Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Peter Tang and Version 1 by Brian J Piper.
Buprenorphine has high affinity for the mu-opioid receptor (MOR), delta (DOR), and kappa (KOR) and intermediate affinity for the nociceptin (NOR). Buprenorphine’s active metabolite, norbuprenorphine, crosses the blood–brain barrier, is a potent metabolite that attenuates the analgesic effects of buprenorphine due to binding to NOR, and is responsible for the respiratory depressant effects. The area under the concentration curves are very similar for buprenorphine and norbuprenorphine, which indicates that it is important to consider this metabolite.
- opioid use disorder
- pain
- opiate
1. Introduction and History
Buprenorphine was first derived from thebaine in 1966 and was subsequently characterized as a partial agonist at the mu-opioid receptor (MOR) [1]. The Committee on Drug Addiction primarily focused on morphine and looked for a way to ensure its multitude of uses without its addictive side effects in the 1920s. Buprenorphine was considered a part of the solution to the 20th century opium problem. Its agonist–antagonist pharmacological character was more fully characterized in 1972 and its potential as an addiction treatment recognized in 1979 [2,3][2][3]. Buprenorphine is a semi-synthetic and lipophilic drug. It has activity at all four major opioid receptors: MOR, kappa (KOR), delta (DOR), and the nociceptin receptor (NOR). Of the four main opioid receptors, three (MOR, DOR, and KOR) were identified in the 1960s and the opioid receptor like (ORL), currently and henceforth designated as NOP, was discovered in the 1990s [4]. In addition to its involvement in nociception, the KOR is widely expressed during prenatal and early postnatal periods including on progenitor, ependymal, and neuronal cells [5], which raises the possibility that a KOR antagonist such as buprenorphine could have an adverse impact on brain development. This may also apply to other (MOR/NOR) opioid receptors that are important for myelination [6]. The NOP is considered an atypical, low affinity receptor for opioid peptides [4]. Although marketed for analgesia and addiction treatment, early research subjects reported that buprenorphine was the “most reinforcing drug they had ever used” [2]. Injectable buprenorphine became commercially available in the US in 1981 [1]. By 1985, it was available in 29 countries [2]. Buprenorphine was originally considered a Schedule V narcotic in the US until 2002 when, after three attempts by the Drug Enforcement Agency, it was rescheduled as Schedule III [1,2][1][2]. US sales of buprenorphine have increased substantially. Buprenorphine was the most commonly used opioid by US veterinarians [7]. This may change as, by morphine mg equivalent, buprenorphine was only the seventh most common opioid in US veterinary teaching institutions [8].
2. Pharmacokinetics and Pharmacodynamics
We now turn our attention to the pharmacokinetics, pharmacodynamics, and their interaction for this semi-synthetic opioid. Many of buprenorphine’s pharmacokinetic properties explain its unique effects [17][9]. Buprenorphine’s metabolism follows non-saturable Michaelis–Menten kinetics [18][10] that furthers its analgesic effects [9][11]. There are two major metabolic pathways in buprenorphine’s metabolism. Buprenorphine undergoes N-dealkylation catalyzed by the hepatic cytochrome P (CYP) 450 (CYP P450-3A4) and glucuronidation, resulting in three major metabolites: buprenorphine-3-glucuronide (B3G), N-dealkylbuprenorphine, and norbuprenorphine-3-glucuronide (N3G). The CYP3A4 system metabolizes buprenorphine to norbuprenorphine through N-dealkylation of the cyclopropylmethyl group [9,19,20][11][12][13]. CYP3A4 is predominantly responsible for this, although CYP2C8 also contributes. The ratio of norbuprenorphine to buprenorphine in urine can provide an index of the recency of buprenorphine administration, the potential use of a CYP3A4 inducer, or the probability of buprenorphine “spiking”, involving submerging the film or tablet in urine in an effort to have a positive immunoassay result [21][14]. Some conclude that norbuprenorphine does not readily cross the blood–brain barrier ([9][11], although see [22][15]). Sheep are used for the similarity of their blood–brain barrier to humans. The large size of sheep contributes to their use in pharmacokinetics investigations. The peak concentration of buprenorphine was half that in the sagittal sinus relative to the arterial quantities, which indicated only intermediate permeability across the blood–brain barrier. In contrast, the peak concentration and time to peak concentration were very similar for samples from the sagittal sinus and arterial blood for norbuprenorphine. Also noteworthy was that the peak sagittal concentration of norbuprenorphine was over twenty-fold higher than that of buprenorphine in this species [23][16]. Among patients receiving buprenorphine/naloxone for two weeks, the twenty-four hour area under the concentration curve was equivalent for buprenorphine and norbuprenorphine [24][17]. Norbuprenorphine is commonly measured in urine analyses because of these high concentrations [25][18]. Norbuprenorphine and buprenorphine are both detectable in meconium, although norburprenorphine’s quantities were six-fold higher [26][19]. Another biological matrix that can provide an index of buprenorphine use is hair. The hair of pregnant women, and a small sample of their offspring, had measurable norbuprenorphine and buprenorphine [27][20]. Norbuprenorphine is then metabolized to N3G [17,28][9][21]. Other metabolites, including hydroxy buprenorphine and hydroxynorbuprenorphine, have been identified. CYP3A4 produces hydroxybuprenorphine [18][10]. The CYP3A4 activity varies between individuals and can be induced, resulting in wide differences in pharmacokinetics [18][10]. Buprenorphine is eliminated in the urine and in feces, accounting for one-third and two-thirds of the eliminated buprenorphine, respectively [18][10]. It is important to note that interactions between different drugs can occur due to the inhibitory effects on CYP3A4, which is often blocked by various medications For instance, when ritonavir, a potent CYP3A4 inhibitor, is administered alone, it can elevate the levels of both buprenorphine and norbuprenorphine without intensifying the adverse effects associated with buprenorphine [15][22]. For instance, when ritonavir, a potent CYP3A4 inhibitor, is administered alone, it can elevate the levels of both buprenorphine and norbuprenorphine without intensifying the adverse effects associated with buprenorphine [24][17]. As cannabis is a CYP3A4 inhibitor, a doubling of buprenorphine concentrations in regular cannabis users [29][23] may impact many patients that use cannabis for chronic pain [30][24]. Buprenorphine has an absolute contraindication in humans to not be combined with the antiretroviral atazanavir, the histamine (H1) blocker azelastine, the typical