1. Introduction
In recent years, the growing prevalence of obesity has become a major global health concern, demanding urgent attention and effective strategies to contrast this multifaceted pandemic issue
[1]. Clinically, obesity is defined as a persistent state of positive energy balance between energy intake and expenditure, resulting in an excessive accumulation of fat mass, especially visceral fat, which leads to an increase in the body mass index (BMI) value. As reported by the World Health Organization (WHO), BMI values between 25 and 29.9 are indicative of overweight or pre-obese conditions, while BMI ≥ 30 is predictive of conclamant obesity
[1][2][1,2]. Therefore, the occurrence of this chronic disorder was not only linked to dietary alterations, but also to the combination of several other parameters including environmental factors, Western lifestyle, and hereditary factors. Additionally, obesity has several serious public health implications mainly related to the fact that this pathology is positively correlated to the onset and progression of several chronic diseases, including type 2 diabetes (T2D), hypertension, cardiovascular disease, congestive heart failure, stroke, rheumatoid arthritis, insulin resistance, and cancer
[3][4][3,4]. In this regard, it was estimated that 44% of cases of type 2 diabetes, 23% of cases of ischemic heart episodes, and up to 41% of diagnosticated cancers are attributable to the obesity/overweight condition
[5]. Moreover, obesity could also lead to psychological problems, such as depression and low self-esteem
[1].
Traditionally, the first multi-disciplinary approaches for obesity treatment were based on diet modification and regular exercise, but when this intervention is not enough to contrast serious obesity conditions, the treatment may rely on several pharmacological remedies
[6]. The conventional drug approach to obesity management is based on different classes of molecules, including appetite suppressants, lipase inhibitors, hormones, metabolic regulators, and inhibitors of gut peptide receptors. Each one of these molecule classes is characterized by a completely different mechanism of action, reflecting the multifactorial etiology of obesity diseases. For example, phentermine, diethylpropion, and liraglutide, a sympathomimetic amine, are synthetic drugs that act at the central nervous system (CNS) level, where they regulate appetite and satiety. Orlistat is a well-known inhibitor of pancreatic lipase
[7], used primarily for weight control since its activity reduces the absorption of dietary fat at the intestinal level. Hormonal regulators, instead, are a class of anti-obesity drugs that target hormonal pathways involved in appetite regulation and energy balance. Examples include the GLP-1 (glucagon-like peptide) receptor agonists such as liraglutide and semaglutide, which increase satiety and promote weight loss. Finally, the last class is metabolic modulators, which include molecules able to alter metabolic processes to promote weight loss. Unfortunately, all the above-considered pharmacological treatments are associated with serious side effects. In this regard, phentermine is available only for short-term treatment in most countries, because prolonged usage could cause a serious case of tolerance and dependency
[8]. In addition, orlistat treatment is associated with various gastrointestinal side effects including oily stools, oily spotting, and flatulence
[9]. In light of these considerations, researchers are increasingly turning to the world of natural compounds to seek new and safe possibilities to contrast this complex disorder, in line with what has already been carried out for the treatment of other pathological conditions with major social implications, such as hypercholesterolemia
[10], diabetes
[11][12][13][11,12,13], cardiovascular diseases
[14][15][14,15], oxidative stress
[16][17][16,17], and inflammation
[18][19][20][18,19,20].
Recently, among natural promising compounds, chalcones, a class of natural flavonoids, attracted scientific interest due to their anti-inflammatory
[21], anticancer
[22], and antimicrobial effects
[23]. Specifically, in nutraceutical research, this class of molecules has attracted considerable attention, especially to its remarkable antidiabetic activity, where phloretin and phlorizin are the main representatives
[24][25][24,25].
