Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Jason Zhu and Version 1 by JOSE ANTONIO SILVA CARVALHO CAMPOS E MATOS.
In General, Glass Powder can be compelling and beneficial for both the concrete industry and the environment if implemented properly, which obviously still requires further studies to have the right understanding of this material.
- glass powder
- glass waste
- green concrete
- recycled materials
1. Glass Production
In recent years, the glass industry has come to prominence as a driving factor for both economic and sustainability balance since it is tremendously valuable, and it has attempted to address both issues. In the United States, only the glass industry was valued at around 31 billion dollars, while worldwide revenue of this section was about 130 billion dollars in 2019 [13,14][1][2]. By 2025, due to the expansion of the United States glass demand, it is anticipated that flat glass sector revenue will reach 44 billion dollars [13,14][1][2]. It has been anticipated that by 2027, the glass industry will surpass 180 billion American dollars [15,16][3][4].
The glass industry is categorized as a market with a relatively high energy consumption griffin [17][5]. Considering that energy costs are a significant expense for the glass industry, the majority of companies have made substantial efforts to optimize their energy efficiency. The raw materials must be melted at approximately 1500–1600 degrees Celsius, and fossil fuels account for 85 percent of the fuel used in furnaces. [18,19][6][7]. The electricity supply provides 15% of the energy for the operation of parts of furnaces and other facilities. Typically, the melting stage requires around 3 MJ/kg of energy [18][6]. There is only a 30 percent share of energy used in the glass-forming process of the entire process of manufacturing glass [19][7].
Besides that, the glass manufacturing process emits greenhouse and toxic gases, such as CO2, NOx, SOx, KOH, NaOH, HF, and HCl, into the atmosphere [20][8]. Direct and indirect emissions could originate from burning fossil fuels, the operation of facilities, and transferring materials and products [20][8]. It is reported that the production of one kilogram of glass contributes to approximately 0.6 kg of carbon dioxide emissions, of which 0.45 kg of this amount are caused by fossil fuel combustion [21][9]. It is estimated that only the manufacturing of flat and container glass could emit approximately 60 million tons of carbon dioxide in one year [21][9]. Over 20 million tons of carbon dioxide are emitted annually by glass production in the European Union [21][9].
One of the environmental benefits of re-melting cullet is that it minimizes CO2 emissions throughout the production process compared to making glass from new raw materials. Yet, the remelting process consumes a great deal of energy and releases a great deal of greenhouse gases [21][9]. Furthermore, it could reduce the cost and consumption of raw materials. It seems that skipping the remelting stage by reusing rather than recycling could be even more beneficial for both the economy and the environment [11,21][9][10]. Approximately 50 billion tons of aggregate are used each year around the world to manufacture glass, electronics, and concrete [11,21][9][10]. The annual demand is expected to reach 82 billion tons by 2060 [11,21][9][10].
As a result of glass’s pozzolanic and filler properties, it could be used as an alternative to cement in the recycling process in order to eliminate the need to remelt and package the material. Approximately 40 to 50 billion tons of sand and gravel are extracted annually throughout the world, with a significant portion being used to produce concrete, according to the United Nations Environment Program (UNEP). Furthermore, similar to the glass industry, the cement industry is also one of the biggest greenhouse gas emitters. In 2018, the International Energy Agency (IEA) reported that cement factories contributed to about 3 billion tons of CO2 (7 percent of the world’s carbon dioxide emissions). It is also indicated that cement replacement with glass powder could reduce GHG emissions by 12% and cement industry energy consumption by 15% [22][11].
To address this massive problem, reducing, reusing, and recycling are the three vital steps towards reaching sustainable goals. As is obvious, the first important step starts with managing the demand and declining waste production by improving the quality of materials. Reusing is the next phase, and the EU has determined that factories must prioritize glass reuse over recycling. Although recycling is superior to manufacturing from scratch by utilizing virgin raw materials, reusing is more sustainable since it eliminates the additional remelting step [23,24][12][13]. In this concern, as a result of producing thicker, higher-quality bottles, the rate of reuse could be increased, thereby reducing the need to recycle or manufacture new bottles [12][14]. As an example, glass milk bottles were collected from the houses and reused up to 18 times before recycling became necessary. It is stated that a glass bottle can be reused up to fifty times without losing significant quality, although flat and construction window glasses have a negligible reuse rate [12][14]. The recycled content of flat glass produced in the UK is around 20–30% [12][14].