antipsychotic bromperidol, the irritable bowel agent eluxadoline, the sleeping sickness agent fexinidazole, the calcium antagonist flunarizine, the antibiotic fusidic acid, kratom, monoamine oxidase inhibitors, naltrexone, the H1 antagonist olopatadine, the anticholinergic orphenadrine, the antihistamine oxomemazine, the depressant paraldehyde, the opioid antagonist samidorphan, or the oncology agent thalidomide [31][25]. Buprenorphine has fewer drug–drug interactions than other opioids that are metabolized through CYP3A4 [1]. Further research needs to be conducted on buprenorphine’s drug interactions, as there is more information for methadone [36][26]. Buprenorphine alone may have a higher ceiling effect than typical MOR agonists, but in combination with benzodiazepines, it could result in a potentially life-threatening drug interaction due to sedation and respiratory depression properties. The mechanism of the respiratory depression is unclear [37,38][27][28]. There is some noted benefit to combining opioids with buprenorphine to produce sub-addictive analgesia [1]. In the postoperative setting, buprenorphine did not impair morphine analgesia (buprenorphine 0.4 μg/kg as an infusion and 0.15 μg/kg as the demand dose) [39][29]. Cancer patients with breakthrough pain receiving transdermal buprenorphine from 35–70 μg/h responded well to an oral morphine to transdermal buprenorphine ratio of 75:1 [40][30]. Additionally, those using high-dose buprenorphine for maintenance therapy did not need to be switched off this opioid for methadone, as the patients morphine responses were not different between the two groups [41][31]. Glucuronide metabolites of buprenorphine are biologically active, contributing to the pharmacology of the drug [9][11]. The glucuronidation rate is roughly the same for buprenorphine and norbuprenorphine in the liver and small intestine. N-dealkylation is one-hundred fold greater in the liver than in the small intestine [42][32]. Conjugated metabolites are excreted in bile and half the buprenorphine administered is eliminated in the feces [28][21]. In bile fistula rats, where the bile flows into a hollow structure when 0.6 mg/kg buprenorphine was administered intravenously, 75% of B3G and 19% of N3G were excreted in bile. In “linked rat models” or intact rats, approximately twice the amount of N3G was found to be excreted compared with B3G. There are differences in excretion due to first-pass effects in enterohepatic circulation [42][32]. It is deconjugated by the colon by bacteria, then reabsorbed [18][10]. Buprenorphine is an atypical opioid as a result of its receptor activity at the MOR [9][11]. Buprenorphine has shown activity at all four opioid receptors [3]. Buprenorphine dissociates from the MOR slowly, resulting in a slow onset and a long duration for the analgesic effects [3]. A 2002 review describes how the MOR partial agonist and KOR antagonist properties of buprenorphine have been well established but that there had been comparatively less research on DOR and NOR [11][33]. Although most opioids show activity at the MOR, DOR, and KOR, buprenorphine is a DOR and KOR antagonist with high affinity [43][34]. Buprenorphine is potent at the MOR and the DOR, with efficacy at the MOR, DOR, and KOR in order of descending efficacy [44][35]. More recent studies of receptor affinity and intrinsic activity in cats have shown that buprenorphine is a MOR, KOR, and NOR receptor agonist and a DOR antagonist [45][36]. The affinity of buprenorphine for NOR (77 nM) was moderate [43][34]. The MOR is primarily responsible for analgesic effects, as well as euphoria, miosis, constipation, and respiratory depression [16][37]. It may have a greater impact at spinal MOR relative to the brain receptors, which is part of what makes buprenorphine classically considered a partial MOR agonist [9][11]. The DOR has minimal antinociceptive effects relative to the MOR but more activity in chronic pain than acute pain. The DOR also participates in analgesic tolerance and physical dependence [16][37]. The KOR has been seen to have analgesic and proanalgesic effects due to opioids, while also contributing to miosis and sedation [16][37]. Buprenorphine’s properties, including low molecular weight, high lipophilicity, and high potency, influence its perceived effects. Potency, the measure of the concentration or quantity of the substance necessary to achieve a predetermined outcome [46][38], differs depending on the formulation [47][39]. A value of ten-fold greater than morphine is generally accepted for pharmacoepidemiological research [48][40]. The drug has a wide tissue distribution and a peak plasma concentration at ninety minutes [17][9]. Buprenorphine is 96% protein bound after absorption [9][11]. Oral absorption is considered to be poor because of first-pass metabolism [9][11]. Transdermal absorption is limited, but there are formulations designed to be more effective. Sublingual administration is considered effective as well [9][11]. Some studies consider buccal formulations to be the most efficient and have the highest non-intravenous bioavailability [9][11]. The formulations available for the management of pain show the anticipated routes of administration effects, with parental forms producing the most rapid onset and transdermal forms producing the longest effects [49][41]. In healthy patients taking buprenorphine/naloxone tablets, they have a peak plasma concentration (Tmax) of 0.75–1.0 h for buprenorphine and 0.5 h for naloxone, demonstrating rapid absorption. Norbuprenorphine plasma concentrations peaked at a Tmax of 1–1.75 h after the buprenorphine/naloxone tablet administration. The plasma terminal half-life (t1/2) was 22–39 h for buprenorphine, 32–44 h for norbuprenorphine, and 1.4–10 for naloxone. Patients who were in withdrawal treatment for opioid dependence had a median Tmax of 0.75–1 h for buprenorphine, a median Tmax of 0.75–1 h for norbuprenorphine, and a median Tmax of 0.5–0.75 [50][42]. In patients with a history of drug addiction but were drug free at the time of the study, buprenorphine with sublingual and buccal routes had a 51.4% and 27.8% bioavailability, respectively [51][43]. The half-life is dependent on the method of administration, with 2 h for intravenous, 26 h for the transdermal patch, 28 h for the buccal film, and 37 h for the sublingual tablet [52][44]. Terminal elimination half-lives were longer for the sublingual and buccal routes of administration than the intravenous route, which may be due to a depot effect from buprenorphine collected in the oral mucosa tissue reservoirs. The time until the maximum concentration occurs was between 0.5 and 3 h sublingually and after 20 min intravenously [18][10]. Norbuprenorphine had mean peak plasma concentrations that vary by individual and route of administration in healthy patients [18,51][10][43]. Intravenous administration of buprenorphine has a 100% bioavailability, buccal has 46–65%, sublingual has 28–51%, and