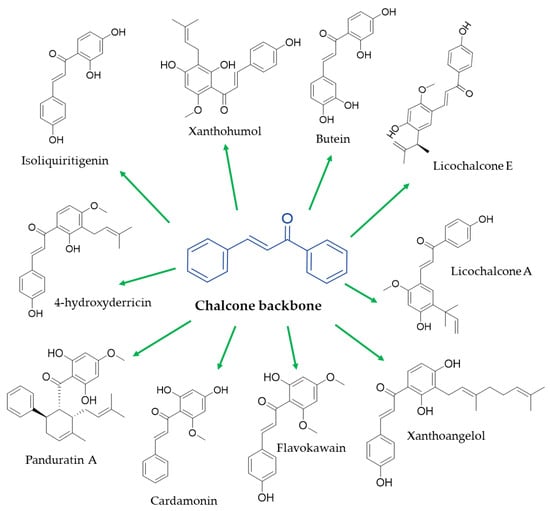
Chemically, chalcones are derivatives of aromatic ketones, 1,3-diphenyl-2-propen-1-ones, a precursor of flavonoid biosynthesis
[26]. Based on the type of subtraction, chalcones are generally classified into hydroxy chalcones, methoxy chalcones, amino chalcones, aryl chalcones, alkyl chalcones, nitrogenous chalcones, and other subclasses less representative
[22] (
Figure 1). Considering that the simple chalcone structural scaffold can be functionalized at multiple sites with different chemical moieties, these molecules could respond to different molecular targets or interfere with different signaling pathways in humans with a specific structure–activity relationship
[27].
Figure 1. Chemical structure of the main natural chalcones with anti-obesity potential.
2. In Vitro Evidence
2.1. Cardamonin
Cardamonin ((2E)-1-(2,4-dihydroxy-6-methoxyphenyl)-3-phenyl-2-propen-1-one) is a dietary polyphenol, belonging to the class of chalcones and mainly isolated from
Alpinia species
[28]. A wide range of biological activities are referred to this molecule, including anti-inflammatory and antitumor activity
[28][29][28,29]. Recently, this compound has attracted considerable attention due to its potential anti-obesity potential. Specifically, in a recent comparative study, 46 different polyphenols were tested on the mouse embryonic fibroblast 3T3-L1 cell model (well-established models for studying the differentiation of preadipocytes to mature adipocytes, i.e., adipogenesis
[30]) in order to evaluate their effects on intracellular lipid accumulation. Specifically, among all the tested polyphenols, the authors described that cardamonin was one of the most active in contrasting the differentiation of preadipocytes in mature adipocytes by the activation of a specific molecular pathway
[31]. Specifically, at a molecular level, the preadipocyte maturation process could be divided into four consecutive steps: growth arrest, clonal expansion, early differentiation, and final differentiation. These phases are modulated with a complex framework of transcription factors, which coordinates the production of several proteins responsible for establishing the mature adipocyte phenotype, involving peroxisome proliferator-activated receptor (PPAR)γ, fatty-acid-binding protein 4 (FABP4), and CCAAT/enhancer-binding proteins (C/EBP) α. During adipogenesis, the initial expression of C/EBPβ promotes the synthesis of C/EBPα and PPARγ, and these two proteins work together to maintain the differentiated phenotype
[32]. In addition to these intracellular pro-obesity master regulators, the obesity progression could be controlled by the regulation of an extracellular signal-regulated kinase (ERK) expression, a member of the mitogen-activated protein kinase family, which modulates several important physiological processes at the cellular level. Particularly, in preadipocytes, the activation of ERK is an essential stage in adipocyte differentiation, and its activation induces the phosphorylation of PPARγ, leading to attenuation of the differentiation process. In this regard, the authors described that cardamonin was not only able to inhibit adipocyte differentiation by activating the ERK pathway, leading to a downregulation of all adipocyte transcription factors, but also to reduce the specific adipokine production normally released by the mature adipose tissue
[31]. Adipokines are multitasked molecules deeply implicated in lipid metabolism, the adipogenesis process, inflammation (reactive protein C), and regulation of energy balance
[33]. Furthermore, other authors have reported that cardamonin could also interfere with other molecular pathways implicated in obesity progression. According to a recent study conducted by Young-Jin et al., cardamonin treatment could inhibit lipogenesis by activating protein-kinase-A-mediated browning in the 3T3-L1 cell line. In mammals, the energy equilibrium is regulated by the presence and secretion of two different types of adipose tissue: white adipose tissue (WAT) and brown adipose tissue (BAT)
[34]. Physiologically, the energy surplus is stored as triglycerides in WAT, which is composed of adipocytes containing large unilocular lipid droplets
