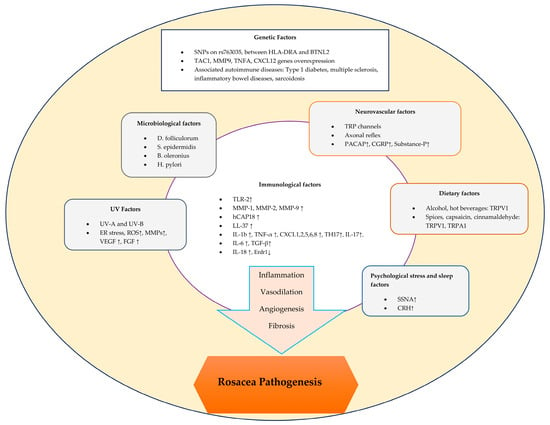
1. Introduction
The pathogenesis of rosacea is complex and involves a variety of factors that can trigger both inflammatory and vascular responses
[1][7] (
Figure 1).
Figure 1.
Schematic representation of factors contributing to the pathogenesis of rosacea.
2. Immunological Factors in Rosacea
2.1. Dysregulation of the Innate Immune System
Rosacea skin has higher levels of TLR-2, KLK-5, cathelicidin, and matrix metalloproteinases (MMPs) than healthy skin
[2][4]. Toll-like receptors (TLRs) are a subset of pattern recognition receptors (PRRs) that recognize damage-associated or pathogen-associated molecular patterns in the skin’s immune system. In particular, TLR-2 is abundant in rosacea skin and has been found in higher levels than in healthy skin
[2][3][4,8]. In rosacea patients, keratinocytes produce pro-inflammatory cytokines and chemokines when TLR-2 is activated by triggering factors, leading to the increased expression of pro-inflammatory cytokine genes such as IL (interleukin)-8, IL-1b, and TNF (tumor necrosis factor)-α
[4][9]. For instance, as an exogenous factor, ultraviolet radiation (UV) activates TLR-2 by inducing endoplasmic reticulum stress. This leads to the production of inflammatory cytokines, which, in turn, trigger inflammatory responses
[2][4]. Moreover, TLR-2 was found to positively mediate a pro-inflammatory response following UV-B irradiation in an in vivo study
[5][10].
IL-8 induces neutrophil chemotaxis in the skin, resulting in the release of proteases such as cathepsin G, elastase, and protease-3
[3][8]. In addition, IL-b and TNF-α also act as the angiogenic factor and vascular endothelial growth factor (VEGF) and may cause the vascular changes seen in rosacea
[3][6][8,11]. In addition, a study has shown that TLR-2 expression in keratinocytes or TLR-2 ligand exposure induces the production and protease activity of the serine protease KLK (kallikrein)-5, resulting in cathelicidin activation
[2][7][4,12]. Cathelicidin, an antimicrobial protein, is found in the lamellar body of keratinocytes and the granules of neutrophils. Human-specific cathelicidin is called human cationic antibacterial protein of 18 kDa (hCAP18), which is the inactive form, and it is cleaved by KLK-5 to the active peptide form called LL-37
[8][9][10][1,13,14]. LL-37 is an antimicrobial peptide that modifies host immunity and growth responses by promoting leukocyte chemotaxis and angiogenesis to rearrange extracellular matrix components. The LL-37 peptide in rosacea skin differs from healthy skin by having shorter fragment forms
[8][10][1,14]. These fragments have been implicated in symptoms characteristic of rosacea, including erythema, vasodilatation, flushing, and telangiectasia, based on observations in studies of injected mice
[11][15].
MMPs indirectly influence the pathogenesis of rosacea by activating the preproenzyme form of KLK5 after cleavage with MMP-9. Specifically, MMP-2 and MMP-9 are increased in the skin of rosacea patients, which could activate KLK5 more and increase LL-37 expression
[8][1]. A study also suggested that mast cell degranulation in individuals with rosacea results in increased levels of MMP-9 and LL-37
[10][14].
