Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Yauheni Shastak and Version 2 by Fanny Huang.
Fish, constantly exposed to environmental stressors due to their aquatic habitat and high metabolic rates, are susceptible to oxidative stress. Astaxanthin, a carotenoid pigment, effectively combats reactive oxygen species, inhibiting lipid peroxidation and maintaining membrane integrity. It significantly enhances reproductive success in fish and improves overall fish health in aquaculture settings.
- astaxanthin
- fish
- antioxidant
1. Introduction
In the domain of aquatic ecosystems, prioritizing the vitality and thriving of fish populations takes precedence. A pivotal determinant influencing fish well-being is oxidative stress [1], a condition characterized by the disproportion between the generation of detrimental reactive oxygen species (ROS) and an organism’s capacity to mitigate their injurious consequences through antioxidants [2]. This intricate equilibrium plays a vital role in upholding the comprehensive health and efficiency of fish, underscoring the significance of researching antioxidants as a fundamental constituent of fisheries science and aquaculture management [3][4][3,4].
One antioxidant that has attracted considerable attention recently is astaxanthin—a naturally occurring carotenoid pigment, also attainable through synthetic means, responsible for the vivid red and orange hues manifested in diverse aquatic organisms, such as salmon, shrimp, and crayfish [5][6][5,6]. Natural sources of astaxanthin primarily include specific microalgae species and red yeast. These organisms produce astaxanthin as a defense mechanism against environmental stressors [7]. Commercially, astaxanthin extraction from these microorganisms employs various techniques, such as supercritical CO2 extraction or solvent extraction [8][9][8,9]. In contrast, synthetic production involves chemical synthesis using specific precursors and controlled reactions in industrial settings [10]. The chemical synthesis of astaxanthin typically entails the Wittig reaction, which necessitates the use of the precursor β-ionone and a carbonyl compound [11].
Astaxanthin is a prevalent pigment employed extensively within the field of aquaculture [10]. Beyond its aesthetic appeal, astaxanthin has emerged as a powerful player in promoting fish health and longevity through its remarkable antioxidant properties [5][10][5,10]. These properties allow astaxanthin to neutralize ROS and mitigate the detrimental impacts of oxidative stress, thereby safeguarding cellular integrity and essential physiological processes [12].
Astaxanthin has earned the moniker “super vitamin E” due to its exceptional free radical scavenging capabilities, which surpass those of other carotenoids including β-carotene, canthaxanthin, lutein, and zeaxanthin, as well as vitamins C and E [13]. The in vitro antioxidant potential of astaxanthin demonstrated a significant superiority, with a potency that surpassed zeaxanthin, lutein, canthaxanthin, and β-carotene by a factor of 10 and even exceeded α-tocopherol by a notable 100-fold in neutralizing toxic ROS without forming pro-oxidants [14]. In comparison to vitamin C, astaxanthin exhibited a potency that was 6000 times greater [15].
Vibrant coloration in fish frequently indicates robust health and optimal physiological performance [16]. Consequently, scientists and conservation experts employ this coloration phenomenon as a visual indicator for tracking the vitality of aquaculture communities and gauging the holistic ecological balance within aquatic environments [17]. The detection of astaxanthin, along with its influence on coloration, presents a valuable mechanism for comprehending the intricate interactions among environmental variables, fish well-being, and the intricate dynamics of ecosystems [18].
Recent research has highlighted the complex relationship between oxidative stress and reproductive processes in fish biology [19]. Oxidative stress can negatively affect gamete quality, leading to compromised structural and functional integrity in sperm and ova [20][21][22][20,21,22]. This can result in reduced fertilization success and developmental anomalies in the offspring. The central player in this interplay is nuclear factor erythroid-2 related factor 2 (Nrf2), a transcription factor regulating antioxidant gene expression that can become disrupted under prolonged oxidative stress, compromising antioxidant defenses [23]. Oxidative stress can also disrupt endocrine pathways related to fish reproduction and is exacerbated by environmental stressors such as pollutants and temperature fluctuations [24][25][26][27][24,25,26,27]. Understanding these dynamics has implications for fish conservation and aquaculture, including the development of biomarkers and antioxidant-based interventions to improve reproductive success.