transdermal has 15% [9][11]. Buprenorphine as a tablet has a bioavailability that is 50–60% that of a buprenorphine solution [53,54][45][46]. Intranasal buprenorphine is 50% bioavailable in humans in a polyethylene glycol 300 and 5% dextrose vehicle, with a maximum concentration at 30 min [55][47]. Buprenorphine’s intranasal bioavailability was 70% with a polyethylene glycol 300 vehicle and 89% with a dextrose vehicle in sheep [55][47]. The half-life in rats following intravenous administration (2.8 h, [42][32]) was very similar to humans. Buprenorphine readily crosses the placenta. However, buprenorphine levels in the third trimester fetal rat brain were only a third of those in the maternal brain [56][48]. Although there is this notion that norbuprenorphine does not readily cross the blood–brain barrier [9][11], this may be age or species dependent. Administration of norbuprenorphine (3 mg/kg) to pregnant rats resulted in higher blood and brain levels in the fetus than in the dam [22][15]. Inhibiting the P-glycoprotein, a drug transporter highly expressed in brain microvessel endothelial cells and placental syncytiotrophoblasts [57][49], increased rat brain uptake of norbuprenorphine seven-fold [58][50]. The fetal plasma norbuprenorphine area under the curve was approximately two-thirds that of maternal mice. The fetal AUC of norbuprenorphine glucuronide was three-fold higher than that of the dam. Although interpretation of this restudyearch is somewhat limited by analysis of the entire mouse gestational day fifteen fetus (instead of isolating plasma or brain), these findings indicate appreciable fetal exposure to buprenorphine’s biologically active metabolites [59][51]. Although buprenorphine and norbuprenorphine are transferred into human breast milk, the quantities were low (1%, [60][52]). In recent years, contrary to traditional receptor theory, it is clear that different ligands for the same receptor can cause different responses [61][53]. For receptor theory models to be useful, they must aid in determining the extent to which drug effects can be interpreted and applied to predict future effects [62][54]. The term “ligand bias” has been used to describe opioid analgesic drugs that elicit a different intracellular response; therefore, their effects are not only the result of receptor binding affinity [44][35]. Buprenorphine differentiates itself from other opioids in mu-receptor activity, with its slow dissociation from the receptor [63][55]. Buprenorphine alone is not responsible for its antagonistic effects, but its varying metabolite concentrations through different forms of drug administration may alter the efficacy of the drug. Traditionally, buprenorphine is described as a partial MOR agonist that is known for limited analgesic effects and developed with the intent for a limited potential for respiratory depression and addiction [15][22]. However, since buprenorphine’s classification in the 1980s and 1990s, what is known about receptor interaction and activation has changed the meaning of the terms “agonist” and “antagonist” [16,62,64][37][54][56]. Importantly, categories such as full agonist, partial agonist, and antagonist may be unsatisfactory, as a drug’s response may land on a continuum [14][57]. Reservations regarding buprenorphine’s clinical use were due to misconceptions about an analgesic “ceiling effect” [9][11]. Until recently, agonists such as buprenorphine have been known for limited intrinsic activity and an inability to produce as large a response at a receptor [15][22]. Initially, it was concluded that all agonists for a receptor will result in different degrees of the same intracellular response [62,64][54][56]. The transduction pathways of a drug activated by an agonist do not act identically for each receptor [4]. Partial agonists are known for their lack of intrinsic efficacy [61][53]. The antinociceptive effect ascribed to buprenorphine is considered mainly mediated by the MOR [65][58]. Bell-shaped dose–response curves for buprenorphine in the 1980s and 1990s showed that there is an optimal range of concentrations for a maximum analgesic effect, with a decrease in activity below or above this range [63][55]. The perception of buprenorphine’s clinical usage may depend on the correct application or interpretation of terms from concepts in receptor theory, such as efficacy and agonist [66][59]. Studies have suggested that different opioid agonists have different downstream effects in the cell when binding and activating the same receptor. Therefore, different opioids cannot be considered equivalent by changing the dose [16][37]. It can no longer be assumed that any ligand activating a receptor will produce a response that is relatively the same, with differences attributed to the agonists’ efficacies [4]. Ligands for a receptor can alter the downstream activity in a pathway, known as biased agonism, ligand-directed signaling, and functional selectivity [67][60]. Opioids that are pure agonists such as morphine or fentanyl produce stronger analgesic effects than drugs such as codeine that have decreased receptor binding [68][61]. However, factors such as affinity and efficacy, as well as variables such as metabolite binding and concurrent receptor binding may alter the perceived effects and receptor activity of buprenorphine [68][61]. The binding affinity of buprenorphine and its metabolites to opioid receptors provides the varied effects seen. Binding affinity is the ability of a drug to bind to a receptor and is measured by the equilibrium inhibitory constant (Ki) [9][11]. Buprenorphine has a high binding affinity at the MOR and KOR, with debated effects [9][11]. Buprenorphine-3-glucuronide has high affinity for the MOR (Ki = 4.9 ± 2.7 μM) and NOR (Ki = 36 ± 0.3 μM). Norbuprenorphine-3-glucoronide had appreciable affinity for the NOR (Ki = 18 ± 0.2 μM) but not the MOR [69][62]. Although norbuprenorphine has a greater efficacy, it is considered a less potent partial agonist than buprenorphine at the MOR [70][63]. A 2002 review described how norbuprenorphine was much less studied than the parent compound but that there was some evidence to suggest that it functioned as a MOR and KOR partial agonist and a DOR and NOR full agonist [11][33]. Competition assays revealed approximately twenty-five-fold lower norbuprenorpine binding to the NOR than was found with buprenorphine [70][63]. All metabolites except nubuprenophine-3-glucuronide have analgesic properties [69,71][62][64]. Buprenorphine alone is not responsible for its analgesic effects, but its varying metabolite concentration through different forms of drug administration may alter the efficacy of the drug. Norbuprenorphine is one of buprenorphine’s better-studied active metabolites and further research must be performed to understand the other metabolites’ pharmacodynamics [11][33]. Norbuprenorphine and buprenorphine have substantially different pharmacological profiles. Norbuprenorphine arises as a result of