2.2. Dysregulation of the Adaptive Immune System in Rosacea
T-Cell Mediated Dysregulation
In the pathogenesis of rosacea, pro-inflammatory cytokines and chemokines play dominant roles in the infiltration of inflammatory cells and trigger immune responses
[3][8]. Among the total T cells in rosacea skin, an increase in CD4+ over CD8+ T cells has been demonstrated. There was also upregulation in the polarizing Th1 and Th17 gene sets, as well as the interferon gamma (IFN-γ) and IL-17A cytokines
[12][16]. Th17 could be responsible for an effect on LL-37 via the expression of abnormal forms that are seen in rosacea
[13][17]. IL-17 induces angiogenesis through the VEGF pathway and has an effect on the expression of LL-37
[6][13][11,17]. Thus, the connection between cytokines and chemokines with the Th1/Th17 pathways and rosacea supports the notion of their association
[3][8].
One study found that the levels of regulatory T cells in rosacea, which are CD4+CD25+ regulatory T cells, were higher in rosacea than in lupus erythematosus. This was a supportive finding, as immune cells in rosacea are more likely to maintain immunological tolerance than in other autoimmune diseases
[3][14][8,18]. A neutrophil chemotactic factor, CXCL8, was found to be increased in rosacea via the upregulation of its mRNA gene modification. CXCL1, CXCL2, CXCL5, and CXCL6 were also upregulated in rosacea patients. These chemokines in rosacea have angiogenic properties that also attract neutrophils and TH17 cells
[12][16].
A recent case report of rosacea-like erythematous papulopustules on the face associated with the COVID-19 vaccine also supports the idea that the adaptive immune system is involved in rosacea. In this re
seapor
cht, a single case was associated with the Vaxzevria (AstraZeneca) COVID-19 vaccine, while the other was related to the Pfizer-BioNTech COVID-19 vaccine. It has been suggested that the pathogenesis could involve elevated levels of cutaneous chemokines like CCL2, CCL5, and CXCL10, inducing chemotaxis and the infiltration of CD4+ T cells, monocytes/macrophages, and polymorphonuclear cells into the skin
[15][19]. In addition, both vaccines were shown to induce, through different mechanisms, neutralizing antibodies against the SARS-CoV-2 spike protein and specific T-cell expansion with the secretion of cytokines such as IFN-γ, IL-2, and IL-10
[15][19]. Furthermore, the SARS-CoV-2 spike protein encoded by COVID-19 mRNA vaccines induces IL-1b secretion in macrophages. Moreover, elevated levels of IL-1 and IL-36 trigger an inflammatory response, leading to pustules in pustular psoriasis
[16][20].
In the study by Salzer et al., erythroid differentiation regulator 1 (Erdr1) was found to be decreased in rosacea patients compared to the controls, whereas IL-18 was found to be increased. It was also found that Erdr1 levels were decreased by IL-18 in rosacea
[17][21]. Erdr1 is expressed in normal skin epithelium and may suppress UV-induced oxidative stress in rosacea by reducing reactive oxygen species (ROS) levels by blocking heat shock protein 90
[3][8]. IL-18 regulates the immune response by activating Th1-mediated responses, as in many other chronic inflammatory diseases, such as psoriasis, atopic dermatitis, and contact dermatitis, and also downregulates the Erdr1 levels in rosacea
[3][17][8,21]. Recombinant Erdr1 may be beneficial for rosacea patients by reducing UV-induced oxidative stress. In a mouse model of rosacea, recombinant Erdr1 also suppressed the expression of VEGF and reduced angiogenesis, giving hope for its use in human rosacea patients
[17][21].
B Cell-Mediated Dysregulation
The relationship between TLRs and B cells involves mechanisms where TLR activation is necessary for certain antigen-specific antibody responses in B cells, and TLR agonists stimulate the differentiation of plasma cells from B cells
[18][22]. As in phymatous rosacea, fibrotic changes in the skin can be stimulated by B cells through the TLR-inducing effect on fibrogenic cytokines such as IL-6 and TGF-β (transforming growth factor beta)
[3][8]. Furthermore, elevated antinuclear antibody titers are frequently detected in patients with rosacea, indicating that the involvement of B cells in the pathogenesis of rosacea cannot be underestimated
[3][19][8,23].
3. Microbiological Factors in Rosacea
Demodex folliculorum is the predominant microbial agent within the skin that is commonly associated with the development of rosacea. This saprophytic mite specifically targets the sebaceous gland area of healthy skin, where it can use epidermal cells and sebum components as nutrients. Facial skin is particularly rich in sebaceous glands, especially the nose, cheeks, forehead, and chin
[20][24], and rosacea skin has a higher density of
Demodex mites compared to normal facial skin
[21][25].