2. Oxidative Stress and Fish Health
Oxidative stress holds significant ramifications for the well-being of aquatic organisms, with fish exhibiting heightened vulnerability to its perturbations [28][29][28,29]. ROS, encompassing entities such as superoxide anions, hydrogen peroxide, and hydroxyl radicals, emerge as natural byproducts of cellular metabolic processes [2]. However, when their generation surpasses the buffering capabilities of antioxidant defenses, the resultant oxidative stress leads to cellular impairment, DNA mutations, and disturbances in pivotal biomolecules [2]. Collectively, these processes contribute to an array of health challenges in fish [30].
The predominant contributor to ROS is the mitochondrial electron transport chain, an essential component of cellular respiration [31][32][33][31,32,33]. Furthermore, internal mechanisms like peroxisomal reactions and inflammatory responses can trigger the production of ROS [34][35][36][34,35,36]. Specifically, peroxisomal reactions can trigger ROS production as a byproduct of processes like fatty acid oxidation and detoxification, generating hydrogen peroxide and superoxide anions [37][38][37,38]. Inflammatory responses activate immune cells, such as neutrophils and macrophages, which produce ROS through enzymes like NADPH oxidase during their antimicrobial defense mechanisms, contributing to ROS production during inflammation [39]. External elements, including exposure to pollutants, heavy metals, and ultraviolet radiation, can intensify ROS generation [40][41][40,41]. The cumulative effect can overwhelm the antioxidant defense system, encompassing both enzymatic antioxidants like superoxide dismutase, catalase, and glutathione peroxidase, as well as non-enzymatic antioxidants such as vitamins C and E, glutathione, and carotenoids [42][43][42,43].
The effects of oxidative stress propagate through various physiological processes in fish, influencing multiple facets of their biology [44]. One of the most pivotal domains influenced is the immune system [34]. ROS, while critical in mounting immune responses against pathogens [45], can paradoxically inhibit immune functioning when their levels surge uncontrollably, rendering fish more susceptible to infections [34][46][34,46]. The impact of oxidative stress further resonates in fish reproduction, exerting influence over sperm quality and egg viability, potentially undermining reproductive success [20][47][20,47]. Moreover, oxidative stress interplays with fish growth and development [48]. The balance between ROS production and antioxidant defenses is particularly precarious during periods of rapid growth, as oxidative stress can slow down the normal process of growth and development [49].
Beyond physiological realms, oxidative stress infiltrates the behavioral patterns and adaptability of fish. Prolonged exposure to oxidative stress can trigger alterations in behavior, manifesting as reduced swimming performance and compromised responses to predator threats [50][51][52][50,51,52]. Physiological stress resulting from oxidative imbalance also reverberates through fish energy metabolism. Damage inflicted upon mitochondria—the cellular powerhouses—by ROS can lead to diminished energy production and compromised endurance in fish, a predicament particularly worrisome for migratory species dependent on sustained energy for extensive journeys [53][54][55][53,54,55].
The increasing anthropogenic pressures on aquatic ecosystems, coupled with genetic selection aimed at achieving rapid growth and efficient fish muscle deposition under commercial rearing conditions, have contributed to a substantial rise in oxidative stress within ichthyoid populations [56][57][58][59][60][56,57,58,59,60]. Pollution, climate change, and habitat degradation converge to amplify ROS production in aquatic environments [60]. Heavy metal contamination in water bodies, for instance, directly augments ROS levels within fish due to metal-catalyzed oxidative reactions [40]. Elevated water temperatures linked to climate change exacerbate oxidative stress by stimulating metabolic rates and intensifying ROS production [61]. As aquatic ecosystems endure these perturbations, comprehending the interplay between oxidative stress and fish health becomes a pressing concern.
Even within controlled environments such as enclosed fish farms or recirculating aquaculture systems that meticulously manage water quality and temperatures, the possibility of oxidative stress persists [62]. This potential arises from factors including unbalanced or insufficient diets, the presence of antinutrients in feeds, challenges associated with handling and transportation throughout various fish production stages, elevated stocking densities, suboptimal management practices, the application of specific medications, the use of oxidative disinfectants, and the influence of genetic predispositions [63][64][65][63,64,65]. Some common oxidative stressors for fish and their permissible limits are shown in Table 1.