When reusing is not possible, recycling becomes the next option, and glass is capable of being recycled to a 100 percent extent. It is reported that recycling one ton of waste glass could prevent around 350 kWh and 246 kg of energy and carbon dioxide, respectively. Using waste glass annually can prevent the use of 1.2 tons of new virgin materials, 25% of energy, and 60% of carbon dioxide emissions [11,12,21][9][10][14]. The global rate of recycling in 2018 was just 21% [11,21][9][10]. America’s recycling rate is approximately 30%, Australia’s 40%, South Africa’s 20%, Portugal and Hong Kong’s 45%, and the United Kingdom’s 50% [11,12,21][9][10][14]. However, in some European Union countries like Germany and Belgium, the recycling rate is about 90% [11[9][10][14],12,21], which is considerably high [21][9]. Globally, in one year, around 200 million tons of produced glasses end up in landfills [2][15]. It seems that partially substituting cement with glass could be beneficial for both the economy and the environment, but there is no research on the life cycle assessment of using glass powder in concrete to show the real advantages of GP for the economy and environment. Figure 1 illustrates the import and export quantities of glass in several key countries. Based on this visual representation, China, the UK, and North Africa show the highest levels of imports, whereas the UK, Switzerland, and the USA exhibit the highest levels of exports, respectively [11,12,21][9][10][14].
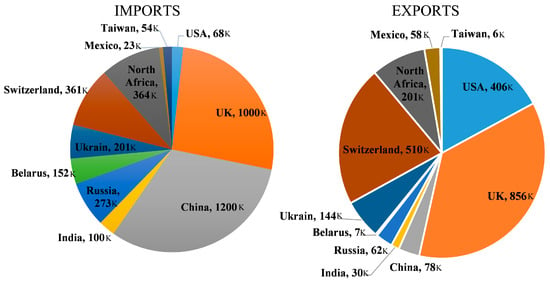
Figure 1.
The import and export amount of glass in some important countries.
2. Physical and Chemical Properties
2.1. Physical Properties
Almost all the previous studies have declared that the particle size of glass waste could lead to different properties in concrete by affecting the cement hydration matrix [1,2,3,4,5,6,7,8,9,10,11,12,25,26][10][14][15][16][17][18][19][20][21][22][23][24][25][26]. Several investigations stated that alkali–silica reaction (ASR) expansions could occur in large particles and result in forming of voluminous gels, which generate intense interior tension and start cracking the concrete [1,2,3,4,5,6,7,8,9,10,11,12,25,26][10][14][15][16][17][18][19][20][21][22][23][24][25][26]. The literature review reveals that using 10–100% of waste glass (soda-lime, electric, and lead glasses) with particle sizes more than 850 μm as an alternative to fine and coarse aggregates could decrease compressive strength between 7–55% [1,2,3,4,5,6,7,8,9,10,11,12,25,26][10][14][15][16][17][18][19][20][21][22][23][24][25][26]. However, contrary results were observed in using glass powder, which looks captivating to have green concrete due to its different filler and pozzolanic characteristics [1,2,3,4,5,6,7,8,9,10,11,12,25,26][10][14][15][16][17][18][19][20][21][22][23][24][25][26]. Generally, glass powder (GP) is characterized by a slippery texture that has steep slopes, sharp corners, and large surface areas with pozzolanic properties [1,2,3,4,5,6,7,8,9,10,11,12,25,26][10][14][15][16][17][18][19][20][21][22][23][24][25][26].
2.2. Chemical Properties
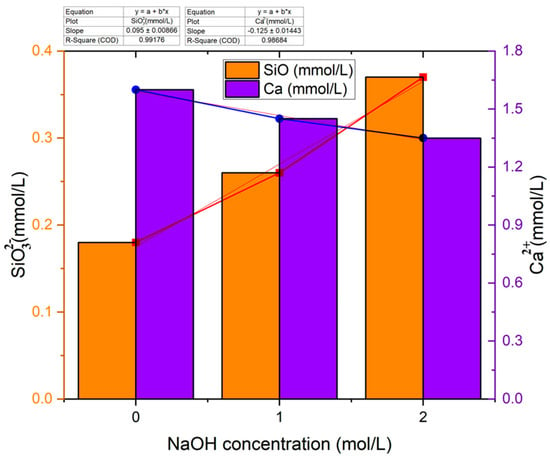
Figure 2 indicates the leaching rate of calcium and silicate in different NaOH solutions [27]. A crucial aspect of evaluating the properties of concrete is analyzing the dissolution states of calcium and silicate ions from glass powder in a NaOH solution. Differences in the leaching behavior of calcium (Ca2⁺) and silicate (SiO₄⁴−) ions in sodium hydroxide (NaOH) could be driven by their chemical properties [27]. Firstly, the difference in their solubility. For instance, calcium is highly soluble in aqueous solutions compared to silicate [27], and secondly, due to the formation of precipitates. For instance, silicate ions can react with NaOH to form various silicate compounds, some of which are poorly soluble in water. These precipitates can limit the leaching of silicate ions into the solution [27]. Last but not least, the pH could have an impact. Sodium hydroxide is a strong base, and its addition to a solution can significantly raise the pH. The high pH environment can influence the solubility of various compounds. In the case of silicate ions, the elevated pH can promote the formation of silicate precipitates, further reducing their leaching into the solution [27]. The research findings reveal that the concentration of calcium ions decreases as the concentration of NaOH solution increases, primarily due to the reaction of calcium with hydroxide ions that leads to the formation of calcium hydroxide [27]. In contrast, the presence of amorphous silicon dioxide in GP promotes the formation of calcium silicate hydration products through interaction with calcium hydroxide. Consequently, the concentration of silicate in GP increases with an increase in the concentration of sodium hydroxide [27].