N-dealkylation catalyzed by cytochrome P450 (CYP3A4) in the liver [19,20][12][13]. The mechanisms and metabolites of this process are illustrated in Figure 1. At the MOR, both norbuprenorphine and buprenorphine are potent partial agonists, with norbuprenorphine having moderate efficacy and buprenorphine having low efficacy. At the NOR, norbuprenorphine has moderate efficacy and buprenorphine has low efficacy, with both substances having low affinity for the receptor. This information was determined using ligand binding experiments and cAMP assays [70][63]. Respiratory depression is induced by norbuprenorphine and mediated by the MOR [72][65]. There is a low risk of respiratory depression with buprenorphine as a monotherapy and this potential effect is rarely considered clinically relevant [73,74][66][67]. Buprenorphine’s active metabolite, norbuprenorphine, was ten-times more potent for causing respiratory depression [72][65]. Buprenorphine was found to be protective against norbuprenorphine’s effect of respiratory depression, both preventing and reversing these effects. An active metabolite of buprenorphine, norbuprenorphine, was alone seen to be responsible for the effects of respiratory depression. Binding experiments show the DOR and, primarily, the MOR as responsible for buprenorphine protecting against norbuprenorphine-induced respiratory depression [17][9]. The intraventricular administration of buprenorphine and norbuprenorphine showed norbuprenorphine’s analgesic activity was 25% that of buprenorphine [75][68]. Norbuprenorphine was 50-fold less potent than buprenorphine through intravenous administration and 4-fold less potent after intraventricular administration in in vivo animal studies. This decrease in potency may be due to poor penetration across the blood–brain barrier compared with buprenorphine ([76][69], although see [21][14]).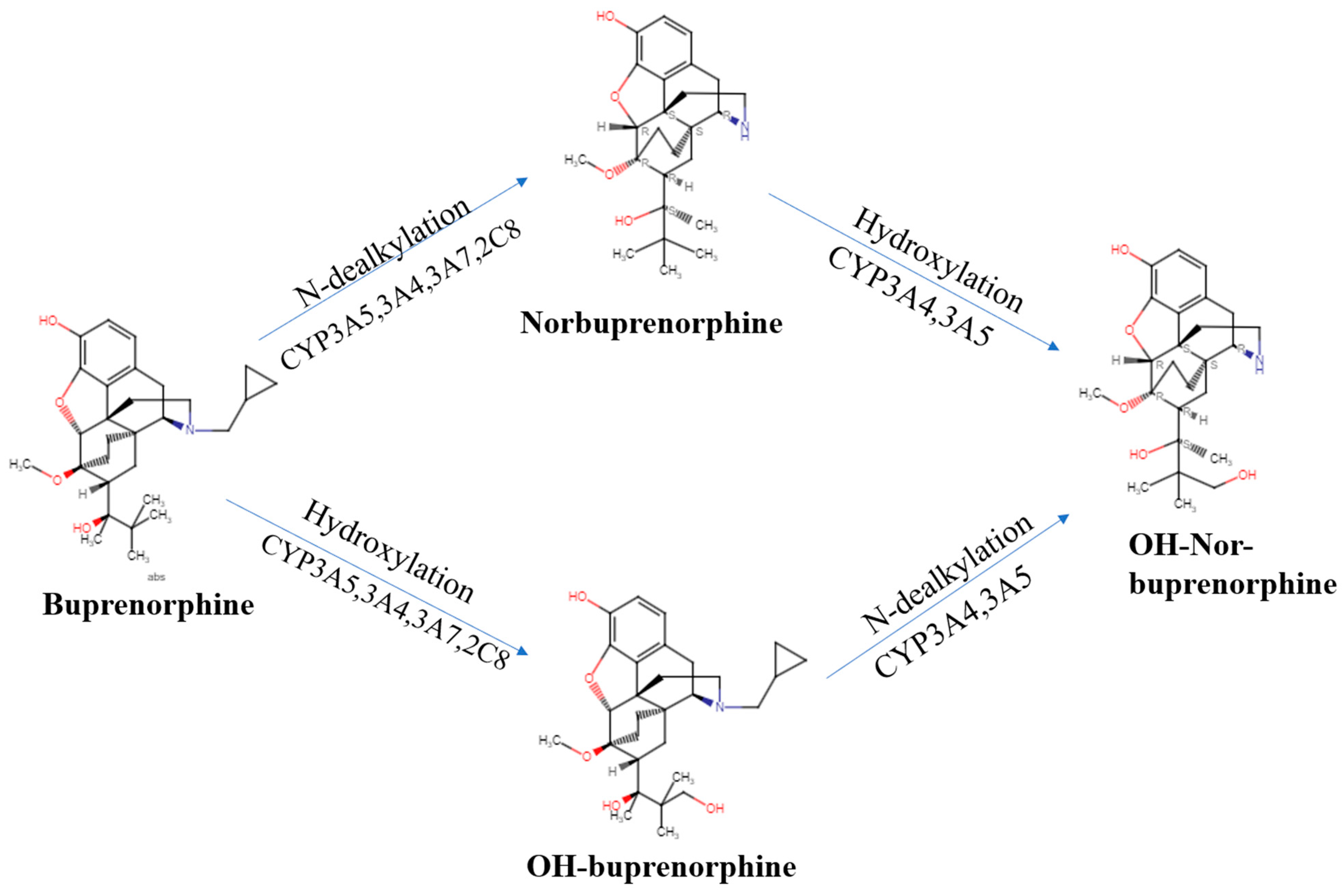
3. Misuse Potential
A growing number of patients are being treated for OUD in the United States using methadone, injectable naltrexone, and buprenorphine. Buprenorphine is the most widely prescribed for OUD in substance use treatment facilities [123][79]. Buprenorphine is a Schedule III drug with a unique mechanism of action that has less potential for misuse than Schedule II drugs (e.g., morphine, oxycodone, fentanyl). The lower abuse potential of buprenorphine may mitigate the number of overdose deaths observed with conventional opioids [81][74]. Buprenorphine is generally perceived to have a low misuse potential alone, particularly when formulated with naloxone [81][74]. Although human research on buprenorphine misuse is informative, there are interpretive difficulties with self-selected samples that often abuse multiple substances and may differ from the general population on a variety of psychiatric, genetic, and socioeconomic variables. Preclinical research allows causal conclusions. Two common preclinical methods to assess misuse potential in nonhuman species are self-administration and conditioned place preference (CPP). Buprenorphine could initiate and maintain self-administration in rhesus monkeys with a morphine history and among one who was opioid naïve [124][80]. Three of four tested baboons intravenously self-administered buprenorphine (1 mg/kg), but at rates half that of codeine [88][81]. The threshold of brain stimulation reward to the median forebrain bundle was reduced by buprenorphine in rats [125][82]. Buprenorphine could maintain self-administration in rats but, unlike with other opioids (fentanyl, oxycodone), use did not escalate over time [126][83]. CPP involves classical conditioning and whether a rodent finds a drug and its associated environment positive, neutral, or negative. Rats formed a CPP to subcutaneous (0.025–0.010 mg/kg) buprenorphine [127][84]. Wild type mice also showed a CPP to a higher (3 mg/kg) dose but MOR knockouts did not [128][85]. The combination of diazepam with a buprenorphine dose (1 mg/kg) that was ineffective by itself produced a CPP [129][86]. There was also a synergistic CPP effect between buprenorphine and cocaine [69][62]. Two other procedures that provide mechanistic insights into the misuse potential for a drug by targeting the nucleus accumbens, a brain structure important for reward, are microdialysis and fast-scan voltammetry. Microdialysis revealed a doubling, albeit over five-hours, in dopamine from the nucleus accumbens following buprenorphine. The combination of buprenorphine and cocaine produced a larger increase in dopamine than only the cocaine [69][62]. Voltammetry showed that buprenorphine could produce an intermediate (25%) nucleus accumbens shell response to buprenorphine that was less than that observed with heroin (60%) [130][87]. It is crucial to appreciate that opioids that act on the MOR, whether as full or partial agonists, also increase dopamine. Chronic administration of 3 mg/kg of buprenorphine to rats using an osmotic minipump greatly potentiated the dopaminergic release in the nucleus accumbens of cocaine [140][88]. Further, escalating doses of buprenorphine to mice decreased striatal D1 and D2 receptors [141][89]. Although there would be value in a direct comparison of the potency of buprenorphine and NBUP for self-administration and CPP in rodents, any effort to develop an “abuse-proof” formulation of BUP will be unsuccessful. With the development of new buprenorphine analogues, the goals will continue to be decreasing the harms relative to those produced by the misuse of illicit or licitly produced heroin, fentanyl, or other opioids.4. Toxicology
Buprenorphine has adverse effects that are similar to other opioids and produces dizziness, nausea, vomiting, sedation, respiratory depression, and constipation. It produces more sweating than codeine, dextropropoxyphene, oxycodone, and pentazocine [18][10]. For obvious ethical reasons, controlled studies of lethality are only completed with experimental animals. The acute toxicity (LD50) of buprenorphine varies based on the method of drug administration [32,33,34,35][90][91][92][93]. When comparing norbuprenorphine and buprenorphine through intravenous administration, the LD50 values are 146.5 and 234.6 mg/kg, respectively, and the norbuprenorphine-to-buprenorphine LD50 ratio was found to be 1/16–1/23 [17][9]. The dose–effect relationship of buprenorphine with respiratory depression suggests limited effects or a plateau of effects over a 0.008–3 mg/kg intravenous dose range [73][66]. In a study with healthy volunteers, intramuscular buprenorphine (0.15–1.2 mg) increased the risk of respiratory depression linearly; however, the effect was not clinically significant [142][94]. With sublingual buprenorphine (1–31 mg), patients reached respiratory depression at doses of 8 mg or more [85][78]. A study on 50 postoperative patients with intravenous buprenorphine (0.4–7.0 mg) showed no signs of respiratory depression for a 24 h period [143][95]. Healthy volunteers with intravenous