Table 1. Some common oxidative stressors for fish and their permissible limits.
| Stressor | Permissible Limit | Effects on Fish | Reference |
|---|---|---|---|
| Oxygen depletion | Dissolved oxygen levels should not fall below 5 mg/L for most freshwater fish. | Low dissolved oxygen can lead to fish suffocation and reduced growth. | [66] |
| Temperature fluctuations | Diurnal fluctuations in water temperature should not exceed a certain threshold, which is species-specific. | Rapid temperature changes can stress fish and impact their metabolism. | [67] |
| Pollutants (heavy metals) | Varies by metal and species. In general, allowable concentrations are low (micrograms per liter or lower). | Heavy metals like lead, mercury, and cadmium can accumulate in fish tissues and harm health. | [68] |
| Pesticides and herbicides | Varies by chemical and species. Generally, very low concentrations are allowed (parts per billion). | These chemicals can disrupt fish physiology and impair reproduction. | [69] |
| Ammonia | Total ammonia nitrogen levels should be below 0.02 mg/L for freshwater fish. | High ammonia can damage fish gills and cause respiratory distress. | [70] |
| pH | Optimal pH ranges from 6.5 to 9.0, depending on the fish species. | Extreme pH levels can stress fish, affecting ion balance and survival. | [71] |
| Salinity | Varies widely by fish species. Some tolerate freshwater, while others require high salinity. | Salinity outside a fish’s tolerance range can cause osmotic stress. | [72] |
| UV radiation | Exposure should be limited, especially in shallow, clear waters. | Prolonged UV exposure can damage fish skin and eyes. | [73] |
| Microorganisms (pathogens) | The presence of pathogens like bacteria, viruses, and parasites should be minimized. | Infections can weaken fish and lead to disease outbreaks. | [74] |
| Toxic algal blooms | Concentrations of harmful algae should be monitored and controlled. | Toxins produced by algae can harm fish and other aquatic organisms. | [75] |
3. Mechanisms of Astaxanthin as an Antioxidant
In recent years, there has been a growing interest in astaxanthin due to its recognized antioxidative properties, particularly within the context of fish physiology [5][76][5,76]. Fish, being aquatic organisms, are continuously exposed to a variety of environmental stressors. To mitigate the potential damage inflicted by oxidative stress, fish have developed complex antioxidant defense mechanisms. Within this framework, the effectiveness of astaxanthin as a robust and multifaceted antioxidant is notable [5]. The antioxidant attributes of astaxanthin are intricately linked to its underlying chemical configuration [77].
3.1. ROS Scavenging Mechanism
Astaxanthin’s structural features, including its conjugated double bond system and extended polyene arrangement, create an optimal framework for electron distribution, enabling effective modulation of ROS activity [78]. The conjugated double bonds also give astaxanthin its characteristic red color, which is often visible in the flesh of certain fish species, such as salmon and trout [79]. Notably, the hydroxyl and keto functional groups within astaxanthin’s molecular structure play a crucial role in facilitating electron transfer to unstable ROS, thereby reducing their reactivity and protecting biomolecules such as lipids, proteins, and nucleic acids from oxidative damage [80]. Importantly, astaxanthin’s potential to neutralize ROS might surpass that of both β-carotene and α-tocopherol [81].
In the context of photosynthesis, singlet oxygen (1O2) can arise from light-interacting chlorophyll molecules in plants [82]. Similarly, in fish, 1O2—a highly reactive and toxic form of ROS—could be generated naturally during cellular metabolism [83]. This reactive species, 1O2, possesses the ability to induce oxidative stress within ichthyoid cells [84]. Similar to its interactions with other ROS, the efficient quenching of 1O2 by astaxanthin can be attributed to its configuration of conjugated double bonds. Additionally, the presence of terminal ionone rings at both ends of the molecule also contributes significantly to this process (Figure 1). These structural features facilitate energy transfer between the excited 1O2 and the astaxanthin molecule [85]. Consequently, this energy transfer dissipates the excess energy of 1O2, rendering it inactive and preventing detrimental interactions with cellular components. In this manner, astaxanthin’s capacity to quench 1O2 contributes to its role as a robust antioxidant in fish tissues [86].
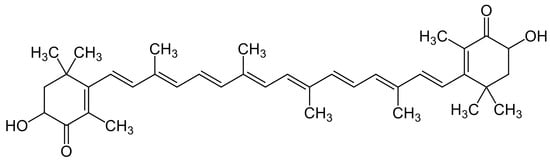
Figure 1. Structural formula for astaxanthin.