2.3. Workability
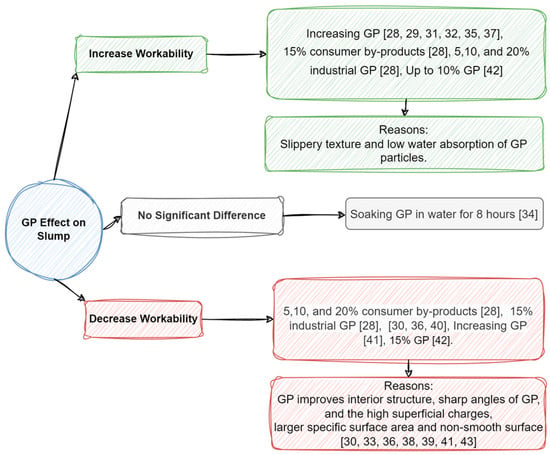
The literature review shows conflicting results regarding the workability of GP as a replacement for cement. The majority of the 15 review articles reported that GP tends to increase the slump, although some contrary results have also been stated [28,29,30,31,32,33,34,35,36,37,38,39,40,41,42][28][29][30][31][32][33][34][35][36][37][38][39][40][41][42]. It is mentioned that the slippery texture and low water absorption of GP could be the reason behind increasing workability compared to cement particles that absorb more water [28,29,31,32,33,34,35,37,42][28][29][31][32][33][34][35][37][42]. Kamali and Ghahremaninezhad (2015) stated that industrial and consumer by-products of glass powder wastes, as a replacement for 5–20% cement in concrete manufacturing, could increase the slump generally with the exception of 15% GP on both types [28]. No reason was given for this behavior. However, it is plausible that this anomaly may have arisen from an experimental error, as similar outcomes were observed in cases of both lower and higher glass powder (GP) usage [28]. Several similar studies reported increasing workability following the use of glass powder [29,31,32,33,34,35,37,42][29][31][32][33][34][35][37][42].
Since the process of releasing ions in the water is time-consuming, soaking before adding it to the mix design might help to activate the pozzolanic properties and compensate for the lower water absorption problem of GPs [34]. In order to explore the impact of immersing glass powder in water, they have conducted an experimental procedure. Based on the study of Elaqra et al. (2019), an initial mixture of GP was blended with a specific quantity of water defined by a w/c ratio of 0.72 for a duration of 8 h [34]. This step has been reported to be crucial as it could facilitate the hydrolysis of the glass powder, leading to the generation of SiO2, CaO, and Na2O, all of which play a vital role in contributing to the formation of calcium silicate hydrate (CSH). Subsequently, the water was gradually introduced to the dry concrete components. Their report included the measurement of free ions through flame atomic absorption spectrophotometry. Specifically, for 10% GP after soaking, the reported results indicated the release of 23.0 mmol/L of Ca ions and 96.3 mmol/L of Na ions into the water [34].
However, some researchers have declared that GP, due to its sharp angles, high superficial charges, large specific surface area, non-smooth surface, and filler effect, could decrease workability by blocking the interior pores and structures [30,33,36,38,39,41,43][30][33][36][38][39][41][43]. The mentioned characteristics appear correct, but time might have a more significant effect than these properties. In other words, while glass powder (GP) exhibits a filler effect, its impact on the slump test might be limited at early stages, as many papers have suggested, due to the delayed activation of GP’s pozzolanic properties. However, using other additives, glass waste type, water-to-cement ratio, type of cement, and size of glass power are some determining parameters that change the final result [28,29,30,31,32,33,34,35,36,37,38,39,40,41,42][28][29][30][31][32][33][34][35][36][37][38][39][40][41][42]. The effects of GP on workability are summarized in Figure 3.
