Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Brian J Piper and Version 2 by Peter Tang.
Buprenorphine has high affinity for the mu-opioid receptor (MOR), delta (DOR), and kappa (KOR) and intermediate affinity for the nociceptin (NOR). Buprenorphine’s active metabolite, norbuprenorphine, crosses the blood–brain barrier, is a potent metabolite that attenuates the analgesic effects of buprenorphine due to binding to NOR, and is responsible for the respiratory depressant effects. The area under the concentration curves are very similar for buprenorphine and norbuprenorphine, which indicates that it is important to consider this metabolite.
- opioid use disorder
- pain
- opiate
1. Introduction and History
Buprenorphine was first derived from thebaine in 1966 and was subsequently characterized as a partial agonist at the mu-opioid receptor (MOR) [1]. The Committee on Drug Addiction primarily focused on morphine and looked for a way to ensure its multitude of uses without its addictive side effects in the 1920s. Buprenorphine was considered a part of the solution to the 20th century opium problem. Its agonist–antagonist pharmacological character was more fully characterized in 1972 and its potential as an addiction treatment recognized in 1979 [2][3][2,3]. Buprenorphine is a semi-synthetic and lipophilic drug. It has activity at all four major opioid receptors: MOR, kappa (KOR), delta (DOR), and the nociceptin receptor (NOR). Of the four main opioid receptors, three (MOR, DOR, and KOR) were identified in the 1960s and the opioid receptor like (ORL), currently and henceforth designated as NOP, was discovered in the 1990s [4]. In addition to its involvement in nociception, the KOR is widely expressed during prenatal and early postnatal periods including on progenitor, ependymal, and neuronal cells [5], which raises the possibility that a KOR antagonist such as buprenorphine could have an adverse impact on brain development. This may also apply to other (MOR/NOR) opioid receptors that are important for myelination [6]. The NOP is considered an atypical, low affinity receptor for opioid peptides [4]. Although marketed for analgesia and addiction treatment, early research subjects reported that buprenorphine was the “most reinforcing drug they had ever used” [2]. Injectable buprenorphine became commercially available in the US in 1981 [1]. By 1985, it was available in 29 countries [2]. Buprenorphine was originally considered a Schedule V narcotic in the US until 2002 when, after three attempts by the Drug Enforcement Agency, it was rescheduled as Schedule III [1][2][1,2]. US sales of buprenorphine have increased substantially. Buprenorphine was the most commonly used opioid by US veterinarians [7]. This may change as, by morphine mg equivalent, buprenorphine was only the seventh most common opioid in US veterinary teaching institutions [8].
2. Pharmacokinetics and Pharmacodynamics
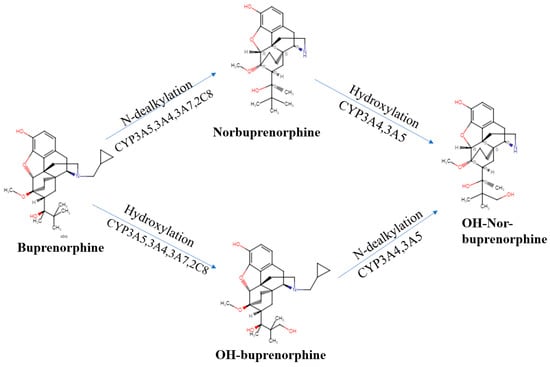
We now turn our attention to the pharmacokinetics, pharmacodynamics, and their interaction for this semi-synthetic opioid. Many of buprenorphine’s pharmacokinetic properties explain its unique effects [9][17]. Buprenorphine’s metabolism follows non-saturable Michaelis–Menten kinetics [10][18] that furthers its analgesic effects [11][9]. There are two major metabolic pathways in buprenorphine’s metabolism. Buprenorphine undergoes N-dealkylation catalyzed by the hepatic cytochrome P (CYP) 450 (CYP P450-3A4) and glucuronidation, resulting in three major metabolites: buprenorphine-3-glucuronide (B3G), N-dealkylbuprenorphine, and norbuprenorphine-3-glucuronide (N3G). The CYP3A4 system metabolizes buprenorphine to norbuprenorphine through N-dealkylation of the cyclopropylmethyl group [11][12][13][9,19,20]. CYP3A4 is predominantly responsible for this, although CYP2C8 also contributes. The ratio of norbuprenorphine to buprenorphine in urine can provide an index of the recency of buprenorphine administration, the potential use of a CYP3A4 inducer, or the probability of buprenorphine “spiking”, involving submerging the film or tablet in urine in an effort to have a positive immunoassay result [14][21]. Some conclude that norbuprenorphine does not readily cross the blood–brain barrier ([11][9], although see [15][22]). Sheep are used for the similarity of their blood–brain barrier to humans. The large size of sheep contributes to their use in pharmacokinetics investigations. The peak concentration of buprenorphine was half that in the sagittal sinus relative to the arterial quantities, which indicated only