[35]. On the other side, BAT is instead formed by adipocytes containing numerous small multilocular lipid droplets, demonstrating high levels of mitochondrial biogenesis that dissipates energy as heat due to it expressing uncoupling protein 1 (UCP1) in the internal mitochondrial membranes. Recently, in view of finding remedies for obesity prevention and treatment, great attention has focused on the browning process, which converts white adipocytes to brown-like (beige or brite) adipocytes
[36]. The beige adipocytes, as well as the brown adipocytes, are characterized by high mitochondrial activity
[37], which leads to increased energy expenditure that may prevent obesity onset and progression. The WAT browning process at the molecular level is modulated by the expression of several brown-adipocyte-specific factors such as PR domain containing 16 (PRDM16), peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1α), and UCP1. In light of these considerations, the authors described that cardamonin induced the expression of the browning marker genes PRDM16, PGC1α, and UCP1 at the mRNA and protein levels. The activation of protein kinase A (PKA) causes the activation of lipolytic enzymes, such as adipose triglyceride lipase (ATGL) and hormone-sensitive lipase (HSL), inducing lipolysis and inhibiting lipid accumulation. Generally, the term lipolysis refers to the hydrolysis of triacylglycerol (TG), which releases free fatty acids (FFAs) that could undergo mitochondrial β-oxidation following trans-membrane transport by carnitine palmitoyltransferase 1 (CPT1). The oxidation of FFA resulted in acetyl-coA, which in turn inhibited the conversion of FFA in TG, but released FFA was used to produce ATP, and consequently led to a reduction in lipid accumulation. Thus, the PKA could be a valuable regulation checkpoint for the browning process. In addition, Seo and collaborators confirmed the cardamonin inhibitory activity on the expression of the adipogenic proteins C/EBPα and FABP4 in a preadipocyte cell line pretreated with differentiation inducers. Furthermore, they have also investigated the cardamomin potential in downregulating the expression of other lipogenic proteins, such as lysophosphatidic acid acyltransferase theta (LPAATθ), lipin 1, diacylglycerol acyltransferase 1 (DGAT1), sterol regulatory element-binding protein 1 (SREBP1), and fatty acid synthase (FAS). Each one of these proteins is differently implicated in lipidic metabolism in the adipocyte; specifically, LPAATθ and DGAT1 are directly involved in triacylglycerol synthesis, while SREBP1 and FAS modulate fatty acid synthesis
[38].
2.2. Licochalcone A
Licochalcone A (LA) (4′,4-Dihydroxy-3-α,α-dimethylallyl-6-methoxychalcone) is the primary active compound of
Glycyrrhiza inflata and
Brassica rapa L. Although LA has been shown to have multiple human pharmacological effects such as anti-inflammatory, antitumor, antifungal, and antiparasitic properties
[39], its anti-obesity potentiality was poorly reported. Quan et al. have described that LA treatment on 3T3-L1 preadipocytes reduced lipid accumulation and downregulated the expression of several factors implicated in adipocyte differentiation and lipogenesis, such as PPARγ and C/EBPα, and in addition, limited the expression of SREBP1c. The reduction in the expression rate of these mediators regulates the fatty acid synthesis (fatty-acid-binding protein, FAS, stearoyl-CoA desaturase 1 (SCD1), and glycerol-3-phosphate acyltransferase (GPAT))
[40]. Moreover, the authors have highlighted LA positive activity also at the mitochondrial level, since the LA treatment on the 3T3-L1 preadipocyte stimulates the gene expression of CPT1, the trans-membrane mitochondrial proteins, responsible for the FFA translocation at the mitochondrial level, reasonably because of collateral inhibition of acetyl-CoA carboxylase (ACC), which is involved in the endogenous fatty synthesis, via AMP-activated protein kinase (AMPK) or the activation of nuclear receptor PPAR-α
[40]. Other authors have also investigated the capacity of LA to induce the browning process in the 3T3-L1 cell line. Specifically, they found that LA treatment was able to upregulate the expression of UCP1, an important browning modulator in 3T3-L1 adipocytes
[41]. Other authors have investigated the LA in vitro inhibition activity on the pancreatic lipase enzyme. Specifically, in a study conducted by Won and colleagues, LA showed relevant lipase inhibitory activity, with calculated IC
50 values of 35 mg/mL (103.4 mM). Particularly, LA-pancreatic lipase inhibition was shown to be a non-competitive inhibition, and there was an estimated K