2.4. Setting Time
Many studies have declared that alternating cement with GP could increase both initial and final setting time, which is antithetical to insignificant numbers of reports [30,40,44,45,46,47,48,49][30][40][44][45][46][47][48][49]. Figure 4 provides the initial and final setting times results of previous experimental work on using glass powder in concrete, respectively [30,40,44,45,46,47,48,49][30][40][44][45][46][47][48][49]. Generally, several samples with different amounts of GP had higher initial and final setting times than control samples. According to the data, as the percentage of GP increases from 5% to 20%, both initial and final setting time increases intensely, and then the rate of increase declines in 25% and 30% GP replacement. Han et al. 2016 indicated that 10% borosilicate GP as an alternative to cement could decrease the initial and final setting time slightly [44]. Kamali and Ghahremaninezhad (2016) found that both fiber and recycled glass waste can be used to reduce initial and final setting times when replacing 20% of cement, with the exception of a slight 11% increase in initial setting time when using fiber [45]. Aliabdo et al. reported a maximum 26% increase in initial setting time with 25% GP substitution for cement but found that 5–10% GP may accelerate hydration, 15% has no effect, and beyond 25% GP can increase final setting time without mentioning reasons behind the occurrence of these adverse behaviors [30].
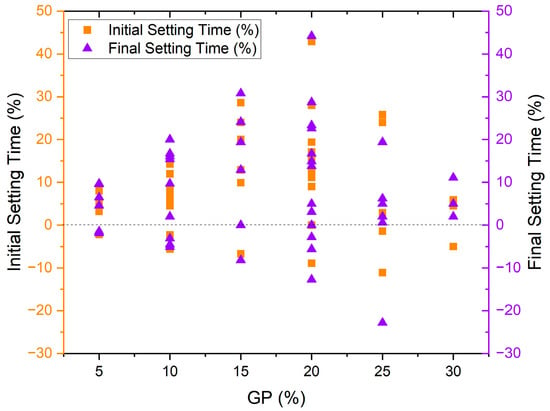
Lu et al. 2017 found that using GP could increase both the initial and final setting time regardless of the size, but smaller GP particles resulting from longer grinding could further increase the setting time [46]. The highest increment occurred at 20% GP replacement with 30 min grinding around 17 and 23% delay in initial and final setting time [46]. The effect of cement type was also investigated, and it was found that both cement types I and II, in combination with 10–30% GP, could delay both initial and final setting time [47]. The higher Al2O3 percentage in cement type II was the reason for more delay in cement hydration [47]. Only one paper has reported that smaller glass powder (GP) particles could lead to a more significant increase in the setting time [46]. Apart from this mentioned article, the remaining studies did not provide any direct correlation between particle size and setting time. Patel et al. 2019 and Huseien et al. 2020 both confirmed that GP could increase both initial and final setting time [48,49][48][49]. Finally, a recent paper indicated that increasing glass powder content in the binder prolonged the setting time by approximately 18% and 30% for the initial and final setting time, respectively [50].
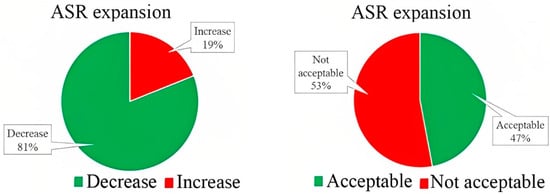
On the contrary, the results of Sharifi et al. 2016 were completely in a different direction and reported that a combination of 5–30% GP with 1.1–1.4% superplasticizer could increase both initial and final setting time in all percentages, although decreasing the SP percentage resulted in lowering the initial setting time [51]. Ibrahim and Meawad 2022 associated the acceleration effect with the glassy surface and lower water absorption of GP, which decreases water demand and improves the structure of hydration products by adhering to the matrix due to its filler effect [40]. The setting time results of previous studies are categorized in Figure 5, showing that around 60% of samples manufactured with GP showed an increment in both initial and final setting time compared to control samples. 25 and 19% of specimens demonstrated no significant differences in initial and final setting time, respectively. Less than one-fifth of the studies stated that glass powder could decline the setting time.
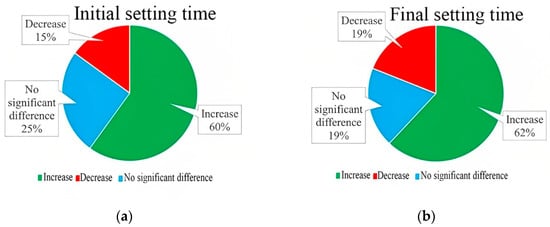
Figure 5.