intermediate permeability across the blood–brain barrier. In contrast, the peak concentration and time to peak concentration were very similar for samples from the sagittal sinus and arterial blood for norbuprenorphine. Also noteworthy was that the peak sagittal concentration of norbuprenorphine was over twenty-fold higher than that of buprenorphine in this species [16][23]. Among patients receiving buprenorphine/naloxone for two weeks, the twenty-four hour area under the concentration curve was equivalent for buprenorphine and norbuprenorphine [17][24]. Norbuprenorphine is commonly measured in urine analyses because of these high concentrations [18][25]. Norbuprenorphine and buprenorphine are both detectable in meconium, although norburprenorphine’s quantities were six-fold higher [19][26]. Another biological matrix that can provide an index of buprenorphine use is hair. The hair of pregnant women, and a small sample of their offspring, had measurable norbuprenorphine and buprenorphine [20][27]. Norbuprenorphine is then metabolized to N3G [9][21][17,28]. Other metabolites, including hydroxy buprenorphine and hydroxynorbuprenorphine, have been identified. CYP3A4 produces hydroxybuprenorphine [10][18]. The CYP3A4 activity varies between individuals and can be induced, resulting in wide differences in pharmacokinetics [10][18]. Buprenorphine is eliminated in the urine and in feces, accounting for one-third and two-thirds of the eliminated buprenorphine, respectively [10][18]. It is important to note that interactions between different drugs can occur due to the inhibitory effects on CYP3A4, which is often blocked by various medications For instance, when ritonavir, a potent CYP3A4 inhibitor, is administered alone, it can elevate the levels of both buprenorphine and norbuprenorphine without intensifying the adverse effects associated with buprenorphine [22][15]. For instance, when ritonavir, a potent CYP3A4 inhibitor, is administered alone, it can elevate the levels of both buprenorphine and norbuprenorphine without intensifying the adverse effects associated with buprenorphine [17][24]. As cannabis is a CYP3A4 inhibitor, a doubling of buprenorphine concentrations in regular cannabis users [23][29] may impact many patients that use cannabis for chronic pain [24][30]. Buprenorphine has an absolute contraindication in humans to not be combined with the antiretroviral atazanavir, the histamine (H1) blocker azelastine, the typical antipsychotic bromperidol, the irritable bowel agent eluxadoline, the sleeping sickness agent fexinidazole, the calcium antagonist flunarizine, the antibiotic fusidic acid, kratom, monoamine oxidase inhibitors, naltrexone, the H1 antagonist olopatadine, the anticholinergic orphenadrine, the antihistamine oxomemazine, the depressant paraldehyde, the opioid antagonist samidorphan, or the oncology agent thalidomide [25][31]. Buprenorphine has fewer drug–drug interactions than other opioids that are metabolized through CYP3A4 [1]. Further research needs to be conducted on buprenorphine’s drug interactions, as there is more information for methadone [26][36]. Buprenorphine alone may have a higher ceiling effect than typical MOR agonists, but in combination with benzodiazepines, it could result in a potentially life-threatening drug interaction due to sedation and respiratory depression properties. The mechanism of the respiratory depression is unclear [27][28][37,38]. There is some noted benefit to combining opioids with buprenorphine to produce sub-addictive analgesia [1]. In the postoperative setting, buprenorphine did not impair morphine analgesia (buprenorphine 0.4 μg/kg as an infusion and 0.15 μg/kg as the demand dose) [29][39]. Cancer patients with breakthrough pain receiving transdermal buprenorphine from 35–70 μg/h responded well to an oral morphine to transdermal buprenorphine ratio of 75:1 [30][40]. Additionally, those using high-dose buprenorphine for maintenance therapy did not need to be switched off this opioid for methadone, as the patients morphine responses were not different between the two groups [31][41]. Glucuronide metabolites of buprenorphine are biologically active, contributing to the pharmacology of the drug [11][9]. The glucuronidation rate is roughly the same for buprenorphine and norbuprenorphine in the liver and small intestine. N-dealkylation is one-hundred fold greater in the liver than in the small intestine [32][42]. Conjugated metabolites are excreted in bile and half the buprenorphine administered is eliminated in the feces [21][28]. In bile fistula rats, where