Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Daniela Minerdi and Version 2 by Peter Tang.
Carbapenems are a group of broad-spectrum beta-lactam antibiotics that in many cases are the last effective defense against infections caused by multidrug-resistant bacteria, such as some strains of Klebsiella pneumoniae, Escherichia coli, Pseudomonas aeruginosa, and Acinetobacter baumannii. Resistance to carbapenems has emerged and is beginning to spread, becoming an ongoing public-health problem of global dimensions, causing serious outbreaks, and dramatically limiting treatment options.
- antibiotic resistance
- carbapenem resistance
- flavin monooxygenases
- Baeyer–Villiger monooxygenases
1. Introduction
Antibiotics, discovered approximately a century ago, are often regarded as miraculous drugs that have revolutionized the treatment of infectious diseases. They have been a cornerstone of modern medicine, and their availability has significantly contributed to public health improvements. However, their effectiveness and widespread use have sometimes been taken for granted. Lately, the efficacy of administered antibiotics has changed dramatically, giving rise to the phenomenon of antibiotic resistance, through which more and more human pathogenic bacteria have acquired the capability to withstand or tolerate the effects of an attack by one or multiple antibacterial molecules. Conventional treatments are becoming ineffective, and the loss of effectiveness in even last-resort antibiotics has become a grave concern in recent years [1]. This development has had significant consequences, including the persistence of infections and an increased risk of their spread to others. Every year, 700,000 people die globally from antibiotic resistance [2] and, by 2050, the United Nations estimates that the superbugs and associated forms of multidrug resistance are projected to cause the deaths of up to 10 million people annually, which is comparable to the number of deaths caused by cancer. Additionally, it is estimated that this global health threat will result in a staggering economic cost of approximately USD 100 trillion [2]. Bacteria causing common blood-stream infections, pneumonia, urinary tract infections are developing antibiotic resistance all over the world. A high percentage of nosocomial infections are caused by multidrug-resistant bacteria (i.e., Gram-negative bacteria resistant to all β-lactam antibiotics, methicillin-resistant Staphylococcus aureus, vancomycin-resistant enterococci). Patients affected by these types of infections not only have a high risk of death but also consume a substantial amount of healthcare resources, leading to significant health and macroeconomic consequences, particularly for emerging economies. The reliance on antibiotics for the treatment of infections places a strain on healthcare systems, resulting in increased healthcare costs and resource allocation challenges. In emerging countries, the economic burden of antibiotic use is particularly profound. Limited healthcare budgets and infrastructure strain are exacerbated by the high demand for antibiotics due to infectious disease prevalence. The cost of acquiring antibiotics, administering treatments, and managing potential complications adds to the economic burden, diverting resources that could be allocated to other critical healthcare needs. About 100 years after the first antibiotic treatment was given to the first infected patient, bacterial infections such as gonorrhea, pneumonia, and tuberculosis have become a threat once again, making the world face the possibility of a post-antibiotic era in which common infections could kill again unless immediate and counteractions are taken. About 70% of all pathogenic bacteria are resistant to at least one commercially available antibiotic [3] and antibiotic resistance may have worsened due to COVID-19 because of the overuse of antibiotics in humans [3].
Effluent from hospitals, agricultural runoff, and wastewater treatment facilities pose potential pathways for the dissemination of antibiotic-resistant bacteria and their associated resistance genes within soil and the surrounding ecosystems. Factors contributing to antibiotic resistance include the excessive and unregulated use of antibiotics, inadequate treatment processes, and the recycling of wastewater. This issue is further exacerbated by the misuse of antimicrobials within the medical, agricultural, and veterinary sectors. Additionally, the widespread presence of antimicrobial resistance within the food supply chain can be attributed to the excessive or inappropriate use of antimicrobials to combat infections in animals, plants, and humans. Moreover, the routine administration of antibiotics for growth promotion and disease prevention in healthy animals is eroding the effectiveness of these medications. Antimicrobial resistance is pervasive at the animal–human–environment interface, with the farm-to-fork continuum emerging as a potential reservoir for resistance development and dissemination. These environments may serve as repositories for genes associated with antibiotic resistance, which could potentially be acquired by pathogens, beneficial microorganisms related to food production, or other entities. Recognizing the food chain as a significant source of antimicrobial-resistant microorganisms highlights the potential for their dispersion at various stages of food production [4]. Until antibiotics had been synthetized, bacterial antibiotic resistance had been a natural phenomenon of adaptation of bacteria to toxic molecules in the environment for more than 2 billion years.
The anthropogenic application of a great amount of antibiotics in medicine and agriculture for many years has led to a significant evolution of the mechanism of resistance in a relatively short period of time.