High densities of
D. folliculorum lead to the release of pro-inflammatory mediators such as TNF-α, IL-1b, IL-8, and LL-37 into the skin. Specifically, the exoskeleton of
Demodex contains chitin, a type of polysaccharide that causes the activation of TLR-2 on keratinocytes, leading to protease activity
[22][26]. In addition,
D. folliculorum was found to be associated with IL-17, which plays a role in papulopustular rosacea by inducing angiogenesis, telangiectasia, inflammation, and pustules
[21][25]. NLRP3 gene expression is also stimulated by mite colonization, leading to the production of IL-1b and the initiation of the inflammatory process
[21][22][25,26]. Moreover,
Demodex mites at high densities have also been shown to affect tissue compatibility. These are HLA Cw2 and Cw4 and cause leukocytes to undergo apoptosis. Immunosuppression in the mite’s microenvironment allows it to survive easily in the skin
[23][27].
Staphylococcus epidermidis is the predominant bacteria that is commensal on the skin
[2][4]. It may play an important role in the pathogenesis of pustular and ocular rosacea, as suggested by a study that isolated
S. epidermidis from the pustules and eyelid margins of rosacea patients
[24][28]. In addition,
S. epidermidis antigens are recognized by TLR-2, and the interaction between
S. epidermidis and TLR-2 leads to the initiation of an immune response. Both
S. epidermidis and TLR-2 induce the expression of AMPs
[8][1].
Another bacterial pathogen associated with rosacea, isolated from the
Demodex mite, is
Bacillus oleronius. It is a Gram-negative, endospore-forming, non-motile bacteria. One study found that patients with erythematous rosacea had higher levels of mononuclear cells to
B. oleronius bacterial antigens in their peripheral blood
[25][29].
Helicobacter pylori, a Gram-negative bacteria found in the gastric mucosa, is the other microbiological agent that causes rosacea symptoms
[26][30].
H. pylori may have an effect on increasing ROS, leading to inflammation in the gut. Among the ROS, nitric oxide (NO) can specifically induce intestinal mucosal inflammation and alter physiological processes in the skin, including vasodilation, inflammation, and immunomodulation, leading to the clinical manifestations of flushing and erythema associated with rosacea
[27][28][31,32]. Through its cytotoxin-associated gene-A (cagA)-encoded cytotoxin,
H. pylori is thought to play a role in inducing the production of pro-inflammatory cytokines such as TNF- α and IL-8, leading to gastric mucosal inflammation and the clinical manifestation of rosacea
[27][28][31,32].
It is demonstrated in the literature that the intestinal microbial population may have an immunomodulatory effect upon non-gastrointestinal systems including the skin. Changes in diet, such as the consumption of alcohol or high glycemic index foods, may cause a shift in the microbiome from a healthy to an unhealthy population
[29][30][33,34]. The prevalence of small intestinal bacterial overgrowth (SIBO) was found to be significantly higher in rosacea patients than in the controls
[31][35]. SIBO may also be linked to the activation of TNF-α, leading to inflammatory symptoms of rosacea
[32][36].
4. Genetic Factors in Rosacea
Genetic factors are implicated in the manifestation of rosacea based on familial studies. In a cohort study evaluating the severity of rosacea between heterozygous and monozygous twins, monozygous twins showed greater severity and a higher correlation with clinical rosacea scores than heterozygous twins
[33][37].
Fifteen genes were found to be over-expressed in the erythematotelangiectatic rosacea group compared to the healthy control group. The genes involved in neuropeptides are CALCA, CALCB, and TAC1. The genes involved in matrix remodeling are COL1, COL3, CYR61, DCN, MMP1, MMP3, and MMP9. Innate Immunity-Related Genes are DEFA1, CXCL12, and CXCR4. The genes involved in inflammatory markers are IL-12 B and TNFA
[34][38]. Mainly, the TAC1, MMP9, TNFA, and CXCL12 genes have been identified as significant, as they encode substances such as P, MMPs, TNF-α, and chemoattractants for mast cells
[35][39].