buprenorphine (0.1 mg/70 kg body weight) demonstrated a ceiling in respiratory depression but not in analgesic efficacy [52][44]. Animal experiments show that the respiratory ceiling occurs at a lower dose (>0.2 mg/ kg) than the analgesic effect ceiling, which will only occur in doses beyond the therapeutic dose range [74,84][67][77]. The respiratory effects are rarely reported in maintenance therapy; however, in situations of abuse, features of opioid poisoning can be present with buprenorphine [17][9]. Experimental and clinical data show that there is a limit of buprenorphine’s maximum depressant effect [91][96]. Although the dose–response curve shows a plateau, the idea that respiratory effects are limited is dangerous, since buprenorphine in combination with drugs such as sedatives can cause fatal respiratory depression [73][66]. Importantly, studies have shown that buprenorphine treatment can be effective in reducing mortality for individuals with OUD and investigations support that ceasing these opioid agonist treatments can lead to higher rates of all-cause mortalities [144][97]. Evidence suggests a protective reaction to fentanyl-induced respiratory depression at 2 ng/mL concentrations and higher. Furthermore, when the MOR occupancy by buprenorphine is sufficiently high, fentanyl is not able to bind and activate the MOR, resulting in a decrease in respiratory depression in those overdosing on fentanyl [145][98]. Buprenorphine, in turn, can be the cause and treatment for respiratory depression. Buprenorphine brings about mild respiratory depression, whereas at high doses fentanyl causes significant respiratory depression and apnea [145][98]. Although buprenorphine has been observed to cause partial respiratory depression, the results indicate that administration of buprenorphine buccal film may have a decreased risk of abuse and respiratory depression compared with the full MOR agonist oxycodone [146][99]. Buprenorphine has an increased potential for misuse when central nervous system depressants such as benzodiazepines are used simultaneously [123][79]. Benzodiazepines are not CYP3A4 inhibitors; however, some, such as diazepam and flunitrazepam, are metabolized through this enzyme. This drug interaction is likely additive or synergistic [21][14]. Interactions between benzodiazepines and opioids, as well as buprenorphine and methadone, have resulted in respiratory depression in animal models and humans [17,147][9][100]. Opioids and benzodiazepines act in combination with different classes of opioid and GABA receptors, but only limited interactions have been reported [17][9]. Benzodiazepine and buprenorphine’s concurrent use causes a decreased reaction time and is associated with an increased risk for emergency room visits for accidental injury [123][79]. It should be noted that many patients with substance use disorders use benzodiazepines during treatment [17][9]. However, whereas one-third of patients are prescribed both buprenorphine and benzodiazepines, approximately another third regularly use illegally obtained benzodiazepines, making it difficult to decrease the risk of substance use relapse. Because of the concern that benzodiazepines might impede opioid maintenance therapy, the US Food and Drug Administration urged withholding opioid agonist treatment if the patient was taking benzodiazepines [123][79]. Pharmacodynamic interaction is the expected cause of buprenorphine–benzodiazepine drug interaction found in humans and animals. However, flunitrazepam–buprenorphine drug interaction is thought to have a pharmacokinetic interaction. Flunitrazepam alters buprenorphine lethality in rats, with a six-fold decrease of its LD50, which appears to be opioid-specific as there was only a two-fold decrease in methadone and no significant effect on morphine [17][9].References
- Davis, M.P.; Pasternak, G.; Behm, B. Treating Chronic Pain: An overview of clinical studies centered on the buprenorphine option. Drugs 2018, 78, 1211–1228.
- Campbell, N.D.; Lovell, A.M. The history of the development of buprenorphine as an addiction therapeutic: Campbell & Lovell. Ann. N. Y. Acad. Sci. 2012, 1248, 124–139.
- Jasinski, D.R. Human pharmacology and abuse potential of the analgesic buprenorphine: A potential agent for treating narcotic addiction. Arch. Gen. Psychiatry 1978, 35, 501.
- Cox, B.M.; Christie, M.J.; Devi, L.; Toll, L.; Traynor, J.R. Challenges for opioid receptor nomenclature: IUPHAR Review 9: Challenges for opioid receptor nomenclature. Br. J. Pharmacol. 2015, 172, 317–323.
- Tan, K.Z.; Cunningham, A.M.; Joshi, A.; Oei, J.L.; Ward, M.C. Expression of kappa opioid receptors in developing rat brain—Implications for perinatal buprenorphine exposure. Reprod. Toxicol. 2018, 78, 81–89.
- Eschenroeder, A.C.; Vestal-Laborde, A.A.; Sanchez, E.S.; Robinson, S.E.; Sato-Bigbee, C. Oligodendrocyte responses to buprenorphine uncover novel and opposing roles of μ-opioid- and nociceptin/orphanin FQ receptors in cell development: Implications for drug addiction treatment during pregnancy. Glia 2012, 60, 125–136.
- Kogan, L.; Hellyer, P.; Rishniw, M.; Schoenfeld-Tacher, R. The US opioid epidemic and its impact on US general practice veterinarians. Front. Vet. Sci. 2019, 6, 222.
- Piper, B.J.; McCall, K.L.; Kogan, L.R.; Hellyer, P. Assessment of controlled substance distribution to U.S. veterinary teaching institutions from 2006 to 2019. Front. Vet. Sci. 2020, 7, 615646.
- Mégarbane, B.; Hreiche, R.; Pirnay, S.; Marie, N.; Baud, F.J. Does high-dose buprenorphine cause respiratory depression? Possible mechanisms and therapeutic consequences. Toxicol. Rev. 2006, 25, 79–85.
- Davis, M.P. Buprenorphine in cancer pain. Support. Care Cancer 2005, 13, 878–887.
- Gudin, J.; Fudin, J. A Narrative pharmacological review of buprenorphine: A unique opioid for the treatment of chronic pain. Pain Ther. 2020, 9, 41–54.
- Iribarne, C.; Picart, D.; Dréano, Y.; Bail, J.-P.; Berthou, F. Involvement of cytochrome P450 3A4 in N-dealkylation of buprenorphine in human liver microsomes. Life Sci. 1997, 60, 1953–1964.
- Kobayashi, K.; Yamamoto, T.; Chiba, K.; Tani, M.; Shimada, N.; Ishizaki, T.; Kuroiwa, Y. Human buprenorphine N-dealkylation is catalyzed by cytochrome P450 3A4. Drug Metab. Dispos. Biol. Fate Chem. 1998, 26, 818–821.
- Elkader, A.; Sproule, B. Buprenorphine: Clinical pharmacokinetics in the treatment of opioid dependence. Clin. Pharmacokinet. 2005, 44, 661–680.
- Griffin, B.A.; Caperton, C.O.; Russell, L.N.; Cabanlong, C.V.; Wilson, C.D.; Urquhart, K.R.; Martins, B.S.; Zita, M.D.; Patton, A.L.; Alund, A.W.; et al. In utero exposure to norbuprenorphine, a major metabolite of buprenorphine, induces fetal opioid dependence and leads to neonatal opioid withdrawal syndrome. J. Pharmacol. Exp. Ther. 2019, 370, 9–17.
- Jensen, M.L.; Foster, D.; Upton, R.; Grant, C.; Martinez, A.; Somogyi, A. Comparison of cerebral pharmacokinetics of buprenorphine and norbuprenorphine in an in vivo sheep model. Xenobiotica 2007, 37, 441–457.