the bile flows into a hollow structure when 0.6 mg/kg buprenorphine was administered intravenously, 75% of B3G and 19% of N3G were excreted in bile. In “linked rat models” or intact rats, approximately twice the amount of N3G was found to be excreted compared with B3G. There are differences in excretion due to first-pass effects in enterohepatic circulation [32][42]. It is deconjugated by the colon by bacteria, then reabsorbed [10][18]. Buprenorphine is an atypical opioid as a result of its receptor activity at the MOR [11][9]. Buprenorphine has shown activity at all four opioid receptors [3]. Buprenorphine dissociates from the MOR slowly, resulting in a slow onset and a long duration for the analgesic effects [3]. A 2002 review describes how the MOR partial agonist and KOR antagonist properties of buprenorphine have been well established but that there had been comparatively less research on DOR and NOR [33][11]. Although most opioids show activity at the MOR, DOR, and KOR, buprenorphine is a DOR and KOR antagonist with high affinity [34][43]. Buprenorphine is potent at the MOR and the DOR, with efficacy at the MOR, DOR, and KOR in order of descending efficacy [35][44]. More recent studies of receptor affinity and intrinsic activity in cats have shown that buprenorphine is a MOR, KOR, and NOR receptor agonist and a DOR antagonist [36][45]. The affinity of buprenorphine for NOR (77 nM) was moderate [34][43]. The MOR is primarily responsible for analgesic effects, as well as euphoria, miosis, constipation, and respiratory depression [37][16]. It may have a greater impact at spinal MOR relative to the brain receptors, which is part of what makes buprenorphine classically considered a partial MOR agonist [11][9]. The DOR has minimal antinociceptive effects relative to the MOR but more activity in chronic pain than acute pain. The DOR also participates in analgesic tolerance and physical dependence [37][16]. The KOR has been seen to have analgesic and proanalgesic effects due to opioids, while also contributing to miosis and sedation [37][16]. Buprenorphine’s properties, including low molecular weight, high lipophilicity, and high potency, influence its perceived effects. Potency, the measure of the concentration or quantity of the substance necessary to achieve a predetermined outcome [38][46], differs depending on the formulation [39][47]. A value of ten-fold greater than morphine is generally accepted for pharmacoepidemiological research [40][48]. The drug has a wide tissue distribution and a peak plasma concentration at ninety minutes [9][17]. Buprenorphine is 96% protein bound after absorption [11][9]. Oral absorption is considered to be poor because of first-pass metabolism [11][9]. Transdermal absorption is limited, but there are formulations designed to be more effective. Sublingual administration is considered effective as well [11][9]. Some studies consider buccal formulations to be the most efficient and have the highest non-intravenous bioavailability [11][9]. The formulations available for the management of pain show the anticipated routes of administration effects, with parental forms producing the most rapid onset and transdermal forms producing the longest effects [41][49]. In healthy patients taking buprenorphine/naloxone tablets, they have a peak plasma concentration (Tmax) of 0.75–1.0 h for buprenorphine and 0.5 h for naloxone, demonstrating rapid absorption. Norbuprenorphine plasma concentrations peaked at a Tmax of 1–1.75 h after the buprenorphine/naloxone tablet administration. The plasma terminal half-life (t1/2) was 22–39 h for buprenorphine, 32–44 h for norbuprenorphine, and 1.4–10 for naloxone. Patients who were in withdrawal treatment for opioid dependence had a median Tmax of 0.75–1 h for buprenorphine, a median Tmax of 0.75–1 h for norbuprenorphine, and a median Tmax of 0.5–0.75 [42][50]. In patients with a history of drug addiction but were drug free at the time of the study, buprenorphine with sublingual and buccal routes had a 51.4% and 27.8% bioavailability, respectively [43][51]. The half-life is dependent on the method of administration, with 2 h for intravenous, 26 h for the transdermal patch, 28 h for the buccal film, and 37 h for the sublingual tablet [44][52]. Terminal elimination half-lives were longer for the sublingual and buccal routes of administration than the intravenous route, which may be due to a depot effect from buprenorphine collected in the oral mucosa tissue reservoirs. The time until the maximum concentration occurs was between 0.5 and 3 h sublingually and after 