A single nucleotide polymorphism (SNP) that is significantly associated with rosacea was identified on chromosome 6 (rs763035), which is intergenic and located upstream of the HLA class II histocompatibility antigen, DR alpha chain (HLA-DRA), and is located downstream of butyrophilin-like 2 (BTNL2), which is mainly associated with histocompatibility complex class I. Both genes were linked through the coexpression of HLA-DRB5 and CIITA (class II, major histocompatibility complex, transactivator)
[36][40]. In papulopustular rosacea, skin immunohistochemistry and an HLA allele analysis demonstrated the presence of HLA-DRA in epidermal Langerhans cells, the presence of BTNL2 in keratinocytes, and the presence both genes in the perifollicular inflammatory infiltrate and endothelial cells. A further genetic analysis of HLA alleles defined three significant replication groups related to rosacea: HLA-DRB1*03:01, HLA-DQB1*02:01, and HLADQA1*05:01. Both of these replication groups and HLA-DRA play roles in antigen presentation from extracellular sources, supporting the effects of microbial agents in the pathogenesis of rosacea
[36][40]. In addition, thymic stromal lymphopoietin (TSLP) gene expression was found to be significantly decreased in papulopustular rosacea, resulting in the recruitment of dendritic cells and pathogenic Th17 cells with an inflammatory microenvironment of IL-17 and IFN-γ
[35][37][39,41].
Rosacea has been shown to have several risk gene loci associated with autoimmune diseases. The HLA allele of the DRB1*03:01-DQB1*02:01-DQA1*05:01 haplotype is linked to rosacea
[35][39]. Such an association between rosacea and these HLA alleles, along with HLA-DRA, supports the idea that extracellular antigen presentation plays a role in the etiology of the condition, suggesting a link with various microbiological agents. Moreover, HLADRB1*03:01 is specifically associated with retinopathy in type 1 diabetes and may be related to the abnormal proliferation of blood vessels observed in the ocular findings of rosacea
[35][36][39,40]. Other genetic loci of shared autoimmune diseases include HLA-DRA, which is associated with rosacea and multiple sclerosis, and BTNL2, which is associated with rosacea, inflammatory bowel disease, and sarcoidosis
[36][38][39][40,42,43].
5. Neurovascular Factors in Rosacea
Facial skin vascular activity is controlled by neuronal (sympathetic, parasympathetic, and nociceptive) and non-neuronal (local inflammatory) mechanisms
[40][44]. Transient receptor potential (TRP) channels activate sensory nerve endings to release vasoactive neuropeptides. There are two calcium channel members of the TRP family called transient receptor potential vanilloid 1 (TRPV1) and ankyrin 1 (TRPA1). TRPV1 and TRPA1 are triggered by spices, alcohol, and temperature changes. In rosacea, these two channels coordinate the inflammatory response by inducing depolarization, which leads to the release of neuropeptides such as the pituitary adenylate cyclase-activated polypeptide (PACAP), substance P, and the calcitonin gene-related peptide (CGRP)
[40][44]. In addition, local mediators trigger the release of vasodilator neuropeptides, CGRP, and substance P by activating skin nociceptors through an axon reflex effect
[41][45]. Moreover, PACAP, substance P, and CGRP cause inflammatory responses by activating mast cells, macrophages, neutrophils, and T cells
[40][44]. Heat can also activate both TRPV1 and TRPA1 in individuals suffering from rosacea. An increased and prolonged expression of TRPs in rosacea patients results in facial flushing, the dysregulation of the vascularity, and neurogenic leukocyte inflammation
[3][8].
6. Dietary Factors in Rosacea
Keratinocytes and sensory nerves have TRPV1 receptors that are activated by substances such as alcohol, spicy foods, hot beverages, vanilla, cinnamon, caffeine, and UV radiation. These channels release substance P and CGRP, resulting in an inflammatory response, dilated arterioles, flushing, and edema in rosacea patients
[42][46]. Alcohol is also converted into metabolites such as acetaldehyde and acetone. These compounds generate histamine, which is recognized to affect the dermal vasomotor system, resulting in flushing
[43][44][47,48].
It is therefore important to note that the excessive consumption of spices could potentially lead to rosacea flares. Capsaicin from chili peppers and cinnamaldehyde from cinnamon can activate TRPV1 and TRPA1 receptors, respectively, causing an intense burning sensation. It has been suggested that spices may activate TRPA1 and TRPV1, resulting in flushing or burning sensations in rosacea patients. According to studies, the dilated microvascular structure and increased blood flow intensity in rosacea patients with papules and pustules may be due to TRPA1 and TRPV1 receptor activation
[45][49].