Initial (
a
) and final (
2.5. ASR Expansion
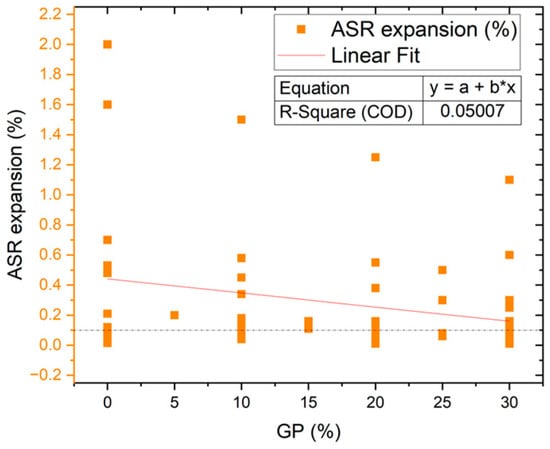
Figure 6 represents the schematic process of the alkali-silica reaction in 3 stages. Dissolving hydroxyl ions and alkali cations of cement and glass powder in the interior pores and reacting with the aggregates’ silica phases (stage 1 in Figure 6) could lead to an alkali-silica reaction (ASR) (stage 2 in Figure 6) and form a voluminous and expansive alkali-silica gel, which cracks the internal structure of cement hydration products and aggregates (stage 3 in Figure 6) [52]. ASR expansion is directly influenced by the size of the glass powder, with larger particles resulting in larger expansion, as shown in Figure 7. The slope of the trend line indicates that increasing the GP percentage may reduce the ASR expansion, but this relationship is not significant and may vary subject to different conditions due to a very low R square value. ASR expansion should not exceed 0.1% [53]. According to Bignozzi et al. 2015, five categories of glass powder with different diameters were tested, with soda-lime glass (SL) having the highest SiO2 + Al2O3 content, resulting in 72.5% ASR expansion and crystal glass (CR) having the lowest amount with around 58.6% ASR expansion [54]. After 14 days, samples made with funnel glass (FNL), end-use fluorescent lamp (LMPS), SL, and control samples had an acceptable expansion, with approximate amounts of 0.08%, 0.07%, 0.06%, and 0.05%, respectively [54]. Kim et al. in 2015 showed that increasing glass powder and combining it with 10% FA could decrease the ASR expansion, with 66%, 70%, and 87.5% reductions in samples manufactured with 10% GP, 20% GP, and 10% GP + 10% FA, respectively [55].
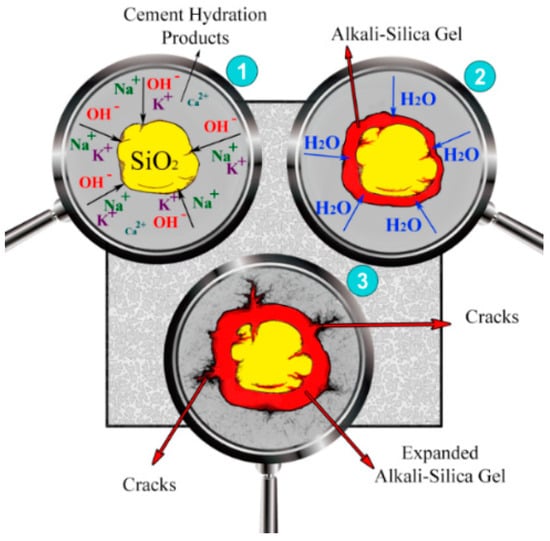
Figure 6.
The schematic process of alkali-silica reaction in 3 stages.
Ke et al. 2018 found that increasing the size of glass powder leads to an increase in ASR expansion, consistent with previous studies [56]. They noted that small sizes may reduce ASR expansion compared to control samples, but larger sizes and higher percentages can lead to opposite results [56]. According to the authors, the pozzolanic reaction transforms portlandite crystals into calcium silicate hydrate gel, enhancing the internal structure by filling voids and pores. However, when larger particles are present, they have the capacity to absorb more water, resulting in increased expansion. This expansion can ultimately lead to the degradation of hydration products and the formation of cracks [56].
Elaqra and Rustom (2019) reported that increasing the percentages of GP up to 30% with sizes smaller than 75 μm could decrease the ASR expansion significantly [34,47][34][47]. However, all the samples except the sample manufactured with 30% GP and cement type I with higher CaO rather than cement type II percentage did not comply with the standard [47]. Lu et al. showed that using 70% glass cullet and 20% GP as aggregate and cement replacement reduced ASR expansion by about 33% at 14 days without cracks after 28 days [46]. Similarly, Hendi et al. (2019) found that only 30% GP with sizes around 1 μm resulted in acceptable ASR expansion [35]
Yang et al. 2020 used up to 30% GP with sizes smaller than 100 μm in combination with 100% glass waste aggregate, 0 and 1% SP in 2 different water-to-cement ratios, including 0.47 and 0.42 [58]. It seems that increasing the water-to-cement ratio and adding a superplasticizer could increase the ASR expansion significantly. Furthermore, in this case, like in previous studies, increasing the GP resulted in lowering the ASR expansion in all samples [58]. They declared that besides forming C-S-H gels that improve the interior structure, GP could control the dissolution of silica by providing higher alumina concentration [58]. Moreover, by using calcium hydroxide in generation gels, the amount of calcium gets reduced, and pozzolanic reactions occur before ASR expansion [58]. In general, calcium hydroxide is likely to react with available amorphous silica in glass powder to form calcium silicate hydrate (C-S-H) gel, resulting in reducing the amount of free calcium hydroxide in the concrete and converting the (Ca(OH)2) crystals into stronger C-S-H gel as well as mitigating the alkali-silica reaction (ASR) concerns. This reaction refines the concrete’s microstructure, leading to a denser and less porous interior structure and ultimately enhancing both mechanical and durability properties, especially during older ages. Fanijo et al. (2021) found that adding more GP or combining it with slag or silica fume could reduce ASR expansion [59]. Slag performed better than silica fume, possibly due to its finer particles that react more easily. In their study, 30% GP resulted in the best ASR test performance [59]. Figure 8 shows that over 80% of samples improved ASR property with GP, but less than 50% met the related standard for acceptable expansion [28,35,36,47,54,55,56,57,58,59][28][35][36][47][54][55][56][57][58][59].