i of 11.2 mg/mL (32.8 mM), with unvaried k
m values for overall different concentrations tested
[42]. Other authors have conducted a comparative study on the ability of different natural lichochalcones, licochalcone A, licochalcone D, and licochalcone E, to inhibit pancreatic lipase activity. Structurally, these molecules differ in the subtraction type in C2 at the level of the A ring, with licochalone D containing an isopropyl group, licochalone E containing a propyl group and no isopentyl group on the B ring, whereas licochalone A contains a hydroxy group
[43]. All these molecules have shown a relevant inhibition of pancreatic lipase activity, resulting in a calculated IC
50 of 4.9, 3.2, and 5.8 μM, respectively
[44]. In addition, the performed structure–activity relationship study also indicated that the presence of both isopentenyl and hydroxyl groups at the A ring level was essential for the noncovalent inhibitory potency of the natural chalcones investigated
[44].
2.3. Butein
Butein (3,4,2′,4′-tetrahydroxychalcone) is a natural chalcone primarily isolated from
Toxicodendron vernicifluum, a traditional Chinese lacquer tree
[45]. Yang et al. revealed that butein treatment inhibited lipid accumulation and adipogenesis in the 3T3-L1 preadipocyte cell line, by downregulating the adipogenic factors PPARγ and C/EBP-α
[46]. The authors described that butein at concentrations of 5, 10, and 25 μM decreased PPARγ expression by 78.8, 68.3, and 31.4%, and C/EBPα concentration by 87.3, 71.7, and 42.1% in 3T3-L1 preadipocytes’ cell line
[46]. The authors described that these results are related to butein capacity to positively modulate the expression of nuclear transcription factor erythroid 2-related factor 2 (Nrf2), a crucial transcription factor, involved in the regulation of several cellular pathways. Mainly, Nrf2 directly upregulates the key C/EBP-α and PPARγ, which as previously described, are involved in maintaining the differentiated phenotype of preadipocytes
[47][48][47,48]; on the other side, Nrf2 is a fundamental transcription factor playing a crucial role in maintaining the homeostatic redox tissue condition. Particularly, Nrf2 modulates the production of many enzymes involved in oxidative stress, such as thioredoxin, glutathione, heme oxygenase-1 (HO-1), quinone oxidoreductase (NQO1) as an antioxidative enzyme, and glutathione S transferases (GSTs) as detoxifying enzymes
[49]. Generally, HO-1 is well known to play a key role in antioxidant processes. This enzyme catalyzes the rate-limiting stage of free heme degradation into iron, carbon monoxide, and biliverdin, all metabolites with powerful antioxidant activity
[50]. Recently, several studies highlighted the HO-1 critical role in the management of metabolic homeostasis, considering its high expression level in the white adipose tissue of genetic or high-fat diet (HFD)-induced obese mice
[51]. Therefore, Nrf2 and its downstream enzyme HO-1 may be a potential target in treating obesity-related diseases. In this regard, Yang et al. have demonstrated that butein treatment on 3T3-L1 results in increased expression of Nrf2, which in turn leads to upregulation of the HO-1 mRNA expression level. A similar experimental design was followed by Wang et al., who also confirmed butein capacity to induce the upregulated HO-1 mRNA expression and the related protein expression in the 3T3-L1 adipocyte cell line
[52]. Additionally, Song et al. conducted a comparative study on the anti-obesity potential of different natural molecules isolated from
Rhus verniciflua Stokes, a lacquer tree with ancient traditional medical usage
[53]. They reported that preincubated C3H10T1/2 cells with adipogenic inducer mixtures were treated with 10 mM of each different molecule studied, in order to investigate their anti-adipogenic activity. Interestingly, they found that butein suppressed lipid umulation and morphological changes during adipocyte differentiation through the suppression in a dose-dependent manner of PPARγ, over the butein concentrations of 10–40 mM. To confirm the butein antiangiogenic potentiality, the authors also compared the butein activity to those obtained upon the treatment with two well-known and established anti-obesity natural molecules, i.e., resveratrol and genistein. Interestingly, they reported that the inhibition of lipid accumulation in C3H10T1/2 cells with resveratrol treatment was obtained at the concentration of 20 µM, with genistein at 40 µM, and surprisingly with butein at 10 µM