- Gruber, V.A.; Rainey, P.M.; Moody, D.E.; Morse, G.D.; Ma, Q.; Prathikanti, S.; Pade, P.A.; Alvanzo, A.A.H.; McCance-Katz, E.F. Interactions between buprenorphine and the protease inhibitors darunavir-ritonavir and fosamprenavir-ritonavir. Clin. Infect. Dis. 2012, 54, 414–423.
- Waters, R.C.; Perez, M. How do I interpret and use quantitative buprenorphine and norbuprenorphine urine levels? Cleve. Clin. J. Med. 2022, 89, 557–560.
- Kacinko, S.; Jones, H.; Johnson, R.; Choo, R.; Huestis, M. Correlations of maternal buprenorphine dose, buprenorphine, and metabolite concentrations in meconium with neonatal outcomes. Clin. Pharmacol. Ther. 2008, 84, 604–612.
- Goodwin, R.S.; Wilkins, D.G.; Averin, O.; Choo, R.E.; Schroeder, J.R.; Jasinski, D.R.; Johnson, R.E.; Jones, H.E.; Huestis, M.A. Buprenorphine and norbuprenorphine in hair of pregnant women and their infants after controlled buprenorphine administration. Clin. Chem. 2007, 53, 2136–2143.
- Ohtani, M. Basic pharmacology of buprenorphine. Eur. J. Pain Suppl. 2007, 1, 69–73.
- Brunton, L.L.; Knollmann, B.C.; Hilal-Dandan, R. (Eds.) Goodman & Gilman’s the Pharmacological Basis of Therapeutics, 13th ed.; McGraw Hill Medical: New York, NY, USA, 2018; ISBN 978-1-259-58473-2.
- Vierke, C.; Marxen, B.; Boettcher, M.; Hiemke, C.; Havemann-Reinecke, U. Buprenorphine-cannabis interaction in patients undergoing opioid maintenance therapy. Eur. Arch. Psychiatry Clin. Neurosci. 2021, 271, 847–856.
- National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Population Health and Public Health Practice; Committee on the Health Effects of Marijuana: An Evidence Review and Research Agenda. The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research; The National Academies Press: Washington, DC, USA, 2017; ISBN 978-0-309-45304-2.
- Lexicomp. Lexicomp Drug Interactions; Lexicomp®: Hudson, OH, USA. Available online: https://www.uptodate.com/drug-interactions/?source=responsive_home#di-druglist (accessed on 19 August 2023).
- McCance-Katz, E.F.; Sullivan, L.E.; Nallani, S. Drug interactions of clinical importance among the opioids, methadone and buprenorphine, and other frequently prescribed medications: A review. Am. J. Addict. 2010, 19, 4–16.
- Reynaud, M.; Petit, G.; Potard, D.; Courty, P. Six deaths linked to concomitant use of buprenorphine and benzodiazepines. Addict. Abingdon Engl. 1998, 93, 1385–1392.
- Coe, M.A.; Lofwall, M.R.; Walsh, S.L. Buprenorphine pharmacology review: Update on transmucosal and long-acting formulations. J. Addict. Med. 2019, 13, 93–103.
- Oifa, S.; Sydoruk, T.; White, I.; Ekstein, M.P.; Marouani, N.; Chazan, S.; Skornick, Y.; Weinbroum, A.A. Effects of intravenous patient-controlled analgesia with buprenorphine and morphine alone and in combination during the first 12 postoperative hours: A randomized, double-blind, four-arm trial in adults undergoing abdominal surgery. Clin. Ther. 2009, 31, 527–541.
- Mercadante, S.; Villari, P.; Ferrera, P.; Porzio, G.; Aielli, F.; Verna, L.; Casuccio, A. Safety and effectiveness of intravenous morphine for episodic breakthrough pain in patients receiving transdermal buprenorphine. J. Pain Symptom Manag. 2006, 32, 175–179.
- Huxtable, C.A.; Macintyre, P.E. An alternative way of managing acute pain in patients who are in buprenorphine opioid substitution therapy programs. Eur. J. Anaesthesiol. 2013, 30, 717–718.
- Ohtani, M.; Kotaki, H.; Uchino, K.; Sawada, Y.; Iga, T. Pharmacokinetic analysis of enterohepatic circulation of buprenorphine and its active metabolite, norbuprenorphine, in rats. Drug Metab. Dispos. Biol. Fate Chem. 1994, 22, 2–7.
- Robinson, S.E. Buprenorphine: An analgesic with an expanding role in the treatment of opioid addiction. CNS Drug Rev. 2006, 8, 377–390.
- Khroyan, T.V.; Wu, J.; Polgar, W.E.; Cami-Kobeci, G.; Fotaki, N.; Husbands, S.M.; Toll, L. BU08073 a buprenorphine analogue with partial agonist activity at μ-receptors in vitro but long-lasting opioid antagonist activity in vivo in mice: BU08073 a long-lasting opiate antagonist. Br. J. Pharmacol. 2015, 172, 668–680.
- Kuo, A.; Magiera, J.; Rethwan, N.; Andersson, Å.; Leen Lam, A.; Wyse, B.; Meutermans, W.; Lewis, R.; Smith, M. In vitro profiling of opioid ligands using the cAMP formation inhibition assay and the β-arrestin2 recruitment assay: No two ligands have the same profile. Eur. J. Pharmacol. 2020, 872, 172947.
- Clark, T.P. The history and pharmacology of buprenorphine: New advances in cats. J. Vet. Pharmacol. Ther. 2022, 45, S1–S30.
- Emery, M.A.; Eitan, S. Members of the same pharmacological family are not alike: Different Opioids, different consequences, hope for the opioid crisis? Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 92, 428–449.
- Waldman, S.A. Does potency predict clinical efficacy? Illustration through an antihistamine model. Ann. Allergy Asthma Immunol. Off. Publ. Am. Coll. Allergy Asthma Immunol. 2002, 89, 7–11; quiz 11–12, 77.
- Centers for Medicare & Medicaid Services. Available online: https://medicaid.utah.gov/Documents/files/Opioid-Morphine-EQ-Conversion-Factors.pdf (accessed on 20 September 2023).
- Piper, B.J.; Shah, D.T.; Simoyan, O.M.; McCall, K.L.; Nichols, S.D. Trends in medical use of opioids in the U.S., 2006–2016. Am. J. Prev. Med. 2018, 54, 652–660.
- Poliwoda, S.; Noor, N.; Jenkins, J.S.; Stark, C.W.; Steib, M.; Hasoon, J.; Varrassi, G.; Urits, I.; Viswanath, O.; Kaye, A.M.; et al. Buprenorphine and its formulations: A comprehensive review. Health Psychol. Res. 2022, 10, 37517.
- Dong, R.; Wang, H.; Li, D.; Lang, L.; Gray, F.; Liu, Y.; Laffont, C.M.; Young, M.; Jiang, J.; Liu, Z.; et al. Pharmacokinetics of sublingual buprenorphine tablets following single and multiple doses in Chinese participants with and without opioid use disorder. Drugs RD 2019, 19, 255–265.