References
- Garside, M. Glass Industry Statistics & Facts. 2019. Available online: https://www.statista.com/topics/4108/glass/ (accessed on 8 August 2023).
- Perry, C. Glass Sector: Joint Industry—Government Industrial Decarbonisation and Energy Efficiency Roadmap Action Plan; BEIS: London, UK, 2017.
- Vinci, G.; D’ascenzo, F.; Esposito, A.; Musarra, M.; Rapa, M.; Rocchi, A. A sustainable innovation in the Italian glass production: LCA and Eco-Care matrix evaluation. J. Clean. Prod. 2019, 223, 587–595.
- Wu, Q.; Zeng, L.; Zhou, Q. Phase I monitoring of optical profiles with application in low-emittance glass manufacturing. J. Qual. Technol. 2018, 50, 262–278.
- Griffin, P.W.; Hammond, G.P.; Norman, J.B. Industrial energy use and carbon emissions reduction in the chemicals sector: A UK perspective. Appl. Energy 2018, 227, 587–602.
- Karellas, S.; Giannakopoulos, D.; Hatzilau, C.-S.; Dolianitis, I.; Skarpetis, G.; Zitounis, T. The potential of WHR/batch and cullet preheating for energy efficiency in the EU ETS glass industry and the related energy incentives. Energy Effic. 2017, 11, 1161–1175.
- Hubert, M. Industrial Glass Processing and Fabrication. In Springer Handbook of Glass; Springer: Berlin/Heidelberg, Germany, 2019; pp. 1195–1231.
- Madeddu, S.; Ueckerdt, F.; Pehl, M.; Peterseim, J.; Lord, M.; Kumar, K.A.; Krüger, C.; Luderer, G. The CO2 reduction potential for the European industry via direct electrification of heat supply (power-to-heat). Environ. Res. Lett. 2020, 15, 124004.
- Griffin, P.W.; Hammond, G.P.; McKenna, R.C. Industrial energy use and decarbonisation in the glass sector: A UK perspective. Adv. Appl. Energy 2021, 3, 100037.
- Del Rio, D.D.F.; Sovacool, B.K.; Foley, A.M.; Griffiths, S.; Bazilian, M.; Kim, J.; Rooney, D. Decarbonizing the glass industry: A critical and systematic review of developments, sociotechnical systems and policy options. Renew. Sustain. Energy Rev. 2021, 155, 111885.
- Hossain, U.; Poon, C.S.; Lo, I.M.; Cheng, J.C. Comparative LCA on using waste materials in the cement industry: A Hong Kong case study. Resour. Conserv. Recycl. 2017, 120, 199–208.
- Blomsma, F.; Pieroni, M.; Kravchenko, M.; Pigosso, D.C.; Hildenbrand, J.; Kristinsdottir, A.R.; Kristoffersen, E.; Shahbazi, S.; Nielsen, K.D.; Jönbrink, A.-K.; et al. Developing a circular strategies framework for manufacturing companies to support circular economy-oriented innovation. J. Clean. Prod. 2019, 241, 118271.
- Cooper, S.J.G.; Hammond, G.P. ‘Decarbonising’ UK industry: Towards a cleaner economy. Proc. Inst. Civ. Eng. Energy 2018, 171, 147–157.
- Kumari, S.; Agarwal, S.; Khan, S. Micro/nano glass pollution as an emerging pollutant in near future. J. Hazard. Mater. Adv. 2022, 6, 100063.
- Jiang, Y.; Ling, T.-C.; Mo, K.H.; Shi, C. A critical review of waste glass powder—Multiple roles of utilization in cement-based materials and construction products. J. Environ. Manag. 2019, 242, 440–449.
- Mohajerani, A.; Vajna, J.; Cheung, T.H.H.; Kurmus, H.; Arulrajah, A.; Horpibulsuk, S. Practical recycling applications of crushed waste glass in construction materials: A review. Constr. Build. Mater. 2017, 156, 443–467.
- Liu, Y.; Shi, C.; Zhang, Z.; Li, N. An overview on the reuse of waste glasses in alkali-activated materials. Resour. Conserv. Recycl. 2019, 144, 297–309.
