You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 1 by PONTI DONATELLA and Version 2 by Fanny Huang.
The activation of members of the Epidermal Growth Factor Receptor (EGFR) family (including ErbB) triggers pathways that have significant effects on cellular processes and have profound consequences both in physiological and pathological conditions. Within the nervous system, the neuregulin (NRG)/ErbB3 signaling plays a crucial role in promoting the formation and maturation of excitatory synapses. Noteworthy is ErbB3, which is actively involved in the process of cerebellar lamination and myelination. All members of the ErbB-family, in particular ErbB3, have been observed within the nuclei of various cell types, including both full-length receptors and alternative variants.
- ErbB receptors
- ErbB3
- Neuregulin (NRG)
- myelination
- ErbB receptors and alternative variants
- glioblastoma
- nucleus
- nucleolus
1. The Family of ErbB Receptors
The Epidermal Growth Factor Receptors (EGFRs) are four tyrosine kinases that can establish a wide network of communication among them and between different cellular pathways. Thus, they regulate and control, in turn, either physiological or pathological conditions. The family of EGFRs consists of four members, ErbB1, ErbB2, ErbB3, and ErbB4 (ErbB: erythroblastic leukemia viral oncogene homolog), also called Her1, Her2, Her3, and Her4 respectively (Her: Human epidermal growth factor). These glycoproteins are transmembrane tyrosine kinase receptors that, once activated, regulate important intracellular pathways involved in cell division, proliferation, differentiation, migration, angiogenesis, apoptosis, and survival. They can also influence cellular behavior in response to extracellular signals [1]. Substantial evidence supports the involvement of ErbB family in the development and progression of various types of cancer. ErbBs are frequently found in elevated levels in tumors [2][3][2,3]. In numerous cells, multiple members of the ErbB receptor family are co-expressed. Upon stimulation with growth factor ligands, these receptors can form both homo- and heterodimers [4]. Specifically, ErbB receptors can arrange into homodimers (ErbB1 and ErbB4) or, predominantly, heterodimers (ErbB1/ErbB2, ErbB3/ErbB2, and ErbB4/ErbB2), initiating unique intracellular signaling pathways. There are more than ten distinct ligands that activate ErbB receptor family members. Among them, three have been categorized as EGF agonists due to their specific binding to ErbB1 (EGF, TGF-α, and amphiregulin), four are specific to ErbB3 and/or ErbB4 (neuregulins (NRGs) also known as heregulin (HRG), while another three are bispecific, capable of binding to both ErbB1 and ErbB4 (betacellulin, epiregulin, and heparin-binding EGF-like) [4][5][6][4,5,6] (Figure 1). The NRG gene family comprises four members: NRG-1, NRG-2, NRG-3, and NRG-4. Within this gene family, numerous isoforms are generated through alternative exon splicing. These isoforms exhibit diverse tissue distributions and have various biological activities [7]. In most cases, these NRG proteins are initially synthesized as precursors and subsequently undergo activation and release from the cell membrane through specific proteolytic processes, [8]. The subsequent activation of various pathways and crosstalk between them, either within the same family or among different receptors, remains an intriguing challenge [9][10][9,10].
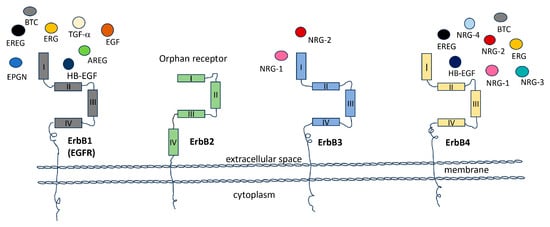
Figure 1. The receptor tyrosine kinases family (RTKs). The members of the Receptor Tyrosine Kinase (RTK) family (ErbB1, ERbB2, ERbB3 and ErbB4), play pivotal roles in a wide array of biological processes, encompassing apoptosis, cell cycle regulation, cell division, cytoskeletal organization, cell differentiation, developmental processes, immune responses, and dynamics within the nervous system. These receptors achieve their functions by interacting with specific ligands, making them some of the most extensively studied receptors in signal transduction and their involvement in oncogenesis. In the diagram, the Epidermal Growth Factor Receptor (ErbB1/EGFR, depicted in grey) is surrounded by its potential ligands, including amphiregulin (AREG), epiregulin (EREG), epigen (EPGN), epidermal growth factor (EGF), heparin-binding epidermal growth factor (HB-EGF), betacellulin (BTC), and transforming growth factor-alpha (TGF-a). ErbB2 (shown in green) is in its active conformation. ErbB3 (colored blue) is presented in conjunction with its ligands neuregulin (NRG-1) and neuregulin-2 (NRG-2), while ErbB4 (in yellow) is surrounded by its specific ligands, which include EREG, HB-EGF, NRG-1, NRG-2, NRG-3, and NRG-4.