- Kuhlman, J.J.; Lalani, S.; Magluilo, J.; Levine, B.; Darwin, W.D.; Johnson, R.E.; Cone, E.J. Human pharmacokinetics of intravenous, sublingual, and buccal buprenorphine. J. Anal. Toxicol. 1996, 20, 369–378.
- Seligman, N.S.; Cleary, B.J.; Berghella, V. Methadone and Buprenorphine Pharmacotherapy of Opioid Use Disorder During Pregnancy. Available online: https://www.medilib.ir/uptodate/show/87238 (accessed on 27 October 2020).
- Nath, R.P.; Upton, R.A.; Everhart, E.T.; Cheung, P.; Shwonek, P.; Jones, R.T.; Mendelson, J.E. Buprenorphine pharmacokinetics: Relative bioavailability of sublingual tablet and liquid formulations. J. Clin. Pharmacol. 1999, 39, 619–623.
- Schuh, K.J.; Johanson, C.-E. Pharmacokinetic comparison of the buprenorphine sublingual liquid and tablet. Drug Alcohol Depend. 1999, 56, 55–60.
- Lindhardt, K.; Ravn, C.; Gizurarson, S.; Bechgaard, E. Intranasal absorption of buprenorphine—In vivo bioavailability study in sheep. Int. J. Pharm. 2000, 205, 159–163.
- Kongstorp, M.; Bogen, I.L.; Stiris, T.; Andersen, J.M. High accumulation of methadone compared with buprenorphine in fetal rat brain after maternal exposure. J. Pharmacol. Exp. Ther. 2019, 371, 130–137.
- Han, L.W.; Gao, C.; Mao, Q. An update on expression and function of P-Gp/ABCB1 and BCRP/ABCG2 in the placenta and fetus. Expert Opin. Drug Metab. Toxicol. 2018, 14, 817–829.
- Auvity, S.; Breuil, L.; Goislard, M.; Bottlaender, M.; Kuhnast, B.; Tournier, N.; Caillé, F. An original radio-biomimetic approach to synthesize radiometabolites for PET imaging. Nucl. Med. Biol. 2020, 90–91, 10–14.
- Liao, M.Z.; Gao, C.; Shireman, L.M.; Phillips, B.; Risler, L.J.; Neradugomma, N.K.; Choudhari, P.; Prasad, B.; Shen, D.D.; Mao, Q. P-gp/ABCB1 exerts differential impacts on brain and fetal exposure to norbuprenorphine. Pharmacol. Res. 2017, 119, 61–71.
- Lindemalm, S.; Nydert, P.; Svensson, J.-O.; Stahle, L.; Sarman, I. Transfer of buprenorphine into breast milk and calculation of infant drug dose. J. Hum. Lact. 2009, 25, 199–205.
- Katzung, B.G. (Ed.) Basic & Clinical Pharmacology, 14th ed.; A Lange medical book; McGraw-Hill Education: New York, NY, USA; Chicago, IL, USA; San Francisco, CA, USA; Athens, Greece; London, UK; Madrid, Spain; Mexico City, Mexico; Milan, Italy; New Delhi, India; Singapore; Sydney, Australia; Toronto, Japan, 2018; ISBN 978-1-259-64115-2.
- Kenakin, T. Principles: Receptor theory in pharmacology. Trends Pharmacol. Sci. 2004, 25, 186–192.
- Dum, J.E.; Herz, A. In vivo receptor binding of the opiate partial agonist, buprenorphine, correlated with its agonistic and antagonistic actions. Br. J. Pharmacol. 1981, 74, 627–633.
- De Lean, A.; Stadel, J.M.; Lefkowitz, R.J. A ternary complex model explains the agonist-specific binding properties of the adenylate cyclase-coupled beta-adrenergic receptor. J. Biol. Chem. 1980, 255, 7108–7117.
- Stahl, S.M. Stahl’s Essential Psychopharmacology: Neuroscientific Basis and Practical Application, 4th ed.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013; ISBN 978-1-107-02598-1.
- Tejwani, G.A.; Rattan, A.K. The role of spinal opioid receptors in antinociceptive effects produced by intrathecal administration of hydromorphone and buprenorphine in the rat. Anesth. Analg. 2002, 94, 1542–1546.
- Ding, Z.; Raffa, R.B. Identification of an additional supraspinal component to the analgesic mechanism of action of buprenorphine. Br. J. Pharmacol. 2009, 157, 831–843.
- Galandrin, S.; Oligny-Longpré, G.; Bouvier, M. The evasive nature of drug efficacy: Implications for drug discovery. Trends Pharmacol. Sci. 2007, 28, 423–430.
- Mitra, R. (Ed.) Principles of Rehabilitation Medicine; McGraw-Hill: New York, NY, USA, 2019; ISBN 978-0-07-179333-9.
- Brown, S.M.; Holtzman, M.; Kim, T.; Kharasch, E.D. Buprenorphine metabolites, buprenorphine-3-glucuronide and norbuprenorphine-3-glucuronide, are biologically active. Anesthesiology 2011, 115, 1251–1260.
- Huang, P.; Kehner, G.B.; Cowan, A.; Liu-Chen, L.Y. Comparison of pharmacological activities of buprenorphine and norbuprenorphine: Norbuprenorphine is a potent opioid agonist. J. Pharmacol. Exp. Ther. 2001, 297, 688–695.
- Butler, S. Buprenorphine—Clinically useful but often misunderstood. Scand. J. Pain 2013, 4, 148–152.
- Ohtani, M.; Kotaki, H.; Nishitateno, K.; Sawada, Y.; Iga, T. Kinetics of respiratory depression in rats induced by buprenorphine and its metabolite, norbuprenorphine. J. Pharmacol. Exp. Ther. 1997, 281, 428–433.
- Dahan, A.; Yassen, A.; Bijl, H.; Romberg, R.; Sarton, E.; Teppema, L.; Olofsen, E.; Danhof, M. Comparison of the respiratory effects of intravenous buprenorphine and fentanyl in humans and rats. Br. J. Anaesth. 2005, 94, 825–834.
- Dahan, A. Opioid-induced respiratory effects: New data on buprenorphine. Palliat. Med. 2006, 20, 3–8.
- Ohtani, M.; Kotaki, H.; Sawada, Y.; Iga, T. Comparative analysis of buprenorphine- and norbuprenorphine-induced analgesic effects based on pharmacokinetic-pharmacodynamic modeling. J. Pharmacol. Exp. Ther. 1995, 272, 505–510.
- Coller, J.K.; Christrup, L.L.; Somogyi, A.A. Role of active metabolites in the use of opioids. Eur. J. Clin. Pharmacol. 2009, 65, 121–139.
- Picard, N.; Cresteil, T.; Djebli, N.; Marquet, P. In vitro metabolism study of buprenorphine: Evidence for new metabolic pathways. Drug Metab. Dispos. 2005, 33, 689–695.
- Lutfy, K.; Eitan, S.; Bryant, C.D.; Yang, Y.C.; Saliminejad, N.; Walwyn, W.; Kieffer, B.L.; Takeshima, H.; Carroll, F.I.; Maidment, N.T.; et al. Buprenorphine-induced antinociception is mediated by μ-opioid receptors and compromised by concomitant activation of opioid receptor-like receptors. J. Neurosci. 2003, 23, 10331–10337.