2. Antibiotic Resistance Mechanisms
The ability of bacteria to withstand the effects of antibiotic molecules can be attributed to several factors, some of which are inherent to the bacteria themselves. This intrinsic resistance arises from specific characteristics within the bacterial structure or physiology. Specifically, the ability to withstand the effects of antibiotic agents may originate from inherent bacterial mechanisms. These mechanisms could result from the absence of a specific target, variations in the composition of the cytoplasmic membrane, or the antibiotic molecule’s incapability to traverse the outer membrane. In parallel with intrinsic resistance, bacteria possess the capability to acquire resistance through diverse mechanisms, which usually encompass a minimum of four distinct methods. These methods not only underscore the adaptability of bacterial species but also emphasize the multifaceted nature of antibiotic resistance development.2.1. Modification of Antibiotic Target
Modification of the target sites of antibiotics represents a frequently observed mechanism of antibiotic resistance. Clinical strains displaying resistance can be identified across all classes of antibiotics, irrespective of their mechanisms of action. This resistance phenomenon arises from a series of genetic mutations that can affect various components, including the gene responsible for encoding the target protein, proteins involved in drug transport, and those associated with drug activation when exposed to the antibiotic [5][6].2.2. Antibiotic Resistance Mediated by Bacterial Enzymes
Bacteria during evolution have adopted several mechanisms of resistance to antibiotics; in some, the cell uses its own genes to survive antibiotic exposure; in others, the bacteria are able to survive thanks to new capacities attained by the acquisition of new genetic material that allow survival. Excellent reviews deal with this subject [6][13]. The aim of this resviearchw is to focus on the mechanisms of resistance mediated by bacterial proteins, specifically on the enzymes that modify drugs in detail. This researchpaper aims to specifically highlight the role of monooxygenases in antibiotic resistance, both in a general context and with a specific focus on the involvement of Baeyer–Villiger monooxygenases in carbapenem resistance. The role of bacterial proteins in the development of antibiotic resistance is versatile and it includes (i) drug alteration (a) enzymes modify the antibacterial drug by destroying its antibacterial activity; (ii) drug alteration (b) that can occur through an enzymatic process where an enzyme covalently transfers various chemical groups to the drug, thereby preventing its binding to its intended target; (iii) target protection (a) proteins bind to the target of the antibiotic molecules, leading to allosteric dissociation of the drug from its intended target; (iii) target protection (b) proteins can bind to the antibiotic target causing a conformational change that allows the functioning of the target protein even in the presence of the drug; and (iv) target bypass where the enzyme target of the antibiotic molecule becomes redundant thanks to the acquisition of a gene that encodes an alternative enzyme that fulfils the function of the drug target [7][14] (Figure 1).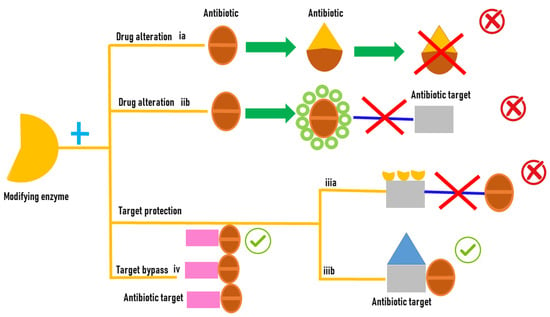
Figure 1.
Antibiotic resistance mediated by enzyme modifications.
2.3. FMOs and Antibiotic Resistance
2.3.1. Tetracyclines
Microbial FMOs are able to modify antibiotics through oxidation, leading to resistance. The class A flavin-dependent monooxygenase tetracycline destructase TetX confers resistance to all clinically relevant tetracyclines, including the broad-spectrum antibiotic tigecycline, which is successfully used against multidrug-resistant pathogens. TetX was isolated from transposons Tn4351 and Tn4400 present in anaerobic bacteria of the genus Bacteroides [11][28]. It is able to regioselectively hydroxylate tetracycline antibiotics to 11a-hydroxy-tetracyclines (Figure 2A). Overexpression in E. coli and protein purification followed by mass spectral and NMR characterization demonstrated that TetX requires NADPH, Mg2+, and molecular oxygen to hydroxylate tetracyclines at C11a. This hydroxylation weakens the binding to magnesium, altering the physical properties of tigecycline and decreasing its affinity to ribosomes. Intramolecular cyclization and non-enzymatic breakdown to non-defined products follow the hydroxylation step [12][29]. The crystallographic structure of TetX from Bacteroides thetaiotaomicron and its complexes with tetracyclines showed the extremely versatile substrate diversity of the enzyme [13][30]. The environmental bacterium Mycobacterium abscessus has emerged as an important human pathogen causing bronchopulmonary infections. It possesses a WhiB7-independent tetracycline-inactivating monooxygenase, MabTetX, that confers a high level of resistance to tetracycline and doxycycline. Both antibiotic molecules are monooxygenated by the purified MabTetX [14][31].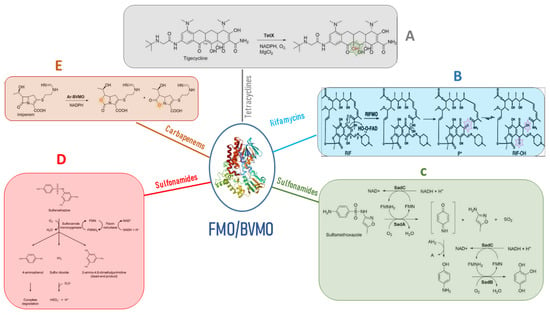
Figure 2. Antibiotic resistance mediated by FMOs’ modifications of antibiotic molecules. (A) the class A flavin-dependent monooxygenase tetracycline destructase TetX hydroxylates tetracycline antibiotics to 11a-hydroxy-tetracyclines inactivating the antibiotic molecule. (B) Rifampicin monooxygenase (RIFMO) catalyzes the hydroxylation of rifampicin (RIF) at the C2 atom inactivating the antibiotic activity of the molecule by preventing key contacts with the RNA polymerase target. (C) the monooxygenases SadA and SadC attack the sulfonamide molecules and release 4-aminophenol, which is converted by the monooxygenase SadB to 1,2,4-trihydroxybenzene that lacks antibiotic activity. (D) sulfonamide monooxygenase and flavin reductase are required for the cleavage of sulfamethazine producing the not active dead-end products and 4-aminophenol. (E) Baeyer–Villiger asymmetric oxygen insertion within the carbapenem ring of imipenem inactivates the antibiotic property of the molecule.