They are functionally dependent and complementary, playing a significant role in heterodimer formation following ligand binding. This process is tightly controlled and is involved in various physiological conditions including embryonic development, as well as aggressive tumor scenarios like mammary lung cancer and glioblastoma [11]. Of particular interest is the relationship between ErbB2 and ErbB3, which, when functioning independently, are incomplete (with ErbB2 lacking the ligand binding site and ErbB3 lacking tyrosine kinase activity). However, when they bind with the NRG, they undergo heterodimerization and generate a potent activation signal. Furthermore, the interaction between ErbB3 and ErbB1 can have important implications for cancer biology and therapeutic strategies. Studies have shown that both ErbB3 and ErbB1 are frequently overexpressed in glioblastoma, the most common and aggressive form of glioma [11]. This receptor’s overexpression can lead to heterodimer formation and the activation of downstream signaling pathways, including the PI3K (phosphatidylinositol 3-kinases)-AKT (PKB—protein kinase B) and MAPK (mitogen-activated protein kinase) pathway, which can promote cell survival, proliferation, and invasion, and can contribute to glioma development and progression [12]. Overall, the interaction between ErbB3 and ErbB1 in glioblastoma is complex and multifaceted. Further research is needed to fully understand the nature and significance of this interaction and its potential as a therapeutic target.
To conclude, this family of receptors, along with its downstream pathway encompassing ligands and targets, holds significant relevance in numerous contexts, both physiological and pathological. Despite the multitude of studies dedicated to unraveling these intricate crosstalk interactions, further endeavors are required to fully comprehend the breadth of this mechanism.
2. ErbB Receptor Structure
Studies of the structures of the EGFR family allow us to understand conserved strategies of action, introducing strengths and weaknesses of the pathways and of targeting both. The general structure of the EGFRs is made of an endo-domain and an ecto-domain. The first domain is in turn composed of a transmembrane domain (23 aa), a juxta membrane domain (40 aa), and a tyrosine kinasic domain with the C-terminal tail (232 aa). The last domain is involved in the interaction between the receptor and the ligands and is composed of the N-terminal glycosylated extracellular domain (620 aa) [13]. After ligand binding, EGFRs interact with each other and form homo- or heterodimers, which then activate the intrinsic tyrosine kinase activity. This leads to the trans-phosphorylation of the receptors’ intracellular domains, which in turn recruit proteins involved in cytoplasmic signaling pathways associated with domain phosphorylation. Otherwise, with a partial homology sequence, the precise domain sequences vary in the EGFRs and lead them to acquire unique features. Higher analogy domain sequences are those that perform more similar functions in the four receptors, hence, less conserved domain sequences are those that perform different functions [10]. The highest grade of homology (64–67%) is verifiable in catalytic domains which form the essential structure for the tyrosine kinase activity [14]. This explains why several molecular inhibitors that bind to STP-binding cassettes target more than one ErbB-receptor [15]. The domains responsible for the interaction with the ligand show 43–45% homology between ErbB3, ErbB2; and ErbB1; in fact, they interact with similar ligands. The extracellular domains of ErbB3 show 50 conserved cysteine residues compared to ErbB2 and ErbB1, as well as ten partially conserved glycosylation sites. Interestingly, up to 30% of the entire molecular weight of ErbB3 is composed of the glycosidic groups. Just one of those glycosylation sites is conserved, which suggests that this modification contributes to the functional uniqueness of this receptor [16]. Glycosylation is important for ligand-dependent and -independent function of ErbB receptors, but also for protein stability and signaling. Mutation in the conserved glycosylation site (Asn to Gln) in a CHO cell line led to auto-dimerization and heterodimerization with ErbB2 even in the absence of the ligand and to the acquisition of neoplastic features by the cells [16]. Glycosylation in Asn-418 hampers tumor progression, stopping aberrant ErbB2-ErbB3 heterodimerization [10]. The proximal site of the transmembrane domain (21 aa) is very conserved. The cytoplasmic domain (277 aa) shows 60–62% sequence homology between ErbB3, ErbB1, and ErbB2 and overlaps with the tyrosine kinase catalytic domain. There is also one binding site for ATP (position 716–721) and a lysine residue with the C-terminal part conserved compared to the other members of the EGFR family. The most divergent region between ErbB3, ErbB2, and ErbB1 comprises 364 aa: Tyrosine residues (position 1197, 1199, and 1262) in proximity of phosphorylation sites and YEYMN repeated sequences (position 1260–1264). These hallmarks make this region the possible auto-phosphorylation site of ErbB3 protein [14]. ErbB4 is unique among ErbB receptors because it goes through alternative splicing. There are four different versions of ErbB4 mRNA obtained by two splicing locations: one in the outer part near the membrane and the other in the inner part, beyond the tyrosine kinase domain.