- Lutfy, K.; Hossain, S.M.; Khaliq, I.; Maidment, N.T. Orphanin FQ/nociceptin attenuates the development of morphine tolerance in rats: Orphanin FQ/nociceptin and morphine tolerance. Br. J. Pharmacol. 2001, 134, 529–534.
- Pergolizzi, J.; Böger, R.H.; Budd, K.; Dahan, A.; Erdine, S.; Hans, G.; Kress, H.-G.; Langford, R.; Likar, R.; Raffa, R.B.; et al. Opioids and the management of chronic severe pain in the elderly: Consensus statement of an international expert panel with focus on the six clinically most often used world health organization step iii opioids (buprenorphine, fentanyl, hydromorphone, methadone, morphine, oxycodone). Pain Pract. 2008, 8, 287–313.
- Hale, M.; Garofoli, M.; Raffa, R.B. Benefit-risk analysis of buprenorphine for pain management. J. Pain Res. 2021, 14, 1359–1369.
- Cremeans, C.M.; Gruley, E.; Kyle, D.J.; Ko, M.-C. Roles of μ-opioid receptors and nociceptin/orphanin fq peptide receptors in buprenorphine-induced physiological responses in primates. J. Pharmacol. Exp. Ther. 2012, 343, 72–81.
- Kögel, B.; Christoph, T.; Straβburger, W.; Friderichs, E. Interaction of μ-opioid receptor agonists and antagonists with the analgesic effect of buprenorphine in mice. Eur. J. Pain 2005, 9, 599–611.
- Yassen, A.; Kan, J.; Olofsen, E.; Suidgeest, E.; Dahan, A.; Danhof, M. Mechanism-based pharmacokinetic-pharmacodynamic modeling of the respiratory-depressant effect of buprenorphine and fentanyl in rats. J. Pharmacol. Exp. Ther. 2006, 319, 682–692.
- Walsh, S.L.; Preston, K.L.; Stitzer, M.L.; Cone, E.J.; Bigelow, G.E. Clinical pharmacology of buprenorphine: Ceiling effects at high doses. Clin. Pharmacol. Ther. 1994, 55, 569–580.
- Park, T.W.; Larochelle, M.R.; Saitz, R.; Wang, N.; Bernson, D.; Walley, A.Y. Associations between prescribed benzodiazepines, overdose death and buprenorphine discontinuation among people receiving buprenorphine. Addiction 2020, 115, 924–932.
- Mello, N.K.; Bree, M.P.; Mendelson, J.H. Buprenorphine self-administration by rhesus monkey. Pharmacol. Biochem. Behav. 1981, 15, 215–225.
- Lukas, S.E.; Griffiths, R.R.; Brady, J.V. Buprenorphine self-administration by the baboon: Comparison with other opioids. NIDA Res. Monogr. 1983, 43, 178–183.
- Hubner, C.B.; Kornetsky, C. The reinforcing properties of the mixed agonist-antagonist buprenorphine as assessed by brain-stimulation reward. Pharmacol. Biochem. Behav. 1988, 30, 195–197.
- Wade, C.L.; Vendruscolo, L.F.; Schlosburg, J.E.; Hernandez, D.O.; Koob, G.F. Compulsive-like responding for opioid analgesics in rats with extended access. Neuropsychopharmacology 2015, 40, 421–428.
- Gaiardi, M.; Bartoletti, M.; Bacchi, A.; Gubellini, C.; Babbini, M. Motivational properties of buprenorphine as assessed by place and taste conditioning in rats. Psychopharmacology 1997, 130, 104–108.
- Marquez, P.; Baliram, R.; Kieffer, B.L.; Lutfy, K. The mu opioid receptor is involved in buprenorphine-induced locomotor stimulation and conditioned place preference. Neuropharmacology 2007, 52, 1336–1341.
- Ma, L.-L.; Freret, T.; Lange, M.; Bourgine, J.; Coquerel, A.; Lelong-Boulouard, V. Benzodiazepines increase the reward effects of buprenorphine in a conditioned place preference test in the mouse. Fundam. Clin. Pharmacol. 2014, 28, 681–689.
- Isaacs, D.P.; Leman, R.P.; Everett, T.J.; Lopez-Beltran, H.; Hamilton, L.R.; Oleson, E.B. Buprenorphine is a weak dopamine releaser relative to heroin, but its pretreatment attenuates heroin-evoked dopamine release in rats. Neuropsychopharmacol. Rep. 2020, 40, 355–364.
- Placenza, F.M.; Rajabi, H.; Stewart, J. Effects of chronic buprenorphine treatment on levels of nucleus accumbens glutamate and on the expression of cocaine-induced behavioral sensitization in rats. Psychopharmacology 2008, 200, 347–355.
- Allouche, S.; Le Marec, T.; Coquerel, A.; Noble, F.; Marie, N. Striatal dopamine D1 and D2 receptors are differentially regulated following buprenorphine or methadone treatment. Psychopharmacology 2015, 232, 1527–1533.
- Belbuca , Food and Drug Administration, Reference ID: 3837517. Revised: 10/2015. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/207932s000lbl.pdf (accessed on 20 August 2023).
- Butrans , Food and Drug Administration, Reference ID: 3534876. Revised: 06/2014. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/021306s015s019lbl.pdf (accessed on 20 August 2023).
- Sublocade , Food and Drug Administration, Reference ID: 4188740. Revised: 11/2017. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/209819s000lbl.pdf (accessed on 20 August 2023).
- Subutex , Food and Drug Administration, Reference ID: 4215177. Revised: 02/2018. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/020732s018lbl.pdf (accessed on 20 August 2023).
- Orwin, J.M.; Orwin, J.; Price, M. A double blind comparison of buprenorphine and morphine in conscious subjects following administration by the intramuscular route. Acta Anaesthesiol. Belg. 1976, 27, 171–181.
- Budd, K. High dose buprenorphine for postoperative analgesia. Anaesthesia 1981, 36, 900–903.
- Kress, H.G. Clinical update on the pharmacology, efficacy and safety of transdermal buprenorphine. Eur. J. Pain 2009, 13, 219–230.
- Santo, T.; Clark, B.; Hickman, M.; Grebely, J.; Campbell, G.; Sordo, L.; Chen, A.; Tran, L.T.; Bharat, C.; Padmanathan, P.; et al. Association of opioid agonist treatment with all-cause mortality and specific causes of death among people with opioid dependence: A systematic review and meta-analysis. JAMA Psychiatry 2021, 78, 979.
- Olofsen, E.; Algera, M.H.; Moss, L.; Dobbins, R.L.; Groeneveld, G.J.; van Velzen, M.; Niesters, M.; Dahan, A.; Laffont, C.M. Modeling buprenorphine reduction of fentanyl-induced respiratory depression. JCI Insight 2022, 7, e156973.
- Webster, L.R.; Cater, J.; Smith, T. Pharmacokinetics of buprenorphine buccal film and orally-administered oxycodone in a respiratory study: An analysis of secondary outcomes from a randomized controlled trial. Pain Ther. 2022, 11, 817–825.
- Gueye, P.N. Buprenorphine and midazolam act in combination to depress respiration in rats. Toxicol. Sci. 2002, 65, 107–114.
More