- Khan, N.N.; Saha, A.K.; Sarker, P.K. Reuse of waste glass as a supplementary binder and aggregate for sustainable cement-based construction materials: A review. J. Build. Eng. 2020, 28, 101052.
- Mehta, A.; Kar, D.; Ashish, K. Silica fume and waste glass in cement concrete production: A review. J. Build. Eng. 2020, 29, 100888.
- Amin, M.; Agwa, I.S.; Mashaan, N.; Mahmood, S.; Abd-Elrahman, M.H. Investigation of the Physical Mechanical Properties and Durability of Sustainable Ultra-High Performance Concrete with Recycled Waste Glass. Sustainability 2023, 15, 3085.
- Guo, P.; Meng, W.; Nassif, H.; Gou, H.; Bao, Y. New perspectives on recycling waste glass in manufacturing concrete for sustainable civil infrastructure. Constr. Build. Mater. 2020, 257, 119579.
- Dong, W.; Li, W.; Tao, Z. A comprehensive review on performance of cementitious and geopolymeric concretes with recycled waste glass as powder, sand or cullet. Resour. Conserv. Recycl. 2021, 172, 105664.
- Mallum, I.; Rahman, A.; Sam, M.; Hasanah, N.; Lim, A.S.; Omolayo, N. Sustainable Utilization of Waste Glass in Concrete: A Review. Silicon 2022, 14, 3199–3214.
- Barret, J.; Cooper, T.; Hammond, G.P.; Pidgeon, N. Industrial energy, materials and products: UK decarbonisation challenges and opportunities. Appl. Therm. Eng. 2018, 136, 643–656.
- Abdelli, H.E.; Mokrani, L.; Kennouche, S.; de Aguiar, J.B. Utilization of waste glass in the improvement of concrete performance: A mini review. Waste Manag. Res. J. Sustain. Circ. Econ. 2020, 38, 1204–1213.
- Mirzahosseini, M.; Riding, K.A. Influence of different particle sizes on reactivity of finely ground glass as supplementary cementitious material (SCM). Cem. Concr. Compos. 2015, 56, 95–105.
- Xu, J.; Zhan, P.; Zhou, W.; Zuo, J.; Shah, S.P.; He, Z. Design and assessment of eco-friendly ultra-high performance concrete with steel slag powder and recycled glass powder. Powder Technol. 2023, 419, 118356.
- Kamali, M.; Ghahremaninezhad, A. Effect of glass powders on the mechanical and durability properties of cementitious materials. Constr. Build. Mater. 2015, 98, 407–416.
- Sharifi, Y.; Afshoon, I.; Firoozjaie, Z. Fresh Properties of Self-Compacting Concrete Containing Ground Waste Glass Microparticles as Cementing Material. J. Adv. Concr. Technol. 2015, 13, 50–66.
- Aliabdo, A.A.; Abd Elmoaty, A.E.M.; Aboshama, A.Y. Utilization of waste glass powder in the production of cement and concrete. Constr. Build. Mater. 2016, 124, 866–877.
- Omran, A.F.; D.-Morin, E.; Harbec, D.; Tagnit-Hamou, A. Long-term performance of glass-powder concrete in large-scale field applications. Constr. Build. Mater. 2017, 135, 43–58.
- Soliman, N.; Tagnit-Hamou, A. Partial substitution of silica fume with fine glass powder in UHPC: Filling the micro gap. Constr. Build. Mater. 2017, 139, 374–383.
- Lee, H.; Hanif, A.; Usman, M.; Sim, J.; Oh, H. Performance evaluation of concrete incorporating glass powder and glass sludge wastes as supplementary cementing material. J. Clean. Prod. 2018, 170, 683–693.
- Elaqra, H.A.; Haloub, M.A.A.; Rustom, R.N. Effect of new mixing method of glass powder as cement replacement on mechanical behavior of concrete. Constr. Build. Mater. 2019, 203, 75–82.
- Hendi, A.; Mostofinejad, D.; Sedaghatdoost, A.; Zohrabi, M.; Naeimi, N.; Tavakolinia, A. Mix design of the green self-consolidating concrete: Incorporating the waste glass powder. Constr. Build. Mater. 2018, 199, 369–384.
- Ali-Boucetta, T.; Behim, M.; Cassagnabere, F.; Mouret, M.; Ayat, A.; Laifa, W. Durability of self-compacting concrete containing waste bottle glass and granulated slag. Constr. Build. Mater. 2021, 270, 121133.
- Balasubramanian, B.; Krishna, G.G.; Saraswathy, V.; Srinivasan, K. Experimental investigation on concrete partially replaced with waste glass powder and waste E-plastic. Constr. Build. Mater. 2021, 278, 122400.
- Ibrahim, K.I.M. Recycled waste glass powder as a partial replacement of cement in concrete containing silica fume and fly ash. Case Stud. Constr. Mater. 2021, 15, e00630.