In contrast to other inflammatory skin diseases such as atopic dermatitis and chronic urticaria
[46][47][50,51], the serum vitamin D levels were found to be higher in rosacea patients compared to the controls
[48][52]. In addition, vitamin D levels are associated with sun exposure, which is a triggering factor in rosacea
[49][53]. However, another study showed that the serum vitamin D levels were significantly lower in rosacea patients than in the controls
[50][54]. Although vitamin D has been demonstrated to enhance TLR-2 and KLK5 mRNA expressions
[51][55] and influence LL-37 pathways that play a role in rosacea
[3][8], larger epidemiologic studies are needed to prove the association between serum vitamin D levels and the presence of rosacea
[49][53]. Also, vitamin B12 (riboflavin) was thought to be a potential critical factor in B complex deficiency, and this deficiency was thought to be associated with rosacea
[52][56]. Zinc is a known antioxidant and anti-inflammatory trace element that may play a role in reducing
Demodex mites or cutaneous bacteria, which play roles in the pathogenesis of rosacea
[53][54][57,58].
In the literature, explanations of caffeine intake and rosacea exacerbation relationships are limited
[52][56]. In a study, regular coffee consumption was associated with a reduction in the occurrence of rosacea. This reduction is proportional to the amount of coffee that is consumed on a daily basis
[55][59]. However, it is thought that the consumption of hot coffee and hot tea, depending on the heat, may increase vasodilation and sympathetic activation, resulting in flushing and telangiectasia
[52][56].
7. UltravioletV Radiation Irradiation Factors in Rosacea
The exacerbation of rosacea by sunlight has been linked to the inflammatory effects of UV radiation on keratinocytes. UV radiation stimulates ROS production in keratinocytes and activates cellular signaling pathways. UV-A radiation increases MMP-1 levels, and ROS also increase MMP-2 mRNA levels and suppress the tissue inhibitor of metalloproteinase-1 (TIMP-1) in human dermal fibroblasts. Thus, ROS cause vascular and dermal matrix damage by upregulating matrix metalloproteinases
[56][60]. Furthermore, UV radiation induces endoplasmic reticulum stress, leading to the activation of TLR-2
[2][4]. Specifically, a study demonstrated the activation of TLR-2 by UV-B radiation
[5][10]. Additionally, another study suggested that UV-B increases the VEGF and fibroblast growth factor (FGF) levels, leading to cutaneous angiogenesis and resulting in telangiectasia in rosacea patients
[57][61]. Consequently, UV-induced increases in ROS and VEGF activity in the skin leads to enhanced inflammatory responses and contributes to the degeneration of collagens and matrix structures in the dermis
[56][60].
8. Psychological Stress and Sleep Factors in Rosacea
Psychological factors play roles in rosacea, where emotions of embarrassment or laughter can initiate flushing
[58][62]. It was shown that rosacea patients had a significantly higher intensity of stress from critical life situations than the control group, and that the emotional status of rosacea patients has an impact on their symptoms
[59][63]. Mental stress induces vasodilatory activities via sympathetic nerve activity (SSNA) in the skin
[60][61][64,65]. An elevated SSNA response was noted in the supraorbital skin of individuals with rosacea following mental stress
[62][66]. In addition, stress induces cortisol-releasing hormone (CRH) that affects pro-inflammatory cytokines such as IL-6, IL-8, and IL-18, which regulate mitogen-activated protein kinase and the nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB) and induce facial erythema. Additionally, CRH type 2 receptors are primarily expressed in blood vessels, acting as a direct vasodilator that is observable in rosacea skin
[63][67]. Through CRH type 1 receptors, CRH induces mast cell degranulation, leading to the release of multiple vasodilatory mediators including histamine and NO
[64][68]. Psychological stress in rosacea patients may modulate TLR signaling, induce ROS, and increase antimicrobial peptide and neuropeptide production, as well as increase other triggering factors such as UV radiation, heat, cold, spicy foods, and microbial factors
[65][69].
In rosacea patients, the impact on appearance can cause a lack of self-confidence, anxiety, and depression, which can lead to psychological stress and affect sleep quality
[66][67][70,71]. Poor sleep quality has been linked to the worsening of chronic inflammatory conditions, including psoriasis
[68][69][72,73]. Regarding these data in the literature, one study found an association between poor sleep quality and rosacea, as well as the severity of rosacea
[70][74]. This is because a lack of sleep can increase the expressions of MMP-9, TLR-2, and CAMP, affecting regional inflammatory factors in the skin
[71][72][73][75,76,77]. In addition, the vascular dysregulation of the skin via increased VEGF expression and angiogenesis occurs in response to poor sleep
[70][74]. Additionally, the investigation of genetic predisposition revealed that single nucleotide polymorphisms (SNPs) in the HTR2A and ADRB1 genes were correlated with sleep regulation in individuals affected by rosacea
[70][74].
