transformation pathway of atmospheric organic matter and is considered an important process in the decomposition of hydrocarbons and polymers
[53][54][53,54]. At present, the degradation of MPs has also been studied. Different types of light are important factors influencing the photodegradation of MPs, with UV light having the greatest effect
[55]. Under strong UV irradiation, MPs can cause UV absorption of unsaturated surface groups, formation of polymer radicals, oxidation, hydrogen extraction, and chain scission or crosslinking
[56][57][56,57]. Ainali et al.
[58] analyzed pyrolysis-gas chromatography–mass spectrometry (Py-GC/MS), Fourier transform infrared spectroscopy (FTIR), X-ray diffraction (XRD), differential scanning calorimetry (DSC), scanning electron microscopy (SEM), and other methods to observe and analyze the degradation process of MPs. FTIR revealed that MPs formed new functional groups during UV irradiation, including carbonyl, vinyl, and hydroxyl/hydroperoxides, and XRD and DSC measurements enhanced the apparent effect of UV irradiation on their crystalline and thermal properties
[58]. SEM found significant morphological changes on the surface of MPs, demonstrating the degradation of plastic properties and their progressive fragility due to UV irradiation
[58]. In addition, the plastic degradation mechanisms of different types of MPs before and after UV irradiation were investigated by Py-GC/MS, demonstrating that the respective quantitative ratios of low molecular weight compounds and relatively high molecular weight hydrocarbons varied with UV irradiation
[58]. Zhu et al.
[37] studied PS-MPs after simulating sunlight for 150 days and observed obvious signs of aging: the surface roughness increased, and the particle size decreased. Ding et al.
[59] found that soils with different properties had different degradation rates during the same photodegradation process. The soil containing clay, iron oxides, and MnO
2 enhanced the degradation rate, while the soil containing organic carbon inhibited the degradation rate. Electrostatic interactions may be the dominant factor influencing the rate of photodegradation of MPs in soils with different properties. Wang et al.
[60] found that natural organic acids in the aqueous environment can promote the aging of PVC microplastics, which may be related to the hydroxyl radicals produced by the photolysis of these organic acids. Although modern technology can realize the degradation of MPs by artificial light sources, according to current research, its degradation efficiency cannot reach 100%. Coupled with its aging degree and the uncertainty of the type of intermediate products in this photochemical system, it may be harmful to the environment, causing secondary damage or even more harmful effects. It has been reported that the residues of photodegraded MPs can cause serious harm to organisms
[61][62][63][61,62,63]. In recent years, some studies have shown that the photocatalytic reaction based on Mie resonance can lead to the coupling reaction of carbon–carbon (C–C) in an aromatic polymer that can achieve a degradation effect
[64][65][64,65]. In addition, Kwon et al.
[66] synthesized different Cu
2−xS nanoparticles (CuS and Cu
1.8S NPs) with localized surface plasmon resonance (LSPR) absorbance in the near-infrared (NIR) region and photodegradation properties leading to polydimethylsiloxane (PDMS) polymer. In addition, photodegradation causes a slight amount of pollution in the environment. The emergence of new technologies offers new ideas for the degradation of polymers. Therefore, future research should focus on the toxicological analysis of intermediates in the photodegradation process and the development and application of new technologies.
2.2.2. Photocatalytic Oxidation
Photocatalytic oxidative degradation is a redox process that uses solar energy as an energy source and utilizes the free radicals generated by semiconductors to react with MPs to break the polymer chain, thereby initiating the degradation process of the MPs in order to achieve the effect of removal
[67]. At present, numerous studies have shown that nano-TiO
2 can be used in the photocatalytic degradation of MPs. The process is accompanied by the generation of hydroxyl, carbonyl, and hydrocarbon groups, resulting in a harder surface
[68][69][68,69]. Nabi et al.
[68] showed in their 2020 paper that the TiO
2 nanoparticle film had a high degradation efficiency of 98.40% for 400 nm PS within 12 h and the highest degradation efficiency for PE after 36 h. In addition, it was mentioned in a paper in 2021 that N–TiO
2 materials synthesized by two routes showed a good degradation effect on MPs
[70]. Not only on nano-TiO
2, Tofa et al.
[70] found in 2019 that zinc oxide (ZnO) nanorods (ZnO-Pt) also have a good effect on the degradation of MPs in water. The use of solar energy as an energy source is the greatest advantage of photocatalytic oxidation. Cao et al.
[71] successfully prepared a series of MXene/ZnxCd1-xS photocatalysts in 2022, which can degrade MPs and utilize light energy to catalyze hydrogen evolution. The optimal photocatalytic hydrogen evolution rate can reach 14.17 mmol/g/h, which solves the pollution and energy problems of MPs in one fell swoop. Zhou et al.
[72] concluded that Cu
2O is an excellent visible-light photocatalyst. In 2021, Zhu et al.
[73] successfully prepared a material (rGO@Fe
3O
4/Cu
2O@ZnO) with strong hydrophobicity, high photocatalytic performance, and recyclability, and its photocatalytic degradation efficiency of acrylamide (AM) reached 97.3%. In addition to this, a large number of studies have reported the study of photocatalysts such as Cu
2O
[74][75][74,75], α-Fe
2O
3 [76][77][76,77], Au
[78], Ag
[79], and Cu
[80] for polymer degradation. Photocatalytic oxidation can utilize solar energy and save energy. It is economically feasible to apply on a large scale, but the photocatalytic oxidation process may release volatile organic compounds (VOCs), which will inevitably have an impact on the environment. In addition, the catalyst is not easy to recycle, and the residue in the water will cause secondary pollution. Therefore, the future application of photocatalytic oxidative degradation technology needs further research.
2.2.3. Electrochemical Oxidation
At present, there are few studies on the effective degradation of MPs by electrochemical oxidation treatment. Miao et al.
[81] proposed a similar electro-Fenton degradation method for MPs based on a TiO
2/graphite (TiO
2/C) cathode, which also generates free radicals through electrode redox to react with the MPs and achieve degradation. This method will not result in secondary pollution, but its electrolysis intermediates are uncontrollable. Therefore, future research should focus on controlling the environmental impact of intermediates and final decomposition products.