20 min intravenously [10][18]. Norbuprenorphine had mean peak plasma concentrations that vary by individual and route of administration in healthy patients [10][43][18,51]. Intravenous administration of buprenorphine has a 100% bioavailability, buccal has 46–65%, sublingual has 28–51%, and transdermal has 15% [11][9]. Buprenorphine as a tablet has a bioavailability that is 50–60% that of a buprenorphine solution [45][46][53,54]. Intranasal buprenorphine is 50% bioavailable in humans in a polyethylene glycol 300 and 5% dextrose vehicle, with a maximum concentration at 30 min [47][55]. Buprenorphine’s intranasal bioavailability was 70% with a polyethylene glycol 300 vehicle and 89% with a dextrose vehicle in sheep [47][55]. The half-life in rats following intravenous administration (2.8 h, [32][42]) was very similar to humans. Buprenorphine readily crosses the placenta. However, buprenorphine levels in the third trimester fetal rat brain were only a third of those in the maternal brain [48][56]. Although there is this notion that norbuprenorphine does not readily cross the blood–brain barrier [11][9], this may be age or species dependent. Administration of norbuprenorphine (3 mg/kg) to pregnant rats resulted in higher blood and brain levels in the fetus than in the dam [15][22]. Inhibiting the P-glycoprotein, a drug transporter highly expressed in brain microvessel endothelial cells and placental syncytiotrophoblasts [49][57], increased rat brain uptake of norbuprenorphine seven-fold [50][58]. The fetal plasma norbuprenorphine area under the curve was approximately two-thirds that of maternal mice. The fetal AUC of norbuprenorphine glucuronide was three-fold higher than that of the dam. Although interpretation of this researchtudy is somewhat limited by analysis of the entire mouse gestational day fifteen fetus (instead of isolating plasma or brain), these findings indicate appreciable fetal exposure to buprenorphine’s biologically active metabolites [51][59]. Although buprenorphine and norbuprenorphine are transferred into human breast milk, the quantities were low (1%, [52][60]). In recent years, contrary to traditional receptor theory, it is clear that different ligands for the same receptor can cause different responses [53][61]. For receptor theory models to be useful, they must aid in determining the extent to which drug effects can be interpreted and applied to predict future effects [54][62]. The term “ligand bias” has been used to describe opioid analgesic drugs that elicit a different intracellular response; therefore, their effects are not only the result of receptor binding affinity [35][44]. Buprenorphine differentiates itself from other opioids in mu-receptor activity, with its slow dissociation from the receptor [55][63]. Buprenorphine alone is not responsible for its antagonistic effects, but its varying metabolite concentrations through different forms of drug administration may alter the efficacy of the drug. Traditionally, buprenorphine is described as a partial MOR agonist that is known for limited analgesic effects and developed with the intent for a limited potential for respiratory depression and addiction [22][15]. However, since buprenorphine’s classification in the 1980s and 1990s, what is known about receptor interaction and activation has changed the meaning of the terms “agonist” and “antagonist” [37][54][56][16,62,64]. Importantly, categories such as full agonist, partial agonist, and antagonist may be unsatisfactory, as a drug’s response may land on a continuum [57][14]. Reservations regarding buprenorphine’s clinical use were due to misconceptions about an analgesic “ceiling effect” [11][9]. Until recently, agonists such as buprenorphine have been known for limited intrinsic activity and an inability to produce as large a response at a receptor [22][15]. Initially, it was concluded that all agonists for a receptor will result in different degrees of the same intracellular response [54][56][62,64]. The transduction pathways of a drug activated by an agonist do not act identically for each receptor [4]. Partial agonists are known for their lack of intrinsic efficacy [53][61]. The antinociceptive effect ascribed to buprenorphine is considered mainly mediated by the MOR [58][65]. Bell-shaped dose–response curves for buprenorphine in the 1980s and 1990s showed that there is an optimal range of concentrations for a maximum analgesic effect, with a decrease in activity below or above this range [55][63]. The perception of buprenorphine’s clinical usage may depend on the correct application