9.Conclusions
Rosacea is a chronic and multifactorial disease with various factors contributing to its pathogenesis. Genetic factors, along with other triggering factors such as microbial elements, UV exposure, diet, neurovascular factors, and stress, as well as immune dysregulation, have been linked to rosacea. In addition to triggering factors, the dysfunctionality of TLR-2, KLK-5, and cathelicidine results in the dysregulation of the adaptive and innate immune system. Induced cytokines and signaling factors culminate in inflammation, angiogenesis, vasodilation, and fibrotic changes (Figure 2).
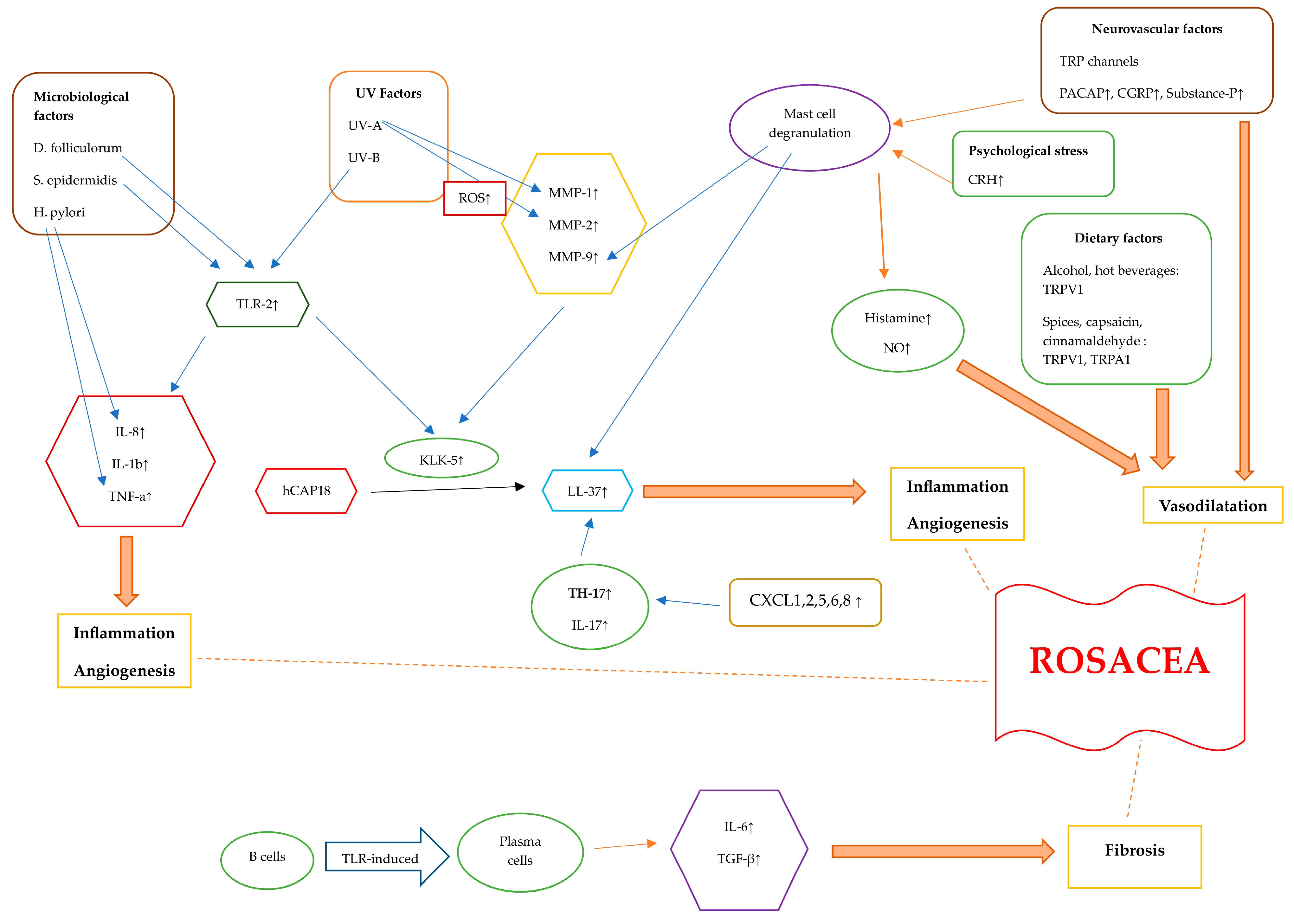
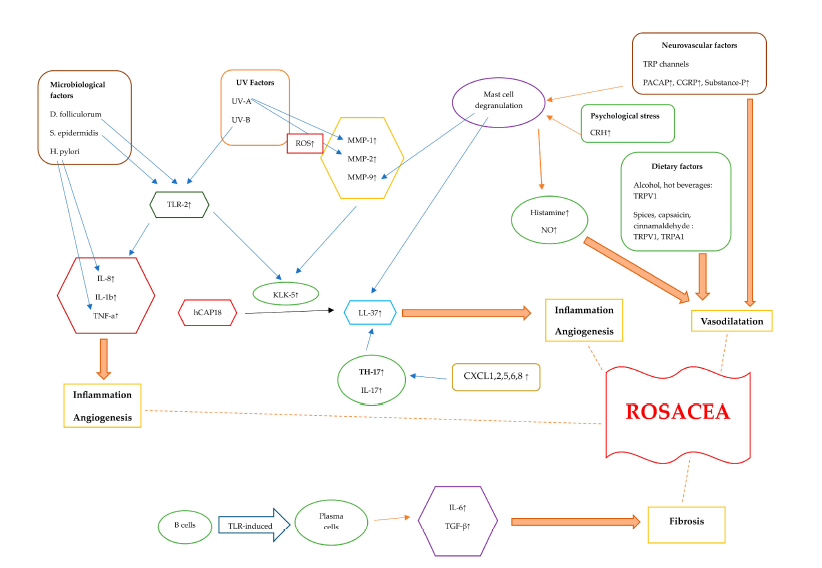
Figure 2. Diagram illustrating the pathogenetic mechanisms of rosacea. Rosacea skin exhibits elevated levels of TLR-2, KLK-5, cathelicidin, and MMPs. The activation of TLR-2 by Demodex, as well as the interaction between S. epidermidis and TLR-2, initiates an immune response. Additionally, exposure to UV-B radiation can activate TLR-2, which then triggers inflammatory responses. TLR-2 induces the production and protease activity of serine protease KLK-5, which results in cathelicidin activation. The inactive form of human-specific cathelicidine is hCAP18, which is cleaved by KLK-5 to form the active peptide, LL-37. LL-37 promotes erythema, vasodilation, flushing, and telangiectasia. CXCL1, CXCL2, CXCL5, CXCL6, and CXCL8 are chemotactic factors that are upregulated in rosacea, attracting TH17 cells. Additionally, TH17 and IL-17 play