[53]. Moreover, the mRNA expression level of the main adipocyte markers, such as PPARγ, aP2, and LPL, upon the pretreatment with resveratrol, genistein, and butein was also investigated. The results indicated that butein was able to reduce the expression of 50% of these obesity markers at the concentration of only 5 µM, while the same results were achieved with 20 and 40 µM of resveratrol and genistein, respectively. In addition, butein at the dose of 10 µM completely suppresses the expression of the above-mentioned agents
[53]. To better understand the molecular mechanism responsible for butein anti-adipogenic effects, the same authors have studied on the 3T3-L1 cell model the capacity of butein to modulate STAT-3, a factor highly required for adipocyte differentiation. Specifically, they reported that after the treatment for 12 h and 24 h of 3T3-L1 with butein, the expression of the main STAT3-regulated genes (
KLF5 and
P53) was downregulated. Moreover, other authors have studied the butein mechanisms of inhibition of the obesity process by regulating AMPK, a serine/threonine protein kinase, involved in a molecular pathway that is generally recognized as an important target for obesity management
[54]. Physiologically, AMPK acts directly in the modulation of cellular energy balance during the 3T3-L1 cell differentiation, by regulating lipid and glucose metabolism
[55]. More specifically, the activation of AMPK leads to the attenuation of expression of the previously cited transcription factors (C/EBPα and PPARγ), but also inhibits the expression of GPAT-1 and increases the expression of CPT-1. This enzyme is involved in the endogenous synthesis of fatty acid, and at the same time inhibits the oxidation of fatty acid through the inactivation of ACC, as previously described being involved in endogenous lipid synthesis
[41]. In this regard, Lim et al. demonstrated that butein treatment of 3T3-L1 cells, in addition to the effects on the C/EBPα and PPARγ expression level also described by others, was able to induce the phosphorylation of AMPK and enhances the level of phosphorylated ACC, a downstream substrate of AMPK, in a dose-dependent manner
[56].
2.4. Panduratin A
Panduratin A (PAN A) is a prenylated flavonoid based on a chalcone skeleton with three oxygenated patterns only at the level of the B ring and a geranyl substituent at the C2-C3 position with the Diels–Alder reaction
[57]. This natural chalcone was mainly isolated from
Boesenbergia pandurata (Roxb.) Schltr. (Syn.
Kaempferia pandurata Roxb.)
, an ancient medicinal plant, largely used for its relevant anti-inflammatory and antioxidant properties. In this context, Kim and colleagues
[58] have studied the potentiality of PAN A as a novel AMPK activator. The regulation of AMPK could be a useful tool for the treatment of obesity due to its important role as an energy sensor at the mammalian cellular level. Thus, they reported that the PAN A treatment on different cellular models used (3T3-L1 adipocytes, HepG2 liver carcinoma cells, and L6 skeletal muscle cells) induced the AMPK-dependent inactivation of ACC, resulting in a decrease in endogenous fat synthesis. More specifically, AMPK could be activated by several modulators including LKB1, CaMKKβ, sirtuin (SIRT 1), and NAD(P)H. Therefore, in order to elucidate the molecular mechanism of AMPK activation by PAN A, the authors investigated the effects of this natural chalcone on the expression of AMPK activators. The authors found that PAN A treatment increased LKB1 translocation from the nucleus to the cytoplasm and the binding rate to AMPK
[58], resulting in its rapid activation. Thus, they assert that the AMPK activation measured was an LKB1-dependent process. Beyond the effects on AMPK induction, PAN A treatment on one side induces a remarkable downregulation of pro-adipogenic mediators (PPARγ, C/EBPα, ACC, FAS, and SREBP1c); on the other side, it induces an upregulation of the expression of genes involved in fatty acid oxidation (PPARα, PGC-1α, CPT-1L, CPT-1M, and UCPs) in all the cell models used (3T3-L1, HepG2, and L6 cells). These results are in line with the findings of other authors, who have observed that PAN A treatment of 3T3-L1 and HepG2 cell lines decreased triglyceride accumulation via activating the AMPK pathway
[59].