3.2. Lipid Peroxidation Inhibition
Lipid peroxidation is a detrimental biochemical process involving the oxidative deterioration of polyunsaturated fatty acids within cellular membranes, leading to the potential disruption of membrane structural integrity and the compromise of vital cellular functions [87]. Astaxanthin demonstrates effectiveness in mitigating lipid peroxidation due to its lipophilic nature, which facilitates its strategic integration into cellular membranes [79]. Its molecular arrangement, characterized by a sequence of linear, polar, nonpolar, and polar components, allows precise integration within the membrane’s lipid bilayer, spanning its entirety. As astaxanthin is incorporated into the lipid bilayers, its polar segments extend into the surrounding aqueous environment, while its hydrophobic region interacts with the hydrocarbon chains of membrane lipids [88][89][88,89]. This molecular disposition hinders the propagation of lipid peroxidation reactions by quenching lipid radicals and stabilizing the lipid bilayer structure [90][91][90,91]. In this configuration, astaxanthin possesses the capacity to intercept reactive molecular species within the lipid core of the membrane and at its interfaces with the surrounding aqueous phase [92]. Moreover, astaxanthin’s antioxidant attributes extend to synergistic interactions with other antioxidants, such as vitamin E, fostering a concerted defense against lipid peroxidation [93][94][93,94]. Specifically, astaxanthin and vitamin E synergize through the possible regeneration of vitamin E by astaxanthin [95].
4. Astaxanthin and Oxidative Stress Regulation in Fish
Over the years, a substantial body of research has emerged, illuminating the capacity of astaxanthin to influence antioxidant enzyme activity and gene expression within fish species [5][23][96][97][5,23,96,97]. Astaxanthin’s potential to modulate these vital aspects of cellular defense mechanisms has garnered significant attention due to its implications for fish health, aquaculture practices, and the broader understanding of oxidative stress regulation in aquatic organisms [98][99][98,99].
Hassanzadeh et al. [97]. conducted a study to examine the potential effects of incorporating synthetic astaxanthin into the diet of rainbow trout. The researchers assessed two different dietary levels: 0.5 and 2 g per kg of feed. Over a duration of 60 days, the fish were fed with these experimental diets and subsequently exposed to paraquat, a known oxidative stress inducer. The results revealed that the inclusion of astaxanthin in the diets led to noticeable improvements (p < 0.05) in growth rates [97]. Additionally, the fish that received astaxanthin-supplemented diets exhibited reduced levels of protein and lipid oxidation, as well as lower peroxide values compared to the control group (p < 0.05) that was exposed to paraquat alone. Furthermore, the rainbow trout fed with astaxanthin displayed an increased expression of genes linked to antioxidant defense mechanisms (p < 0.05). This suggests that astaxanthin likely contributed to enhancing the overall antioxidant system of the fish.
In their 2023 research, Shabanzadeh and colleagues [23] conducted a comprehensive investigation into the impact of commercial synthetic astaxanthin on various parameters in rainbow trout when faced with diazinon exposure. The study encompassed a dietary regimen where fish were administered varying concentrations of astaxanthin (0.0, 0.5, 2.0, and 5.0 g/kg) for a span of 60 days, followed by a subsequent diazinon challenge. The outcomes demonstrated significant improvements (p < 0.05) in growth patterns across all astaxanthin-enriched diets as compared to the control group, which did not receive supplementary astaxanthin. The investigation further probed the serum antioxidant status of the fish. The findings exhibited that those fish that were fed diets enriched with astaxanthin, especially at the 2.0 and 5.0 g/kg levels, displayed enhanced serum antioxidant levels (p < 0.05). This enhancement was evident through a decrease in malondialdehyde levels, a well-established marker of oxidative stress, and an increase in overall antioxidant activity. Central to the study was the analysis of gene expression patterns. The researchers documented a substantial up-regulation of vital antioxidant-related genes in the kidneys and liver of fish that received astaxanthin supplementation (p < 0.05), particularly in the highest dosage group. The genes included superoxide dismutase, catalase, glutathione peroxidase, glutathione S-transferase, and Nrf2. This observation suggests that astaxanthin may have the potential to activate these genes, bolstering the fish’s ability to counteract the oxidative stress prompted by diazinon exposure.