- Esmaeili, J.; Al-Mwanes, A.O. A review: Properties of eco-friendly ultra-high-performance concrete incorporated with waste glass as a partial replacement for cement. Mater. Today: Proc. 2021, 42, 1958–1965.
- Ibrahim, S.; Meawad, A. Towards green concrete: Study the role of waste glass powder on cement/superplasticizer compatibility. J. Build. Eng. 2021, 47, 103751.
- Ahmad, J.; Zhou, Z.; Martínez-García, R. A study on the microstructure and durability performance of rubberized concrete with waste glass as binding material. J. Build. Eng. 2022, 49, 104054.
- Chu, S.; Li, L.; Shen, P.; Lu, J.; Poon, C.S. Recycling of waste glass powder as paste replacement in green UHPFRC. Constr. Build. Mater. 2022, 316, 125719.
- Ammari, M.S.; Tobchi, M.B.; Amrani, Y.; Mim, A.; Bederina, M.; Ferhat, A.A. Influence of glass powder incorporation on the physical-mechanical properties of sand concrete. World J. Eng. 2021, 20, 314–324.
- Han, W.; Sun, T.; Li, X.; Sun, M.; Lu, Y. Using of borosilicate glass waste as a cement additive. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2016, 381, 11–15.
- Kamali, M.; Ghahremaninezhad, A. An investigation into the hydration and microstructure of cement pastes modified with glass powders. Constr. Build. Mater. 2016, 112, 915–924.
- Lu, J.-X.; Duan, Z.-H.; Poon, C.S. Fresh properties of cement pastes or mortars incorporating waste glass powder and cullet. Constr. Build. Mater. 2017, 131, 793–799.
- Elaqra, H.; Rustom, R. Effect of using glass powder as cement replacement on rheological and mechanical properties of cement paste. Constr. Build. Mater. 2018, 179, 326–335.
- Patel, D.; Tiwari, R.P.; Shrivastava, R.; Yadav, R.K. Effective utilization of waste glass powder as the substitution of cement in making paste and mortar. Constr. Build. Mater. 2019, 199, 406–415.
- Huseien, G.F.; Hamzah, H.K.; Sam, A.R.M.; Khalid, N.H.A.; Shah, K.W.; Deogrescu, D.P.; Mirza, J. Alkali-activated mortars blended with glass bottle waste nano powder: Environmental benefit and sustainability. J. Clean. Prod. 2019, 243, 118636.
- Mahajan, L.S.; Bhagat, S.R. Utilization of Pozzolanic Material and Waste Glass Powder in Concrete. Lect. Notes Civ. Eng. 2023, 260, 201–206.
- Sharifi, Y.; Afshoon, I.; Firoozjaei, Z.; Momeni, A. Utilization of Waste Glass Micro-particles in Producing Self-Consolidating Concrete Mixtures. Int. J. Concr. Struct. Mater. 2016, 10, 337–353.
- Cota, F.P.; Melo, C.C.D.; Panzera, T.H.; Araújo, A.G.; Borges, P.H.R.; Scarpa, F. Mechanical properties and ASR evaluation of concrete tiles with waste glass aggregate. Sustain. Cities Soc. 2015, 16, 49–56.
- ASTM C1260-21; Standard Test Method for Potential Alkali Reactivity of Aggregates (Mortar-Bar Method). ASTM: West Conshohocken, PA, USA, 2021.
- Bignozzi, M.; Saccani, A.; Barbieri, L.; Lancellotti, I. Glass waste as supplementary cementing materials: The effects of glass chemical composition. Cem. Concr. Compos. 2014, 55, 45–52.
- Kim, J.; Yi, C.; Zi, G. Waste glass sludge as a partial cement replacement in mortar. Constr. Build. Mater. 2015, 75, 242–246.
- Ke, G.; Li, W.; Li, R.; Li, Y.; Wang, G. Mitigation Effect of Waste Glass Powders on Alkali–Silica Reaction (ASR) Expansion in Cementitious Composite. Int. J. Concr. Struct. Mater. 2018, 12, 67.
- Lu, J.-X.; Zheng, H.; Yang, S.; He, P.; Poon, C.S. Co-utilization of waste glass cullet and glass powder in precast concrete products. Constr. Build. Mater. 2019, 223, 210–220.
- Yang, S.; Lu, J.-X.; Poon, C.S. Recycling of waste glass in dry-mixed concrete blocks: Evaluation of alkali-silica reaction (ASR) by accelerated laboratory tests and long-term field monitoring. Constr. Build. Mater. 2020, 262, 120865.
- Fanijo, E.O.; Kassem, E.; Ibrahim, A. ASR mitigation using binary and ternary blends with waste glass powder. Constr. Build. Mater. 2021, 280, 122425.
More