In summary, EGFRs share a common structural framework and a universal mechanism of activation and signaling, consisting of the following steps: ligand binding, homo- or heterodimerization, activation of the intrinsic tyrosine kinase activity, trans-phosphorylation of the intrinsic domain, and recruitment of associated proteins. It is worth noting that sequence variations lead the receptor to acquire distinctive characteristics alongside their shared functions. Among these shared features, certain targeting strategies can be advantageous or detrimental. These diversifications are prominently exemplified in the glycosylation sites of ErbB3, which can affect both ligand-dependent and -independent functions, as well as stability and signaling. Additionally, tyrosine residues near the phosphorylation site play a role as auto-phosphorylation sites, further illustrating the complexity of these receptors.
3. ErbB Receptors and Neural Development
Neuregulin/EGFR signaling is heavily involved in various aspects of neural development, from synapse formation to cellular differentiation, myelination, and establishment of pathways involved in psychiatric disorders. The members of the ErbB receptors family have been implicated in various aspects of neural development. NRG/ErbB signaling has been implicated in neural development, including circuit generation, axon lining, neurotransmission, and synaptic plasticity. Several members of this signaling network are encoded by genes involved in psychiatric disorders [17]. NRG/ErbB signaling also contributes to synapse formation and promotes the formation and maturation of excitatory synapses on GABAergic interneurons [18][19][20][21][18,19,20,21]. NRGs function by activating ErbB tyrosine kinases, such as ErbB2, ErbB3, and ErbB4 [22]. Specifically, the ErbB2 and ErbB4 receptors are involved in glial differentiation, which is necessary for the radial migration of neurons in the cortex and cerebellum, respectively [23]. Undifferentiated neural stem cells in the nervous system express high levels of ErbB1 or ErbB2 [24]. Following neural development and maturation, the level of ErbB1 decreases, and ErbB4 expression is elevated, both of which are limited to midbrain dopaminergic neurons and GABAergic cells [25]. In the glial lineage, ErbB1 expression is replaced by ErbB3 during development. The interactions of NRGs expressed on the neuronal surface with ErbB3 in oligodendrocytes promote their differentiation and myelination [26]. ErbB1 is distributed in dopaminergic neurons in the substantia nigra compacta, as well as in the ventral tegmental area [24]. Similarly, ErbB4 is expressed in the same regions but not always in the same dopaminergic cell population [25]. NRG1 has been linked to neural development and brain activity homeostasis. Nevertheless, the simultaneous knockout of ErbB2 and ErbB4 has no effect on cortical and cerebellar lamination [27][28][27,28]. Conversely, ErbB4 plays a critical role in the assembly of GABAergic circuitry [29][30][31][32][29,30,31,32], while ErbB3 is necessary for myelination in the central and peripheral nervous systems [33][34][35][33,34,35] (Figure 2). Although ErbB3 is expressed in the developing brain, its role in central nervous system development remains unknown due to the embryonic lethality caused by null mutation [36]. The NRG-ErbB signaling pathway appears to be common among mammals, functioning in both mice and humans. NRG plays a crucial role as a neuronally-released factor that activates microglia in the central nervous system (CNS). This activation is observed in response to experimental peripheral nerve injuries and degenerative diseases in both human and mouse models.
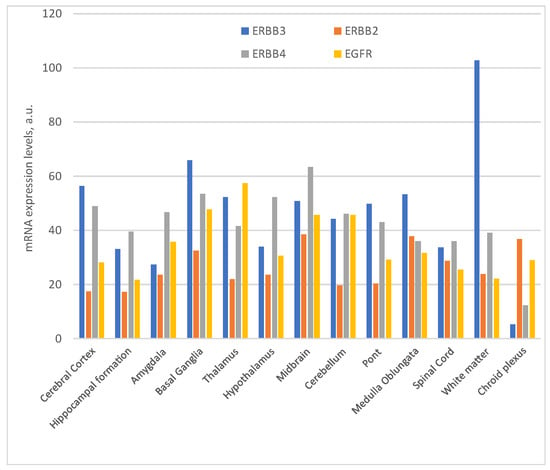
Figure 2. Expression of ErbBs mRNA in the Central Nervous System (nTPM: normalized transcript expression) https://www.proteinatlas.org/ (access on 2 October 2023).
In brief, the signaling pathways activated by NRGs and ErbB receptors play a crucial role in various aspects of neural development, maturation, and the maintenance of proper function and homeostasis, both in the central nervous system and the peripheral nervous system. These receptors are dedicated to distinct functions, leading to their expression in different locations and at various developmental stages, all highly coordinated. However, a comprehensive understanding of the precise roles that these receptors play in the brain remains a formidable challenge, as variations in their expression can potentially result in significant disorders.