In 2019, Kalinowski et al. [96]. conducted a 13-week trial involving juvenile rainbow trout to investigate the impacts of two diets: a control diet and another enriched with 100 mg/kg of synthetic astaxanthin. The researchers employed intermittent hyperoxia exposure to induce stress responses. The introduction of astaxanthin supplementation demonstrated notable effects on reducing oxidative stress. This was evident through a decrease in thiobarbituric acid-reactive substances, indicative of lipid peroxidation products in muscle and liver cells. While hyperoxia triggered a decline in liver antioxidant enzyme activities, the inclusion of astaxanthin led to increased glutathione reductase activity, corroborated by higher mRNA levels (p < 0.05). Moreover, astaxanthin supplementation improved the ratio of reduced glutathione to oxidized glutathione (p < 0.05). This improvement was attributed to enhanced glutathione recycling and de novo synthesis, facilitated by the up-regulation of related enzyme mRNA expression. Additionally, the astaxanthin-enriched diet prompted elevated mRNA expression of liver glucokinase and glucose-6-phosphate dehydrogenase, suggesting potential activation of the NADPH-producing pentose phosphate pathway, which counteracts oxidative stress. In essence, this study highlights the potential of dietary astaxanthin to alleviate oxidative stress in the context of hyperoxia-induced conditions.
Zhu et al. [5] conducted an investigation into the influence of astaxanthin on the antioxidative function and associated traits of coral trout. The research employed four distinct diets (designated as A0, A1, A2, and A3) containing varying levels of astaxanthin (0, 0.05, 0.1, and 0.2 g/kg) sourced from Haematococcus pluvialis powder. Over the course of an 8-week feeding trial, the supplementation of astaxanthin demonstrated no statistically significant (p > 0.05) impacts on the growth of coral trout. However, a notable enhancement in antioxidant activity was observed within the A2 diet group, characterized by elevated levels of catalase, superoxide dismutase, and glutathione peroxidase activities, along with heightened total antioxidant capacity levels present in both the serum and liver (p < 0.05). Subsequent to a challenge involving Vibrio harveyi, the A2 diet group exhibited increased levels of serum and liver acid phosphatase, lysozyme activities, and complement contents (p < 0.05). Furthermore, the expression of genes in the liver associated with antioxidant enzymes, immune responses, and defense mechanisms was found to be up-regulated within the A2 group (p < 0.05). Remarkably, the A2 diet group displayed a notably improved survival rate following exposure to the V. harveyi challenge (p < 0.05). To conclude, the inclusion of 1.0 g/kg of H. pluvialis powder rich in astaxanthin (equivalent to 0.091 g/kg astaxanthin) resulted in the augmentation of antioxidant capacity, immune response, and resistance against the V. harveyi challenge among coral trout.
Xie et al. [98] conducted a comprehensive investigation into the influence of astaxanthin on the growth performance and antioxidant functions of golden pompano (Trachinotus ovatus) through both in vivo and in vitro experiments. During the in vivo phase, fish were subjected to diets with or without astaxanthin supplementation (0 and 200 mg/kg) over a span of 6 weeks. The inclusion of astaxanthin in the diet resulted in statistically significant enhancements in weight gain and specific growth rate, accompanied by a notable reduction in the feed conversion ratio within the astaxanthin-supplemented group (p < 0.05). This supplementation also triggered elevated hepatic total antioxidant capacity and augmented levels of reduced glutathione within the astaxanthin-fed cohort. Conversely, there was a decrease observed in superoxide dismutase levels due to the astaxanthin supplementation. The in vitro aspect of this study involved the exposure of isolated hepatopancreas cells to oxidative stress instigated by hydrogen peroxide (H2O2). The viability of these cells exhibited a decline upon H2O2 treatment, which was effectively counteracted by the introduction of astaxanthin supplementation (p < 0.05). Oxidative stress induced a reduction in both total antioxidant capacity and reduced glutathione levels, while concurrently leading to an elevation in malondialdehyde concentrations. Nevertheless, the addition of astaxanthin served to ameliorate these effects and resulted in a reduction of malondialdehyde levels (p < 0.05). Collectively, these findings strongly indicate that astaxanthin plays a pivotal role in enhancing hepatic antioxidant capacity, both in vivo and in vitro, by effectively mitigating the deleterious impacts of ROS-induced damage.