or interpretation of terms from concepts in receptor theory, such as efficacy and agonist [59][66]. Studies have suggested that different opioid agonists have different downstream effects in the cell when binding and activating the same receptor. Therefore, different opioids cannot be considered equivalent by changing the dose [37][16]. It can no longer be assumed that any ligand activating a receptor will produce a response that is relatively the same, with differences attributed to the agonists’ efficacies [4]. Ligands for a receptor can alter the downstream activity in a pathway, known as biased agonism, ligand-directed signaling, and functional selectivity [60][67]. Opioids that are pure agonists such as morphine or fentanyl produce stronger analgesic effects than drugs such as codeine that have decreased receptor binding [61][68]. However, factors such as affinity and efficacy, as well as variables such as metabolite binding and concurrent receptor binding may alter the perceived effects and receptor activity of buprenorphine [61][68]. The binding affinity of buprenorphine and its metabolites to opioid receptors provides the varied effects seen. Binding affinity is the ability of a drug to bind to a receptor and is measured by the equilibrium inhibitory constant (Ki) [11][9]. Buprenorphine has a high binding affinity at the MOR and KOR, with debated effects [11][9]. Buprenorphine-3-glucuronide has high affinity for the MOR (Ki = 4.9 ± 2.7 μM) and NOR (Ki = 36 ± 0.3 μM). Norbuprenorphine-3-glucoronide had appreciable affinity for the NOR (Ki = 18 ± 0.2 μM) but not the MOR [62][69]. Although norbuprenorphine has a greater efficacy, it is considered a less potent partial agonist than buprenorphine at the MOR [63][70]. A 2002 review described how norbuprenorphine was much less studied than the parent compound but that there was some evidence to suggest that it functioned as a MOR and KOR partial agonist and a DOR and NOR full agonist [33][11]. Competition assays revealed approximately twenty-five-fold lower norbuprenorpine binding to the NOR than was found with buprenorphine [63][70]. All metabolites except nubuprenophine-3-glucuronide have analgesic properties [62][64][69,71]. Buprenorphine alone is not responsible for its analgesic effects, but its varying metabolite concentration through different forms of drug administration may alter the efficacy of the drug. Norbuprenorphine is one of buprenorphine’s better-studied active metabolites and further research must be performed to understand the other metabolites’ pharmacodynamics [33][11]. Norbuprenorphine and buprenorphine have substantially different pharmacological profiles. Norbuprenorphine arises as a result of N-dealkylation catalyzed by cytochrome P450 (CYP3A4) in the liver [12][13][19,20]. The mechanisms and metabolites of this process are illustrated in Figure 1. At the MOR, both norbuprenorphine and buprenorphine are potent partial agonists, with norbuprenorphine having moderate efficacy and buprenorphine having low efficacy. At the NOR, norbuprenorphine has moderate efficacy and buprenorphine has low efficacy, with both substances having low affinity for the receptor. This information was determined using ligand binding experiments and cAMP assays [63][70]. Respiratory depression is induced by norbuprenorphine and mediated by the MOR [65][72]. There is a low risk of respiratory depression with buprenorphine as a monotherapy and this potential effect is rarely considered clinically relevant [66][67][73,74]. Buprenorphine’s active metabolite, norbuprenorphine, was ten-times more potent for causing respiratory depression [65][72]. Buprenorphine was found to be protective against norbuprenorphine’s effect of respiratory depression, both preventing and reversing these effects. An active metabolite of buprenorphine, norbuprenorphine, was alone seen to be responsible for the effects of respiratory depression. Binding experiments show the DOR and, primarily, the MOR as responsible for buprenorphine protecting against norbuprenorphine-induced respiratory depression [9][17]. The intraventricular administration of buprenorphine and norbuprenorphine showed norbuprenorphine’s analgesic activity was 25% that of buprenorphine [68][75]. Norbuprenorphine was 50-fold less potent than buprenorphine through intravenous administration and 4-fold less potent after intraventricular administration in in vivo animal studies. This decrease in potency may be due to poor penetration across the blood–brain barrier compared with buprenorphine ([69][76], although see [14][21]).