2.5. Isoliquiritigenin
Isoliquiritigenin (ILG, 2′,4′,4′-trihydroxy chalcone), a chalcone compound belonging to the flavonoid family, consists of two benzene rings linked through an α,β-unsaturated carbonyl group
[60]. ILG is usually isolated from
Glycyrrhiza uralensis (licorice),
Allium ascalonicum,
Astragalus membranaceus,
Dalbergia odorifera,
Dianthuschinensis,
Glycine max L.,
Sinofranchetia chinensis [60][61][62][60,61,62]. This molecule was largely studied for its relevant health-promoting effects, including anticancer, anti-inflammatory, antimicrobial, antioxidative, antiviral, antidiabetic, and anti-depressive activity
[63]. In a study conducted by Zeng et al. (2022), it was reported that ILG is advantageously associated with diminishing cholesterol levels in different cellular models
[64]. The mechanism proved to be assertive through downregulation of expression of Niemann Pick C1 Like 1 (NPC1L1), which is a protein responsible for intestinal absorption of cholesterol. Interestingly, NPC1L1 is not only a relevant molecular target for hyperlipidemia therapy but also for obesity management, considering that the cholesterol uptake rate is known to be one of the main elevating factors of obesity. In this regard, Zeng and colleagues
[64] used the colon cancer cell line (Caco-2) and liver cancer cell line (HepG2) cell model to explore the ILG-NPC1L1 downregulatory activity. Auspiciously, they observed that this natural product (at the dose of 100 μmol/L) was as efficient as the pharmaceutical complex of Ezetimibe (EZ, used as positive control) in the limitation of the cholesterol uptake rate. It is known that EZ is marketed as a cholesterol absorption inhibitor that takes over the function of NPC1L1 and also has some pleiotropic effects
[65]. Furthermore, the authors reported that in both the cellular models, NPC1L1 expression was modulated by ILG treatment in a dose-dependent manner, and at the same treatment dose, the higher inhibitory effects were observed in Caco-2 cells in comparison with the HepG2 cells. Additionally, the anti-obesity function of ILG was explored in another study accomplished by Park et al. (2016)
[66]. Specifically, they explored the capacity of ILG, at two different dosages (75 or 100 µM), to regulate the insulin-stimulated adipogenesis in 3T3-L1 cells. It is known that adipocyte maturation is strongly regulated by the insulin signaling pathway
[67]. Insulin is one of the most important adipogenic hormones responsible for modulating several transcription factors involved in adipocyte differentiation and lipid accumulation, such as PPARγ C/EBPα, as well as the expression of profibrogenic genes, such as FABP4, also known as aP2, and the translocation of glucose transporter 4 (GLUT4) at the adipocyte level. The authors described that not only was ILG able to inhibit the production of all the above-mentioned adipogenic molecular agents, but was especially able to inhibit the insulin-stimulated adipocyte differentiation through the regulation of oxygen species (ROS) production that occurs during this process. Specifically, increased production of intracellular ROS occurs during the initial phase of insulin-stimulated adipogenesis, leading to the activation of NADPH oxidase 4. ROS blocked the activation of protein-tyrosine phosphatase 1B (PTP1B), which is a pivotal modulator of phosphorylation-dependent insulin signaling, resulting in an increased adipocyte differentiation rate. The authors demonstrated that ILG in virtue of its intrinsic antioxidant potential (related to two functional hydroxyl groups) was able to inhibit the oxidation of PTP1B, and consequently the activation of its related downstream signaling pathway
[68]. In addition, Moon et al. (2020)