In addition to influencing the activity of antioxidant enzymes and gene expression, astaxanthin operates through complex interactions with redox-sensitive signaling pathways in fish [99]. The presence of oxidative stress not only jeopardizes cellular integrity but also plays a crucial role in cellular signaling, impacting a range of physiological processes. Astaxanthin’s capacity to regulate redox-sensitive pathways assumes a dual role: safeguarding against oxidative damage and participating in signaling cascades [77][100][77,100]. Remarkably, the impact of astaxanthin on the nuclear factor-erythroid 2-related factor 2 (Nrf2) pathway has attracted considerable attention [23]. The Nrf2 pathway functions as a central overseer of the cellular antioxidant response, coordinating the activation of various genes responsible for cellular protection against oxidative stress [101][102][101,102]. Astaxanthin has demonstrated the ability to stimulate the Nrf2 pathway, leading to an increase in the expression of antioxidant enzymes [23]. Furthermore, astaxanthin’s interactions with other signaling pathways, such as mitogen-activated protein kinases (MAPKs) and nuclear factor-kappa B (NF-κB), underscore its potential to regulate broader cellular responses that extend beyond antioxidative defenses [103][104][103,104].
Astaxanthin’s role in modulating oxidative stress in fish presents promising implications for both aquaculture and environmental applications. In the realm of aquaculture, where stressors encompass a spectrum from physical handling and transportation to suboptimal water conditions, the addition of astaxanthin holds potential as a strategy to enhance fish resilience [98][105][98,105]. Through its capacity to augment antioxidant enzyme activity and influence gene expression, astaxanthin supplementation may offer a means to alleviate the adverse effects of stress on fish well-being and overall performance [106]. Furthermore, investigating how astaxanthin interacts with redox-sensitive signaling pathways could uncover innovative avenues for advancing aquaculture methodologies, fostering sustainable fish production, and minimizing ecological repercussions. Table 2 presents the effects of astaxanthin on oxidative status and growth across various fish species.
Table 2. Effects of astaxanthin on oxidative status and growth across various fish species 1.
| Fish Species | Astaxanthin Supplementation Levels in Feed | Form | Challenge 2 | Astaxanthin Effects on Oxidative Status | Astaxanthin Effects on Growth | Reference |
|---|---|---|---|---|---|---|
| Rainbow trout (Oncorhynchus mykiss) | 0.5, 2.0 g/kg | Synthetic | Yes | Reduced MDA and peroxide values and upregulation of the expression of antioxidant-relevant genes in fish fillet | Improved final weight and FCR | [97] |
| Yellow catfish (Pelteobagrus fulvidraco) | 0.08 g/kg | Synthetic | Yes | Higher levels of catalase activity and reduced MDA in the liver | Improved final weight and specific growth rate | [107] |
| Rainbow trout (Oncorhynchus mykiss) | 0.5, 2.0, 5.0 g/kg | Synthetic | Yes | Reduced MDA and improved T-AOC in blood serum, upregulation of the expression of antioxidant-relevant genes in the liver | Improved final weight and specific growth rate | [23] |
| Characin (Hyphessobrycon eques Stein-dachner) | 0.01, 0.02, 0.04 g/kg | Synthetic | Yes | Improved antioxidant capacity as measured by total antioxidant status and superoxide dismutase activity | No effect | [108] |
| Rainbow trout (Oncorhynchus mykiss) | 0.1 g/kg | Synthetic | Yes | Decrease in TBARS in muscle and liver cells; increased glutathione reductase activity; improved ratio of GSH to GSSG | Numerical improvement in final weight | [96] |
| Discus fish (Symphysodon aequifasciatus) | 0.2 g/kg | Synthetic | Yes | Improved antioxidant defense status | No effect | [109] |
| Coral trout (Plectropomus leopardus) | 0.05, 0.1, 0.2 g/kg | Natural | No | Elevated levels of catalase, superoxide dismutase, and glutathione peroxidase activities increased T-AOC in the serum and liver | No effect | [5] |
| Golden pompano (Trachinotus ovatus) | 0.2 g/kg | Synthetic | No | Elevated hepatic T-AOC and augmented levels of GSH to GSSG | Improved weight gain, specific growth rate and FCR | [98] |
| Rainbow trout (Oncorhynchus mykiss) | 0.1 g/kg | Synthetic and natural | No | IncreasedNrf2/HO-1 signaling and antioxidant enzyme activity | Improved final body weight and FCR | [110] |
1 Please note that these effects may vary depending on factors such as dosage, fish species, duration of supplementation, type of challenge, and individual fish physiology; 2 induced oxidative stress; MDA = malondialdehyde; T-AOC = Total antioxidant capacity; TBARS = Thiobarbituric acid-reactive substances; GSH = Reduced glutathione; GSSG = Oxidized glutathione; FCR = feed conversion ratio; Nrf2 = Nuclear factor erythroid-2 related factor 2; HO-1 = heme oxygenase-1.