roles in inducing the expression of LL-37. When triggered by external stimuli, TLR-2 activation enhances the expression of pro-inflammatory cytokine genes like IL-8, IL-1β, and TNF-α . Helicobacter pylori induces the production of pro-inflammatory cytokines such as TNF-a and IL-8, leading to inflammation. TLRs lead to the differentiation of B cells into plasma cells that generate fibrogenic cytokines, including IL-6 and TGF-β . UV radiation stimulates the production of ROS. UV-A radiation increases MMP-1 levels, and ROS also increase MMP-2 levels. Alcohol, hot beverages, capsaicin, cinnamaldehyde, and spices trigger TRPV1 and TRPA1 receptors. These channels release substance P and CGRP, leading to an inflammatory response and vasodilatation. PACAP, substance P, and CGRP initiate an inflammatory response through the activation of mast cells. Mast cell degranulation leads to elevated MMP-9 and LL-37 levels. Stress causes the release of CRH, which, in turn, causes mast cell degranulation. This leads to the release of several vasodilatory mediators such as histamine and NO. Abbreviations: TLR, Toll-like receptor; KLK-5, kallikrein-5; MMP, matrix metalloproteinase; TNF-α, tumor necrosis factor alfa; TRPV1, transient receptor potential vanilloid 1; TRPA1, transient receptor potential ankyrin 1; PACAP, pituitary adenylate cyclase-activated polypeptide; CGRP, calcitonin gene-related peptide; TGF-β , transforming growth factor beta; ROS, reactive oxygen species; CRH, corticotropin-releasing hormone; NO, nitric oxide.