[69] evaluated the impact of low-dose ILG treatment on the differentiation process of white adipocytes into functional beige ones in human adipose-derived stem cells (hASCs), stem cell lines directly isolated from human fat tissue. The following was established well: the crucial roles of brown adipocytes (mitochondria-rich multilocular cells) and beige adipocytes of subcutaneous white adipose tissue containing thermogenin (UCP1) in energy dissipation, lipid and glucose oxidation, and uncoupling the mitochondrial respiration
[70]. The authors reported that ILG treatment promotes brown adipogenesis by increasing the expression of both UCP1 and PRDM16, as previously described with two relevant browning markers. In addition, the authors reported that the ILG treatment led to a downregulation of the c-Jun N-terminal kinase (JNK) expression rate, a specific kinase implicated in adipocyte differentiation. Finally, the authors assessed that low-dose ILG induced the beige adipocyte potential of hASCs via JNK inhibition. Additionally, the inhibition of monoamine oxygenase-B (MAO-B), a key enzyme for cholinergic transmission, could find a therapeutic application for obesity management
[71]. Specifically, MAO-B inhibitors have, to an unheard-of degree, shown a relevant anti-obesity potential via the reduction in adipose tissue, and promotion of weight loss. In light of these considerations, Prajapati and colleagues investigated the ILG capacity to inhibit the enzymatic activity of human monoamine oxidase (hMAO-A and hMAO-B) in a vitro enzymatic assay
[72]. The authors reported that ILG was able to contrast the MAO-A and MAO-B enzymatic activity with calculated IC
50 values of 0.68 and 0.33 µM, respectively. Additionally, the conducted study on the enzymatic kinetic mechanism indicated that ILG was able to inhibit the MAO activity in a competitive way.
2.6. Xanthohumol
Xanthohumol (XN, 3′-(3,3-dimethylallyl)-2′,4′,4-trihydroxy-6′-methoxychalcone) is a natural prenylated chalcone, mainly extracted from the hops plant,
Humulus lupus. Samuels and colleagues investigated the XN anti-obesity potential on 3T3-L1 adipocytes and primary human subcutaneous preadipocyte models
[73]. According to their experimental protocol, initially, in both the cell systems used, the differentiation was induced, followed by treatment with XN at different concentration levels, dorsomorphin as an AMPK inhibitor (positive control), or 5-Amionimidazole-4-carboxamide (AICAR) as an AMPK activator. Their results indicate that XN acts as a bridging agent in white adipocytes as suggested with the increased synthesis of beige markers CIDE-A and TBX-1. After 24 h of treatment, XN activated AMPK, leading to the upregulation of UCP1, phosphorylation of ACC, and HSL expression. In addition, they reported that XN acts positively at the mitochondrial level, inducing mitochondrial biogenesis, as evidenced with increased mitochondrial content, enhanced expression of PGC-1α, and the thermogenic protein UCP1
[73]. In addition, Miyata et al. investigated the ability of XN in Huh7 cells, a human hepatoma line, to inhibit SREBP, by impairing the translocation of the SREBP cleavage-activating protein complex from the endoplasmic reticulum to Golgi
[74]. Instead, Rayalam and colleagues have studied the XN obesity potential in combination with another natural compound, guggulsterone (GS), in 3T3-L1 adipocytes
[75]. They reported that XN and GS, tested separately at a treatment dose of 1.5 mM (XN) and 3.12 mM (GS), can each reduce lipid accumulation by 26 ± 4.5% (
p < 0.001), while their combination results in a 78.2 ± 1.8% (
p < 0.001) reduction in lipid accumulation, indicating possible synergistic effects. Additionally, a similar XB effect on 3T3-L1 adipocytes was also reported by Rossi et al.
[76], who described that XN up to 50 µM could reduce adipocyte proliferation, but that this resulted in adipocyte hypertrophy without an improvement in the metabolic profile.